Introduction
In the 21st century, malaria remains one of the biggest global issues in public health. Although international aid and Government spending in an enhanced governance context have an impact positively on the efficiency of malaria prevention programmes [Reference Atake1], the remarkable progress in the fight against the disease still falls short of the expected results because of the huge gap between the needs and the available resources. Indeed, in 2013, the funding of malaria control programmes represented only 53% of US$5.1 billion needed to achieve the global objectives regarding the control and eradication of malaria [Reference Zelman2]. Thus, millions of people at risk still do not have access to diagnostic tests and therapeutic combinations based on artemisinin, which resulted in 198 million new cases and 584 000 deaths around the world [3, 4].
The endemic disaster of malaria plagues mainly low-income countries and affects the poorest and the most marginalised communities. These communities are also those who have the least access to preventive measures and diagnostic/therapeutic services [3–5]. As a result, malaria remains the leading cause of mortality in sub-Saharan Africa. In 2015, the region was home to 89% of global malaria cases and 91% of malaria deaths [Reference Atake1]. Among all persons at risk, pregnant women and children under 5 years are the most vulnerable [5, Reference Maitland6].
In Guinea, malaria is the most common cause of consultation, hospitalisation and death in public and private health facilities regardless of age [7]. In 2012, the Demographic and Health Surveys (DHS) showed that the overall prevalence of malaria among children aged 6–59 months was 44% with large variations according to the area of residence (53% in rural areas vs. 18% in urban areas; 66% in Faranah vs. 3% in Conakry). Although not investigated at that time, the geographical disparity was nonetheless raising alarming concerns [7].
The major concern that emerged from the DHS was that more than 60% of children under 5 who had had fever within the 2 weeks that preceded the survey had not received any treatment. However, in its vision of ‘a society in good health’ prior to this survey, the National Health Policy (NHP) had defined several essential targets of good health that included: a universal access to quality care without barriers (geographic, economic or socio-cultural), the community accountability for a better involvement of the population in the management of their health problems, and the availability of trained, motivated and valued health staff [8].
Thus, the need to reorganise and implement a national response challenged Guinea health authority while it was going through the worst health crisis in its history: the Ebola virus epidemic. In 2013, a national initiative led to the implementation of a National Strategic Plan (NSP) for malaria [8]. One of the essential components of the NSP was the prevention and rapid management of malaria among pregnant women and children under 5 and one objective was to achieve rapid management of malaria cases in 80% of children under 5.
To evaluate the results at the mid-term of the NSP, a National Malaria Control Intervention Coverage Survey (NMCICS) was conducted in 2016. The present study is a secondary use of data from this survey. It aims to describe the socio-demographic and health determinants of rapid management of fever (RMF) in children under 5.
Methodology
Type of study
A descriptive study was carried out on a sample of 4876 Guinean children under 5 living in 2874 households and participants in the NMCICS. This is a secondary analysis of data from the cross-sectional survey conducted between 17 February and 18 March 2016 to assess the coverage of anti-malaria interventions in 2013 in Guinea. The NMCICS sponsored by the National Malaria Control Program (NMCP) targeted all Guinean households and members of these households, especially women aged 15–49 years who were pregnant and/or supporting at least one child under 5.
Data collection
A two-stage cluster sampling was used for household selection. The primary sampling unit was the Guinean census geographic entities (named: ‘Zone de Dénombrement’) as defined by the map of the third General Census of Population and Housing [9]. To determine the required sample size, the survey selected an input indicator (the proportion (26.7%) of pregnant women who slept under insecticide-treated mosquito net according to the DHS) associated with the most weakly represented subpopulation in the population. The introduction of the value of this indicator in the formula that determined the size of the sample with a cluster effect of 1.5 and a non-response rate of 10% led to a sample size of 5280 households. This size was proportionally distributed by administrative region and area of residence (3200 rural and 2080 urban) according to their respective demographic weights. This study involved all children under 5, members of one of the households sampled.
The survey selected 83 officers (70 investigators and 13 supervisors) and trained them to administer two questionnaires (household and individual) that were tested in the pilot phase, harmonised and validated by the steering committee of the NMCICS. The survey used a semi-structured interview approach with a two-part questionnaire administered and completed by an interviewer. The first part of the questionnaire related to the household (structure, way of life, etc.) was addressed to the head of household. The second part related to each woman and each of her children (maternity, schooling, health including fever and malaria, etc.) was addressed to women aged at least 15 years or to men (in a household having no woman aged at least 15 but in which at least one child lives).
