The changes in sleep patterns are a frequent complaint during pregnancy due to multiple physical, hormonal and physiological changes(Reference Neau, Texier and Ingrand1,Reference Pien and Schwab2) . Evidence indicates that total sleep time, daytime sleepiness and insomnia tend to increase in the first trimester of pregnancy, while in the second trimester, there is a tendency to decrease the overall quality of sleep(Reference Signal, Paine and Sweeney3,Reference Sedov, Cameron and Madigan4) . In the third trimester, there is a greater disturbance of sleep, with an increased risk of insomnia compared with early pregnancy(Reference Kızılırmak, Timur and Kartal5). It is estimated that the prevalence of poor sleep quality among pregnant women is between 29 % at the beginning of pregnancy and 79 % at the end of it(Reference Gelaye, Barrios and Zhong6,Reference Mindell, Cook and Nikolovski7) . Such data confirm the worsening of sleep complaints throughout the gestational period(Reference Sedov, Cameron and Madigan4,Reference Gelaye, Barrios and Zhong6) .
Research has shown that sleep duration and quality have a significant impact on eating patterns(Reference Theorell-Haglöw, Lemming and Michaëlsson8–Reference Crispim, Zimberg and dos Reis10). Although studies addressing this topic in pregnant women are scarce, existing research has indicated that a good sleep pattern (adequate duration and better quality) is associated with better diet quality(Reference Al-Musharaf11–Reference Bennett, Cain and Blumfield14). This includes a higher adherence to scores of the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH), which was associated with good sleep quality (P < 0·05)(Reference Shiraseb, Mirzababaei and Daneshzad15). Several mechanisms could explain the association between altered sleep patterns and changes in food intake. Shorter sleep duration may provide more opportunities for individuals to consume food, change the timing of food consumption and induce hedonic eating(Reference Capers, Fobian and Kaiser16–Reference Kim, DeRoo and Sandler20). Additionally, sleep deprivation may be linked to increased concentrations of the hunger-stimulating hormone ghrelin and decreased levels of the appetite-suppressing hormone leptin(Reference Taheri, Lin and Austin21,Reference Jakubowicz, Froy and Wainstein22) .
During pregnancy, the deterioration of sleep and the occurrence of insomnia(Reference Signal, Paine and Sweeney3,Reference Sedov, Cameron and Madigan4,Reference Mindell, Cook and Nikolovski7) are compounded by hormonal changes, such as increased progesterone levels and leptin resistance(Reference Ladyman, Augustine and Grattan23), which can lead to heightened appetite and increased food consumption(Reference Godos, Grosso and Castellano24). Moreover, poor sleep during pregnancy can impact chrononutrition patterns through associations between sleep and food consumption(Reference Loy, Loo and Godfrey25–Reference Messika, Toledano and Hadar27). Recent studies involving pregnant women and chrononutrition, which explore the relationship between the circadian clock, metabolic physiology and nutrition(Reference Loy, Loo and Godfrey25,Reference Chen, Loy and Chen26) , have demonstrated that eating during times that go against the body’s natural circadian rhythms, such as the inactive/sleep phase, negatively affects nocturnal metabolism, resulting in worsened glycaemic, insulinemic and lipidemic responses, as well as greater weight gain(Reference Loy, Loo and Godfrey25–Reference Facco, Grobman and Reid28).
Based on the above, identifying factors associated with food intake during the gestational period is crucial, as excessive energetic consumption and poor diet quality can contribute to maternal obesity. Maternal obesity, in turn, increases the risk of adverse conditions during pregnancy, such as hypertension, pre-eclampsia(Reference Rasmussen and Yaktine29), gestational diabetes(Reference Saldana, Siega-Riz and Adair30), a higher frequency of emergency caesarean delivery(Reference Haugen, Brantsæter and Winkvist31) and complications in newborn development(Reference Saldana, Siega-Riz and Adair30–Reference Fall32). Considering the limited research on this topic, we hypothesise that a worse sleep pattern throughout pregnancy, especially in the later stages, may be associated with unhealthy eating practices and excessive gestational weight gain. Thus, the aim of this study was to investigate the associations between sleep duration and quality with food intake, chrononutrition patterns, and weight gain during pregnancy.