According to the current Guinean legislation, this secondary data exploitation does not change the routine management of patients and does not require the opinion of an ethics committee. Nevertheless, a favourable opinion was obtained from the ethics committee on the NMCICS protocol and each person participating in the survey (head of household and woman or child's legal representative) signed an informed consent.
Operational definition of variables
RMF was defined in terms of recourse to primary care for the treatment of fever which did or did not lead to treatment for malaria. In the present study, the types of recourse are: no recourse (R0), use of a health facility (hospital, health centres, clinic, etc.) or community health officers trained by the NMCP for the prevention of malaria (R1), use of a pharmacy (R2), use of a traditional healer (R3). Each type of recourse is described by the estimated proportion of cases among children who had fever within the 2 weeks that preceded the survey. A focus was put on R0 and R3 to highlight the potential determinants of self-medication and traditional treatment regarding malaria.
Diagnosis of malaria was confirmed by a proof of diagnosis (paraclinical diagnostic test results) and malaria management in medical records or in a health sheet (medical prescription). The prevalence of malaria was estimated among the children included in the study and among children with reported fever.
The studied factors were selected among the socio-demographic characteristics collected by the survey. The main factors concerned: (i) the household and the child's socio-demographic characteristics: household size (⩽ 3, 4–6, ⩾7 people), child's mother age (<25, 25–34, 35–44, ⩾45 years), highest education level in the household (none, primary, secondary, technical and higher), head of household occupation (employee, farmer, self-employed, unclassified, student or unemployed), area of residence (rural, urban), administrative region (Boké, Conakry, Faranah, Kankan, Kindia, Labé, Mamou, N'Zérékoré), distance between the household and the nearest health facility (0–5 km-public, 0–5 km-private, >5 km-public or private) and child age (0–12, 13–36, 37–60 months); (ii) history in the national projects for malaria prevention: mother has been pregnant during the past 2 years and received during pregnancy, one insecticide-treated net (ITN) accompanied each dose of an intermittent preventive treatment (IPT) by sulfadoxine/pyrimethamine (not pregnant, pregnant and not ITN and not IPT, ITN + incomplete IPT: <3 doses, ITN + complete IPT: ⩾3 doses); (iii) the level of awareness regarding the availability of drugs and their free access in hospitals or from community-based agents (0: no information, 1: only know it is free or only know it is available, 2: know at once that drugs are free and available).
Statistical analysis
The main household and children characteristics are described in terms of average and standard deviation (quantitative variables) or proportion (qualitative variables). Student t test was used to compare averages between groups and Pearson's χ 2 test or Fisher's exact test to compare frequencies between groups.
Cartographic analyses (prevalence of fever, malaria prevalence among children with reported fever, proportion of children in care within 24 h, malaria prevalence among children in care within 24 h) were used for comparisons between administrative regions or between rural and urban areas within these regions. The map coordinates stemmed from the SOGEFI (https://www.sogefi-sig.com/ressources).
For the analysis of the relationship between the type of recourse for treatment of fever and its predictors, RMF (as a discrete variable) was classified into three unordered modalities (recommended recourse (R1 + R2), traditional healer's recourse (R3), no recourse (R0)). A two-level hierarchical model (subject level and household level) was chosen to take into account the correlation induced by the household effect. A mixed polytomic unordered regression model was preferred.
A multivariate analysis was performed using a mixed-effect model that included covariates whose association strength was ⩾80% in the univariate analysis. In addition, there was a strong correlation between the IPT and the level of awareness and between the area of residence and the occupational activity; the model was thus extended with two terms of interaction, one for each couple of correlated covariates. Only significant interaction terms at the level of P < 0.01 in the univariate analysis (performed with a mixed polytomic unordered regression model) were retained for the final model. The effect of each variable was evaluated by the likelihood ratio test.
Model analyses were run using the generalised linear and latent mixed models programme [Reference Skrondal and Rabe-Hesketh10]. Differences were considered as significant at the 5% level of significance and all computations were run using STATA software, version 14 (College Station, Texas, USA).
Results
The present study included 4876 children under 5 from 2874 households. The mean number of children under 5 per household was 1.7 ± 0.9 (1.8 ± 1.0 in rural areas vs. 1.5 ± 0.8 in urban areas, P < 0.001). Among the 2874 households with at least one child under 5, the mean household size was 7.3 ± 3.8 (7.4 ± 3.6 in rural vs. 7.2 ± 4.0 in urban areas, P = 0.22). In these households, the study found 3429 eligible women whose number of children (under 5) varied from 1 to 7 (mean: 1.4 ± 0.6 per woman, 1.4 ± 0.7 in rural vs. 1.3 ± 0.6 in urban areas, P < 0.001). The mean age of these women was 28.4 ± 7.9 years (28.3 ± 7.8 in rural vs. 28.4 ± 8.1 urban areas, P = 0.77).