Materials and methods
Study design and ethical aspects
This is a prospective cohort study conducted in a low-risk prenatal care outpatient clinics of the public health service in the city of Uberlândia, Minas Gerais, Brazil. The inclusion criteria were pregnant women with a single fetus who had their first prenatal consultation at these clinics up to the 12th week, were not shift workers and had no previous chronic non-communicable diseases. Exclusion criteria were pregnant women under 18 years of age, those who tested positive for HIV, syphilis, toxoplasmosis, rubella, cytomegalovirus, chickenpox, and pregnancies with malformed or anomalous fetuses.
The study was approved by the Human Research Ethics Committee (protocol: 1199829/2015) of the Federal University of Uberlândia. All procedures are in accordance with the principles of the Declaration of Helsinki, and all pregnant women signed the Informed Consent Form.
Sample selection
During the study period, 142 women in the first trimester of pregnancy were invited to participate. A total of eleven pregnant women declined to participate, ten were excluded because they did not meet the age criteria and twenty-one did not complete all assessments. Therefore, the final sample consisted of 100 pregnant women.
Data collection
Data collection took place during 2016, with assessments conducted once during each gestational trimester: first trimester (≤12 weeks), second trimester (20th–26th weeks) and third trimester (30th–37th weeks). Initially, a questionnaire was administered to collect demographic data, including the mother’s age, marital status, education and physical activity practices.
On a quarterly basis, the pregnant women were evaluated regarding food consumption, weight gain and sleep variables (duration and quality). They were also asked about episodes of nausea in the last 30 d and the frequency of these episodes. Results from the oral glucose tolerance test conducted between the 24th and 28th weeks of gestation were collected from the medical records of pregnant women. In this test, pregnant women were instructed to follow an 8-h fasting period. At the time of the sample collection, they were asked to confirm adherence to this fasting period. The oral glucose tolerance test involved measuring the baseline fasting serum blood glucose level, followed by the ingestion of a 75 g of glucose solution dissolved in 300 ml of water. Subsequent serum blood glucose levels were measured at 1-h and 2-h intervals after the consumption of the glucose solution(Reference Metzger and Gabbe33,Reference Alberti and Zimmet34) .
Demographic data
For the collection of sociodemographic data, a structured questionnaire was administered. Pregnant women were queried regarding their age (in complete years), marital status (married, living with a partner, single and widowed, with the results presentation grouping the options ‘married or living with a partner’), parity (no children, one child, two children, and three or more children), schooling (high school or below, above high school), and work away from home (yes) in the first, second and third trimesters.
Assessment of sleep quality and duration
To assess sleep quality and duration, the Pittsburgh Sleep Quality Index (PSQI)(Reference Buysse, Reynolds and Monk35,Reference Backhaus, Junghanns and Broocks36) was used. The PSQI has been translated and validated into Portuguese(Reference Bertolazi, Fagondes and Hoff37) and is a widely used and validated instrument to measure the subjective quality of sleep during pregnancy, treated as linear data(Reference Qiu, Gelaye and Zhong38,Reference Zhong, Gelaye and Sánchez39) . Sleep quality was classified as poor (score greater than or equal to 5 points) or good (score less than 5)(Reference Bertolazi, Fagondes and Hoff37). The components of the PSQI instrument include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disorders and use of sleep medication.
To assess sleep duration, the mean self-reported sleep duration was calculated considering weekdays and weekends using the formula: [(current sleep duration of the current weekday × 5) + (current sleep duration of the weekend × 2)]/7(Reference Reutrakul, Hood and Crowley40).
Anthropometric assessment
The height of pregnant women was measured only in the first assessment and used in all other trimesters. Pre-pregnancy weight was self-reported and used to calculate the pre-pregnancy BMI (kg/m2), which was classified according to the cut-off points proposed by the WHO: underweight (<18·5 kg/m²), normal weight (18·5–24·9 kg/m²), overweight (25·0–29·9 kg/m²) and obese (30·0–39·9 kg/m²)(Reference Rasmussen and Yaktine41).
Weight was measured using a scale with a precision of 0·1 kg (Welmy®). Height was measured to the nearest 0·1 cm using a wall-mounted stadiometer (Welmy®). Gestational weight was measured in the three trimesters, and BMI was classified according to the Atalah curve(Reference Samur, Castillo and Santoro42) for the gestational age.