Within the 2 weeks that preceded the survey, 1491 children (31.2%) had a bout fever. This prevalence was higher among children from households with at least seven members (33.1%, P < 0.001) or those living in rural areas (33.1%, P < 0.001). However, the prevalence of fever was lower in children with at least one parent with high level of education (26.3, P = 0.03). It varied significantly from one administrative region to another, with a disparity between urban and rural areas (Table 1). Similarly, the prevalence of malaria was significantly higher in the region of Nzérékoré than in other regions (20.5%, P < 0.001). It was also higher among children whose mothers had a complete IPT during their last pregnancy (17.3%, P < 0.001) (Table 1).
Table 1. Distribution of fever and malaria prevalence according to the socio-demographic characteristics of the participant children
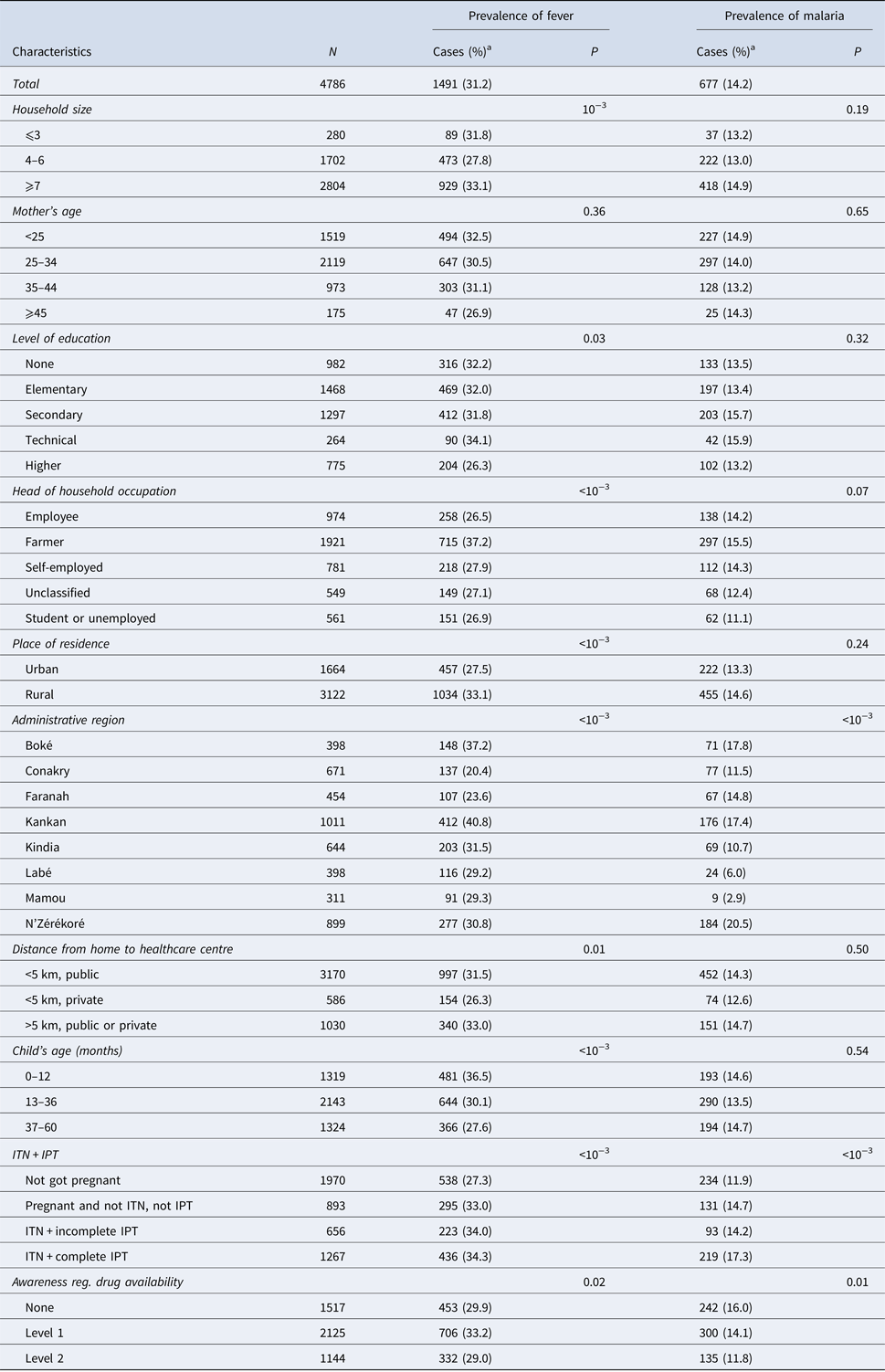
a Values are number (line percentage); ITN, insecticide-treated nets; IPT, intermittent preventive treatment.