Gestational weight gain was assessed according to the recommendations of the Institute of Medicine(Reference Rasmussen and Yaktine41). The adequacy of gestational weight gain was assessed in each trimester as follows: first, the recommended weight gain in each trimester was calculated considering the number of gestational weeks corresponding to the interval between assessments, except for the first trimester, in which weight gain was considered to be in the range of 0·5–2 kg. Subsequently, weight gain in each trimester was assessed using the current value of the measured weight subtracted from the weight value in the previous trimester, or the pre-pregnancy weight in the case of the first trimester.
Assessment of food consumption
Dietary intake was determined using three 24-h recalls (R-24h) in each trimester, on non-consecutive days, including a weekend day, totalling nine dietary recalls throughout pregnancy. The R-24h with implausible data, defined as energy intake less than 500 kcal/d or more than 3500 kcal/d(Reference Loy, Wee and Colega43), were excluded from the average consumption calculations. All variables were calculated using the average of the R-24h in each quarter.
To classify the types of meals (breakfast, morning snack, lunch, afternoon snack, dinner and evening snack), perceptions of the type of meal were considered(Reference Trancoso, Cavalli and Proença44), and the type of meal was also analysed based on frequently consumed foods by the Brazilian population at each meal(Reference Sato, Fujimori and Szarfarc45). Energy intake and nutrient intake were calculated using the Dietpro® software, version 5i, with the Brazilian Table of Food Composition(46) as a reference, along with information from food labels and the Table of the United States Department of Agriculture (USDA – United States Dietetic Association 2005)(47). The dietary variables used were energy (kcal), fat (kcal and %), protein (kcal and %) and carbohydrate (kcal and %). The decision to use the dietary data was made a priori.
Chrononutrition variables
The number of eating episodes was determined by the number of energetic events ≥50 kcal/d, with time intervals between food and/or beverage consumption of ≥15 min(Reference Gibney and Wolever48). The times of meals and snacks were reported for each eating episode. Eating duration was calculated as the period from the first energetic intake after awakening to the last energy intake before sleep onset(Reference Gill and Panda49). Night fasting was determined by calculating the hours between the first and last eating episodes of each day and individually subtracting this time from 24 h(Reference Marinac, Natarajan and Sears50).
Meal times for the following meals were analysed: breakfast (h:min); morning snacks (h:min); lunch (h:min); afternoon (h:min); dinner (h: min); nighttime snack (h:min); first meal (h:min); and last meal (h:min). The time-related eating patterns included: number of meals; eating duration (hours); energetic midpoint (h:min); and fasting hours.
Statistical analysis
The sample size was determined using G * Power software version 3.1(Reference Faul, Erdfelder and Lang51). The calculation of the sample size was based on the linear regression, with an effect size of 0·25, α level of 0·05, power of 95 % one group, one measure, correlation between repeated measures of 0·5 and non-sphericity correction ε of 1. Based on these specifications, a total sample of ninety-four women was required.
Statistical analyses were performed using IBM SPSS Statistics 20 software. The Kolmogorov–Smirnov test was performed to test the normality of the data. Data are presented as mean and standard deviation.
Linear regression analyses were conducted to associate sleep duration and quality with food intake during the gestational period. For each dependent variable (total energy content and macronutrients, percentage of meals and energy content, meal times, snacks, and chrononutrition variables), a separate model was performed to assess their association with the independent variables (sleep duration and quality) in each gestational trimester. All models were adjusted for age, education, frequency of nausea in the last 30 d and pre-pregnancy BMI. Statistical tests with P < 0·05 were considered significant.
Results
Most pregnant women were married or lived with a partner (77 %) and had a high school education level (74 %), with a mean age of 27·3 years. A total of 37 % of pregnant women had a pre-pregnancy BMI classified as overweight (overweight and obesity combined). Approximately 80 % of the pregnant women did not engage in physical activity (83 % during the first trimester and 80 % during the third trimester), 56 % of the pregnant women worked during the first trimester and 43 % worked during the third trimester (Table 1).
Table 1. Sociodemographic data and lifestyle of pregnant women by gestational trimesters (n = 100)
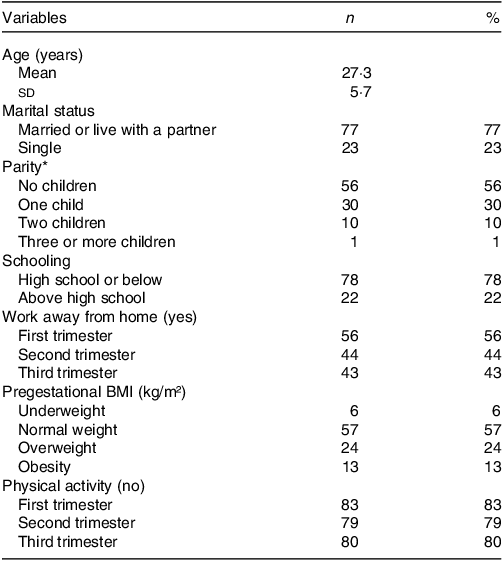
Values are presented as mean and sd for normally distributed data, or n (%).
*Data are missing for three (3 %) pregnant women.
Six pregnant women were diagnosed with gestational diabetes, but due to their favourable clinical progress and well-controlled blood glucose levels, they continued to receive care at the same clinic and remained part of the study.
Table 2 presents the associations between sleep duration and sleep quality with anthropometric variables. During the first trimester of pregnancy, we found a positive association between the PSQI sleep quality score and BMI (β = 0·420, P < 0·001), and a negative association between the PSQI sleep quality score and weight gain (β = –0·263, P = 0·030).
Table 2. Associations between sleep duration and sleep quality score PSQI and anthropometric variables (n = 100/per quarter)
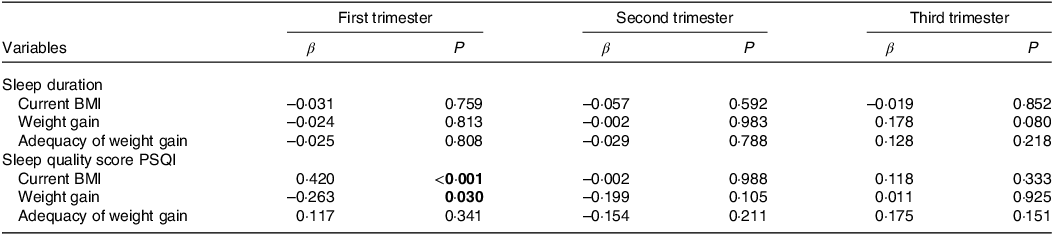
PSQI, Pittsburgh Sleep Quality Index.
Linear regression analysis model adjusted for age, education, frequency of nausea in the last 30 d and gestational BMI. Significant values are assumed as P < 0.05. Significant associations shown in bold. Independent variable: sleep duration and sleep quality score.
Table 3 shows the associations between sleep duration and food consumption during pregnancy. Positive associations were found between sleep duration and dinner time (β = 0·228, P = 0·025) in the first trimester and with morning snack time (β = 0·315, P = 0·026) in the second trimester. A negative association was also found between sleep duration and dinner time in the third trimester (β = –0·223, P = 0·026).
Table 3. Associations between sleep duration and food consumption (n = 100/per quarter)
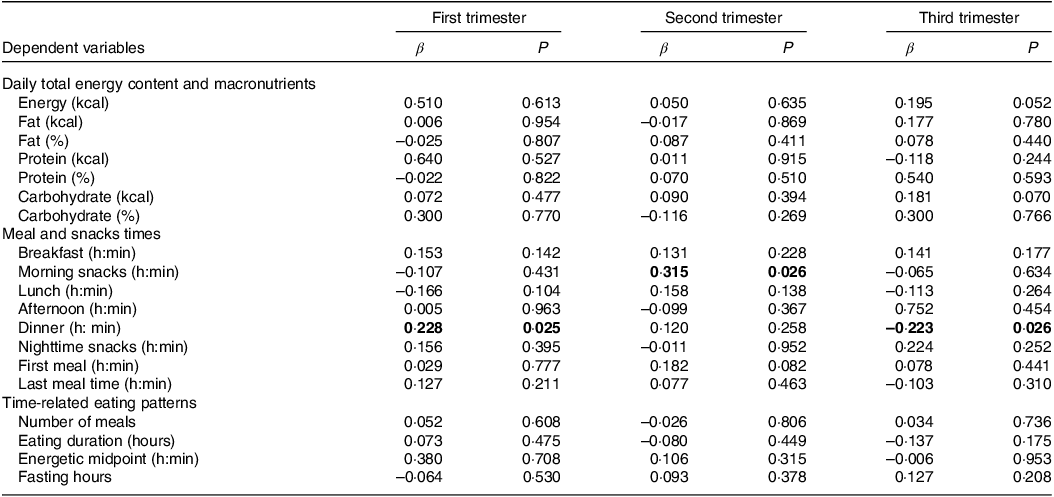
Linear regression analysis model adjusted for age, education, frequency of nausea in the last 30 d and gestational BMI. Significant values are assumed as P < 0.05. Significant associations shown in bold. The duration of eating was determined in the interval between the first and the last energetic event. Fasting hours were determined by calculating the hours between the first and last eating episodes of each day and subtracting this time from 24 h. Independent variable: sleep duration.