The prevalence of malaria among children with reported fever was significantly higher in urban than in the rural areas in the region of Boké, Kindia, Labé, Mamou and Faranah (P < 0.001). However, this malaria prevalence after fever was higher in rural than in urban areas in the regions of Kankan (42.1% vs. 31.1%, P < 0.01) and N'Zérékoré (64.3% vs. 62.5%, P < 0.01) (Fig. 1).

Fig. 1. Distributions of the prevalence of fever and the prevalence of malaria in febrile children by place of residence (urban vs. rural) and administrative region.
Among the children with fever, 9.6% used a traditional therapy and 71.5% used a hospital or community service (Table 2). Compared with parents without any educational level (68.0%) or having attended university (69.5%), parents with secondary (75.1%) or technical (78.9%) education had a significantly higher use of hospital/community services (P = 0.02). The proportion of recourse to the pharmacist is high in urban areas, whereas the recourse to a traditional healer remains high in rural areas (P < 0.001).
Table 2. Distribution of the types of recourse for treatment of fever according to the socio-demographic characteristics of the participant children
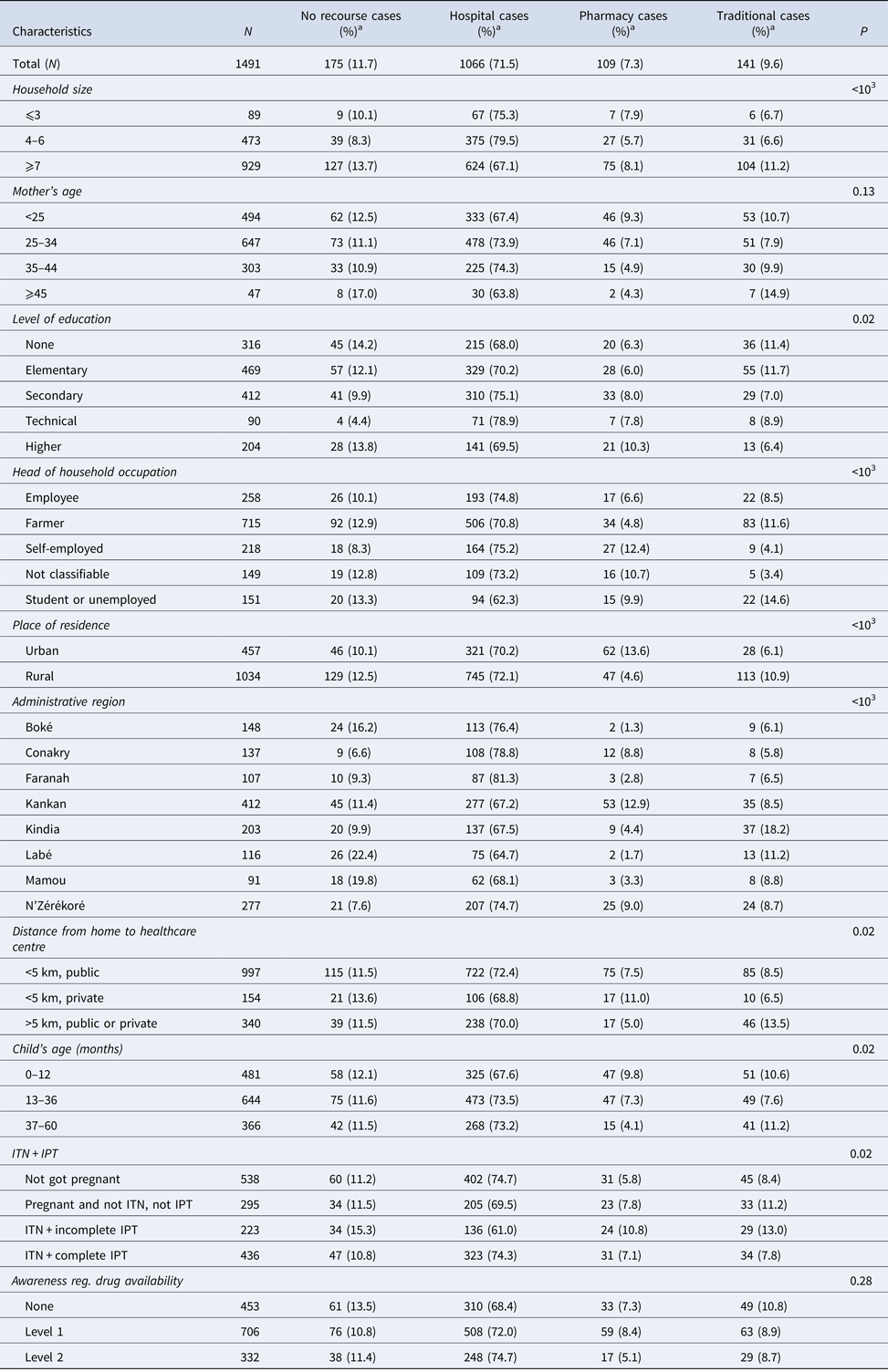
a Values are number (line percentage); ITN, insecticide-treated nets; IPT, intermittent preventive treatment.
The mixed and multivariate polytomic regression (Table 3) shows an association between the use of traditional healers and the household size. The odds ratio (OR) of using a traditional therapy was 1.5 times higher in households located more than 5 km from a health centre (adjusted OR 1.5, confidence interval (CI) 1.0–2.3, P = 0.04) and 2.2 times higher when the household was located in a rural area (adjusted OR 2.2; CI 1.5–3.3, P < 0.001). The OR of using a traditional therapy was halved when the level of education increased by one increment (adjusted OR 0.5, CI 0.4–0.6, P < 0.001).