Table 4 shows associations between the PSQI sleep quality score and food consumption during the gestational period. During the third gestational trimester, positive associations were found between the PSQI score and the consumption of total energy content (β = 0·243, P = 0·044) and total fat (β = 0·291, P = 0·015), and a negative association with the time of the first meal (β = –0·255, P = 0·034). Also, in the third gestational trimester, associations were found between the PSQI sleep quality score and the number of meals (β = 0·252, P = 0·037), the average energetic intake per d (β = 0·243, P = 0·044), and total fasting in hours (β = –0·255, P = 0·034).
Table 4. Associations between PSQIª sleep quality score and food consumption (n = 100/per quarter)
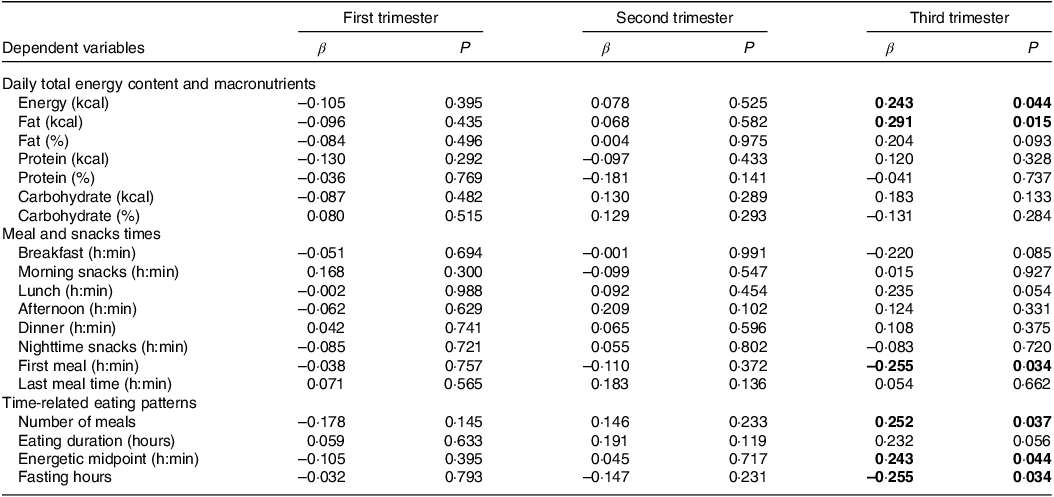
PSQI, Pittsburgh Sleep Quality Index.
Linear regression analysis model adjusted for age, education, frequency of nausea in the last 30 d and gestational BMI. Significant values are assumed as P < 0.05. Significant associations shown in bold. The duration of eating was determined in the interval between the first and the last energetic event. Fasting hours were determined by calculating the hours between the first and last eating episodes of each day and subtracting this time from 24 h. Independent variable: sleep quality score.
Online Supplementary Table S1 shows the energetic and macronutrient intake, meal and snacks times and time-related eating patterns (n = 100 per quarter).
Discussion
The present study evaluated the association between sleep duration and quality with food consumption and weight gain in each gestational trimester. To the best of our knowledge, this is the first cohort study with pregnant women to provide results on the associations between sleep variables and food consumption and body weight. Overall, longer sleep duration was associated with a later dinner in early pregnancy and an earlier dinner in late pregnancy, as well as with a later morning snack in the second trimester of pregnancy. Additionally, poor sleep quality was associated with a higher BMI in early pregnancy and with higher energy and fat intake, an earlier timing of the first meal, and several other chrononutrition variables, including a greater number of meals, higher energetic midpoint, and shorter fasting time in late pregnancy. Most of these results confirm our initial hypothesis that a worse sleep pattern throughout pregnancy is associated with unhealthy eating practices. These findings are relevant, as poor diet quality and an unfavourable sleep pattern negatively impact maternal(Reference Rasmussen and Yaktine41) and newborn health(Reference Fall32,Reference Moreno-Fernandez, Ochoa and Lopez-Frias52) .
Our results showed that worse subjective sleep quality is associated with a poorer pattern of food consumption (higher energetic and fat consumption) in the last gestational trimester (Table 4). These findings suggest that poorer sleep quality impacts unhealthy eating practices. Among the few studies on this subject, Van-Lee et al. (2017)(Reference Van-Lee, Chia and Loy12) also found, in a cross-sectional study, that pregnant women with good sleep quality (PSQI ≤ 5) reported better diet quality (P = 0·032) and better intake of vegetables, fruits, and rice compared with pregnant women with poor sleep quality (PSQI ≥ 5). In a recent cohort study with 140 pregnant women, it was observed that sleep quality worsened in the middle and at the end of pregnancy (both P = 0·001), and energy consumption in the second (P = 0·048) and third trimester (P = 0·044) was positively associated with worse sleep quality(Reference Al-Musharaf11). A meta-analysis on the same topic, but which analysed a non-pregnant population, observed that poor subjective sleep quality was associated with greater consumption of processed foods rich in simple sugars(Reference Godos, Grosso and Castellano24).
In this study, we found that pregnant women with worse sleep quality were associated with higher BMI and lower gestational weight gain, both in the first trimester. This result suggests that a history of overweight/obesity prior to pregnancy seems to have an impact on worse sleep quality during pregnancy, in addition to favouring unwanted weight gain during pregnancy and weight retention in the postpartum period. Therefore, the eutrophic state can be a protective factor for adequate weight gain and better gestational outcomes. Our findings are relevant because in the first trimester, little or no weight gain is recommended(Reference Rasmussen and Yaktine41). Similar results were found in a recent prospective study with pregnant women, in which worse sleep quality and short sleep duration in early pregnancy were associated with a higher proportion of women with high BMI (>25 kg/m²), and in late pregnancy, poor sleep quality was associated with greater fat gain(Reference Hill, Lipsky and Betts53).
Chronobiological disorders have recently been identified as risk factors for morbidities such as obesity and diabetes mellitus(Reference Messika, Toledano and Hadar27). Chrononutrition disorders are related to the frequency and content of meals according to the sleep–wake cycle, sleep disorders related to sleep quality, and chronoobesity disorders, such as abnormal weight gain due to sleep deprivation and inadequate eating times(Reference Messika, Toledano and Hadar27). Our study found a greater occurrence of associations between worse sleep quality and a worse chrononutrition pattern in the third trimester of pregnancy, which is shown in the literature as the period of pregnancy with the greatest complaints of sleep deprivation(Reference Sedov, Cameron and Madigan4).
Meal times are closely linked to health markers(Reference Gallant, Lundgren and Drapeau54) and may promote an important circadian misalignment in physiological, endocrine, metabolic and behavioural aspects(Reference Garaulet and Gómez-Abellán55). From this perspective, eating late at night has been related to dysregulation of the hunger and satiety mechanism(Reference McHill, Phillips and Czeisler56). Teoh et al. (2023)(Reference Teoh, Kaur and Shafie57) found results similar to ours regarding the relationship between sleep and chrononutrition patterns in pregnant women, and in a study with 114 pregnant women in Malaysia, in the adjusted linear regression model, the authors found that a lower frequency of meals and fat intake during dinner were significant predictors of poor sleep quality (β = −0·266, P = 0·035 and β = −0·232, P = 0·026, respectively).