Table 3. Associations of subject demographic characteristics with the types of recourse for treatment of fever according to the multivariable polytomous mixed regression modelling (N = 1491; base = recommended recourse)
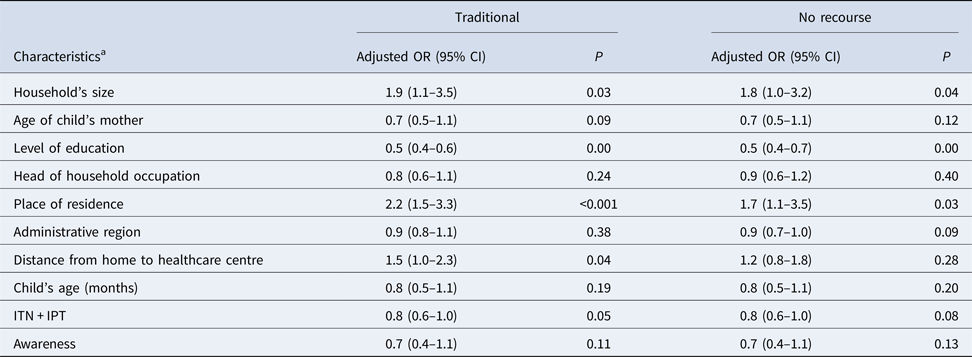
a All characteristics have shown significant relationships (P < 0.001) in the univariate analysis. In each of the following characteristics, the first modality is the reference: household's size (⩽3, 4–6, ⩾7 people); age of child's mother (<25, 25–34, 35–44, ⩾45 yrs); Level of study (None, primary, secondary, technical, higher); Head of household occupation (employee, farmer, self-employed, unclassified, student or unemployed); area of residence (rural, urban); administrative region (Boké, Conakry, Faranah, Kankan, Kindia, Labé, Mamou, N'Zérékoré); distance from home to healthcare centre (0–5 km-public, 0–5 km-private, >5 km-public/private); child's age (0–12, 13–36, 37–60 months); IPT = intermittent preventive treatment (not pregnant, pregnant and not ITN and not IPT, ITN + incomplete IPT, ITN + complete IPT); level of awareness (0: no information, 1: only know it is free or only know it is available, 2: know at once that drugs are free and available).
However, the decision to avoid the recourse for the management of fever was not related to the distance to a health centre (adjusted OR 1.2, CI 0.8–1.8, P = 0.28) or the level of awareness on the availability of antimalarial drugs (adjusted OR 0.7, CI 0.4–1.1, P = 0.11).
Overall, the proportion of febrile children who received a treatment within the recommended timeliness (24 h) was 74.9% (74.1% in rural vs. 76.6% in urban areas, P = 0.30). In urban areas, this proportion of rapid response varied significantly from one region to another ranging from 68.2% in Faranah to 79.2% in Labé or N'Zérékoré (79.2%, P < 0.001). Similarly, this difference between rural areas was also significant and ranged from 68.2% in Faranah to 100% in Mamou (P < 0.001). The difference seen between urban and rural areas was significant only between Kankan and Mamou (Fig. 2). Among the children with reported fever and admitted within 24 h, the prevalence of malaria was higher in urban than in rural areas in six regions but not in Kankan (where the inverse was seen) and N'Zérékoré (where the difference in prevalence was not significant, P = 0.46).
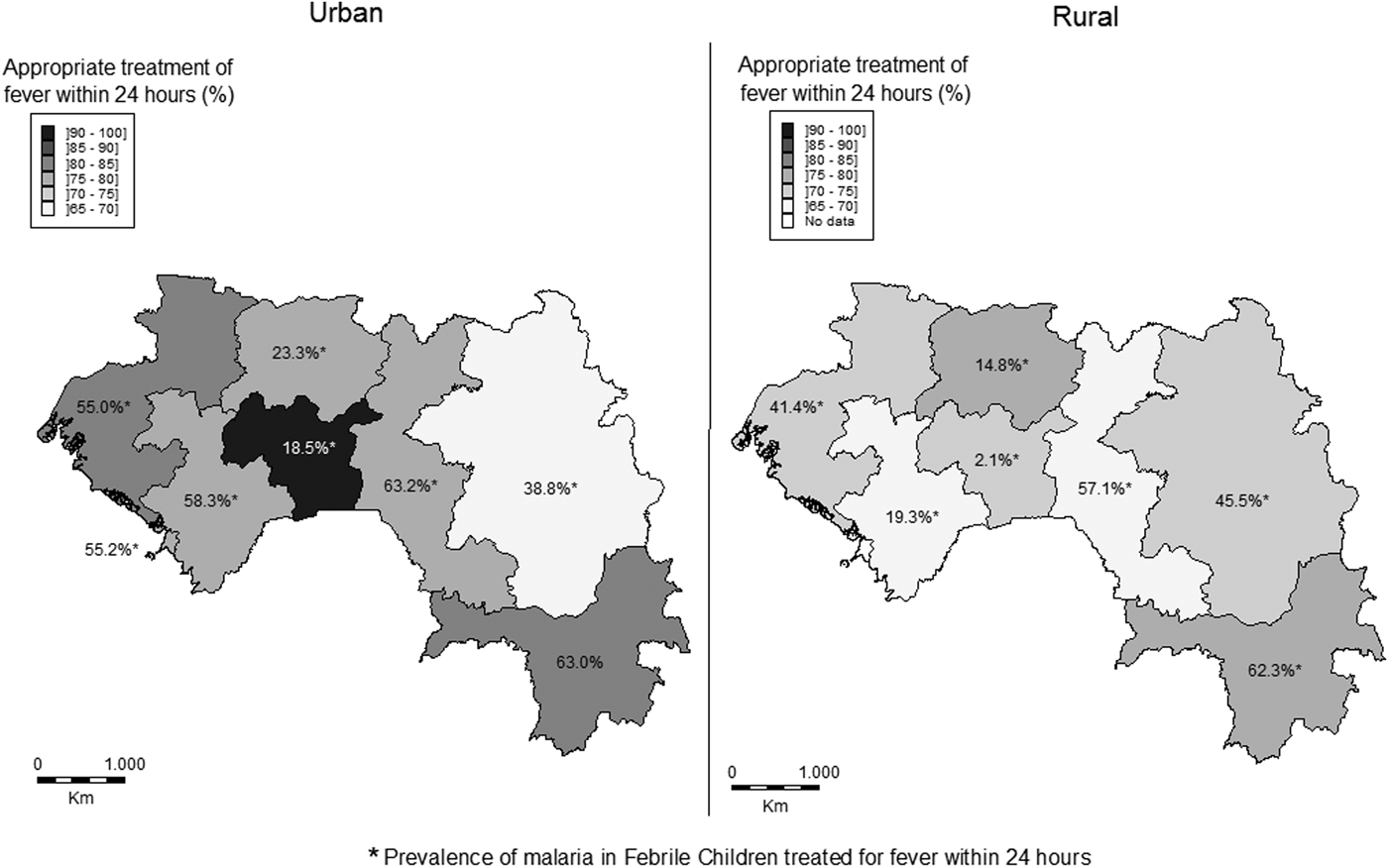
Fig. 2. Distributions of the proportions of children who received treatment for fever within 24 h according to Guinea national guidelines and the prevalence of malaria in febrile children who received appropriate treatment for fever within 24 h.