Findings from the present study also revealed that longer sleep duration was associated with a later dinner time in early pregnancy and an earlier dinner time in late pregnancy. This opposite direction of associations in different trimesters may have occurred due to the dynamic way in which the sleep pattern and food consumption course and possibly relate to each other throughout pregnancy. As reported in the literature, insomnia often occurs in early pregnancy(Reference Sedov, Cameron and Madigan4,Reference Kızılırmak, Timur and Kartal5) along with hormonal changes, such as increased progesterone(Reference Ladyman, Augustine and Grattan23,Reference Czyzyk, Podfigurna and Genazzani58) , both of which can favour greater daytime sleepiness, a frequent complaint in this early stage of pregnancy(Reference Signal, Paine and Sweeney3,Reference Tsai, Lee and Lin59) . This possible irregular sleep dynamics for the light cycle of the day may impact on routine eating times and favour late food consumption and consequent higher nighttime energetic intake(Reference Gontijo, Balieiro and Teixeira60), which may also contribute to inadequate glucose metabolism and excess gestational weight gain(Reference Loy, Chan and Wee61,Reference Parrettini, Caroli and Torlone62) . Longitudinal studies(Reference Gontijo, Balieiro and Teixeira60,Reference Bo, Musso and Beccuti63,Reference Maukonen, Kanerva and Partonen64) have found associations between nighttime energy intake and an increased risk of excess body weight, and there are also some studies of nighttime food consumption with pregnant women who reported negative outcomes for maternal–fetal health(Reference Gontijo, Balieiro and Teixeira60,Reference Chandler-Laney, Schneider and Gower65–Reference Englund-Ögge, Birgisdottir and Sengpiel67) . Evidences report that in the last gestational trimester, in addition to hormonal and metabolic changes, anatomical changes also occur(Reference Neau, Texier and Ingrand1,Reference Parrettini, Caroli and Torlone62) , with the growth of the uterus and consequent progressive lordosis(Reference Betsch, Wehrle and Dor68), a characteristic finding of pregnancy to compensate for the weight of the abdomen. Consequently, there are more reports of breathing difficulties, locomotion and fatigue(Reference Neau, Texier and Ingrand1,Reference Betsch, Wehrle and Dor68) in this period, and these changes may still collaborate for an earlier night rest, but with greater complaints of night awakenings and/or fragmented sleep and insomnia, which may favour an earlier dinner or not making dinner.
There are some limitations in our study. The evaluations were made through questionnaires, which are subjective and depend on the motivation and memory of the participants. However, to obtain data to minimise memory bias, respondents were trained before participating in the research. In addition, sleep was subjectively assessed, and studies using objective measures of sleep, such as polysomnography, would allow a greater understanding of the dynamics and architecture of sleep. Nevertheless, the PSQI is a valid instrument for population studies(Reference Qiu, Gelaye and Zhong38,Reference Zhong, Gelaye and Sánchez39) . Furthermore, despite the majority of pregnant women being in their first pregnancy or having only one child, we acknowledge that the number of children can influence the interpretation of sleep data, which can also be considered a study limitation. Our results are based on only 100 pregnant women who had regular consultations in the public health system, and the generalisation of the results to pregnant women at the population level cannot be made.
We conclude that most of the associations found in the present study show that poor sleep is associated with higher energetic and fat consumption and higher BMI. Longer sleep duration was associated with a later dinner in early pregnancy and an earlier dinner in late pregnancy, as well as with a later morning snack in the second trimester of pregnancy. Further studies should explore the mechanisms that underlie these changes in sleep, dietary patterns, chrononutrition and weight gain during pregnancy.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/S0007114523002908
Acknowledgements
The authors thank all of the pregnant women who agreed to participate in this study.
Nothing to declare.
The authors’ responsibilities were as follows: L.C.T.B., C.A.G., W.M.F., Y.C.P.M. and C.A.C. conceptualised and designed the study; L.C.T.B., C.A.G., G.P.T. and W.M.F. collected the data; N.C.S., L.C.T.B., C.A.G., Y.C.P.M. and C.A.C. analysed and interpreted the data; N.C.S. wrote the initial manuscript; N.C.S., L.C.T.B., C.A.G., G.P.T., W.M.F., Y.C.P.M. and C.A.C. reviewed the manuscript and approved the final manuscript.
The authors declare no conflicts of interest.