Discussion
The NSP aims mainly at reducing malaria morbidity and mortality especially in pregnant women and children under 5 [8]. The sample of children analysed in this study may be considered representative of all children under 5 living in Guinea. As a reminder, 2874 households were drawn from 5780 representative Guinean household and their characteristics were found consistent with those of the Guinean population. However, the average number of children under 5 per household seems very low when considering the average household size. Indeed, other household members are likely to be other children over the age of 5, cousins over the age of 5 or any other person in the household who meets the household membership criteria at the time of the survey. The average household size here is similar to that observed in the DHS of 2012 (8.5 people per household) [7].
The high prevalence of fever seen over a 2-week period revealed the heavy burden caused by fever and fever-associated diseases. In sub-Saharan Africa, the biweekly prevalence of fever among children under 5 was estimated at 26.7% [Reference Prasad11]. The high prevalence of malaria in the region is a significant contributor to this prevalence of fever. The prevalence of fever could be underestimated in this study because of information bias related to possible cases of fever not reported at the time of the survey. The links between this high prevalence of fever and household size, parental education level and occupational activity established in this study are consistent with those found in the literature [Reference Prasad11–Reference Mathanga13]. Also, as already mentioned by Dambach et al. [Reference Dambach14], the links established with the area of residence, the administrative region and the ‘home to health centre distance’ could be interpreted in terms of impact of environmental factors on the density of malaria vectors in rural areas of West Africa.
Previous large-scale interventions for malaria prevention have proved very effective in reducing the prevalence of fever in communities [Reference Abegunde15]. In children under 5 in the tropics, malaria has probably the highest association with fever [Reference Carlucci16]. Although it should be considered with caution because of the random nature of the links established in this study, the high prevalence of fever among children whose mothers had a complete IPT during the last pregnancy compromised one of the major preventive actions of the NMCP. Indeed, in addition to the IPT, pregnant women admitted to the pre-natal consultation and their children under 5 had benefited from an extensive programme of distribution of ITNs. Most evaluation studies have shown that the regular use of these nets has a positive impact on controlling the prevalence of fever [Reference Damien12, Reference Mathanga13]. The results of the present study should justify an assessment of the observance of the national programme of net distribution initiated by the NMCP in 2012 [8].
In the present study, the evidence for a link between fever and malaria is provided by the regional distribution of fever prevalence and the prevalence of malaria among febrile children. Regardless of the area of residence (rural or urban), these two prevalences were found higher in administrative regions encompassing malaria high-risk areas. Indeed, although malaria is endemic in Guinea, seasonal fluctuations in the intensity of transmission are common in areas of high rainfall (south-eastern of the low coast and forest regions) and in highly irrigated areas (Savannah and the plain of Upper Guinea) [17].
In its vision of a ‘Guinean society in which everyone living there has good health’, Guinea's health policy advocated universal access to quality care without geographical, economic or socio-cultural barriers and greater community accountability for better involvement of the populations in the management of their health problems. It also advocated the availability of trained, motivated and valued health personnel; an adequate supply of health facilities with high-quality inputs; a substantial sector funding; an appropriate work environment; and a healthcare system oriented toward the satisfaction of the needs of the communities [8, 17]. However, recent studies on the effect of the Ebola epidemic on the management of malaria have shown a significant reduction in the demand for malaria care in the country's health structures because of the symptom similarity between Ebola virus disease and malaria. As a reminder, from December 2013 to December 2015, the epidemic of the Ebola virus (the worst health crisis in Guinea) killed 2536 people out of 3805 confirmed cases. Some studies concluded that this decline in malaria-related care would threaten the control of the disease in Guinea [Reference Brolin Ribacke18–Reference Plucinski20]. This threat is reflected here by the still high recourse to traditional healers for the management of fever and by the no-recourse which, according to Kassam et al. [Reference Kassam21], would be associated with a tendency to self-medication and by non-confidence in traditional healers.
Yet, the impact of economic and socio-cultural barriers on the decision to seek treatment should not be overlooked. Indeed, in Guinea, in a socio-sanitary context dominated by household poverty and lack of a social security system, the recourse to traditional healers is not merely the result of lack of information or instruction. The literature suggests that, in rural areas, in addition to cultural habits, difficulties in accessing adequate care could also be explained by a lack of means and/or health structures; which favours inevitably the recourse to traditional healers [Reference Adera22, Reference Rutebemberwa23].
This is reflected here by the high proportion of recourse in large households and in rural areas. The probability of calling for traditional healer is almost doubled each time the household size increases by one person. This tendency is reinforced by the modelling results that showed higher probabilities of recourse to traditional healers in rural vs. urban areas. However, in terms of proportion, the use of social and health structures was also higher in rural areas compared with urban areas, certainly because of the high proportion of use of pharmacies in urban areas. Indeed, in the absence of pharmacy in rural areas, people who need healthcare have no other choice than traditional healers and community health officers. This strong recourse of a community health officer could demonstrate the effectiveness of the new strategies put in place by the NMCP and focused on local actions. The relevance of these actions, especially in rural areas in Guinea, has been already demonstrated [Reference Rutebemberwa23, Reference Yansaneh24]. However, to be more effective, these new control and prevention strategies should be extended to traditional healers. Although it is unconventional to date in Guinea, the recourse to traditional healers in case of fever remains a questionable approach in developing strategies for malaria indicators control. Trained, these traditional healers could be true relays of information for a fast orientation of the children to health facilities.
This study highlights the persistence of a geographical barrier: a high prevalence of fever was seen in children living more than 5 km away from a health facility and the high probability of recourse to a traditional healer. Remoteness is an important factor in deciding whether or not to attend a health centre. According to the WHO, for adequate accessibility, a health centre should be within a 1–2 h walk, that is, about 5–10 km away [25]. Regarding primary care in Guinea, the existence of almost deserted rural areas is a serious strategic constraint [Reference Comolet26]. Any measure to reduce the unequal distribution of staff and means over the national territory is welcome and needed. This finding may help the NMCP refine its thinking about additional strategies to optimise outreach services toward remote populations.
It should be recognised, however, that despite these community-based outreach activities, the disparity in the rapid response is still visible between administrative regions and between places of residence (although the difference is not statistically significant between rural and urban areas in most administrative regions). The high risk of malaria in case of fever varies between areas; this may partly explain this disparity. Moreover, although their involvement is sometimes contrary to the guidelines for managing malaria, pharmacies are highly effective in delivering information and rapid care to the public [Reference Malik27, Reference Ndo, Menze-Djantio and Antonio-Nkondjio28]. However, the present study found a low recourse to pharmacies in rural areas, most probably because of the virtual absence of pharmacies in rural Guinea. Also, there are some urban areas in Guinea with very limited access to pharmacies. In terms of rapid response, the presence of pharmacies which is specific to certain urban areas, in particular to the city of Conakry, could have a considerable impact on the disparity between regions or places of residence. Reducing inequalities in rapid response is inevitably a new challenge requiring additional efforts and means. These efforts and resources should lead to the establishment of health facilities (hospitals and pharmacies) in areas where healthcare provision is still very precarious.
Conclusion
A high prevalence of fever and a high risk of malaria in case of fever were observed, especially in areas at high risk of malaria (south-east of the low coast, Savanah and plains of Upper Guinea, the forest region). Although a large proportion of cases of fever/malaria are managed within 24 h (rapid response), the use of traditional healers for the management of fever is still a major concern in Guinea. New control and prevention strategies should be extended to traditional healers.
In addition, the rapid management of isolated or malaria-associated fever still faces regional disparities. The reduction of gaps between different regions or between different areas in the same region is a new challenge requiring additional efforts and resources, including the establishment of health facilities in areas where healthcare provision is still very precarious.
Acknowledgement
The NSP was implemented with the financial support of several development partners, including the Global Fund to Fight HIV/AIDS, Tuberculosis and Malaria, The United States Presidential Elimination of Malaria (PMI) Initiative. Catholic Relief Services (CRS) and NMCP initiated the ENACIP survey in the context of the mid-term evaluation of the results achieved in the implementation of the NSP. Funding was provided by a grant from the Global Fund through the New Financing Model managed by CRS. The authors are grateful to Jean IWAZ (Service de Biostatistique, CHU de Lyon), Michaela LO (AgroParisTech) and Koligna Zoumanigui (National Malaria Control Program, Conakry) for the thorough editing of the manuscript and to all the collaborators who took part in the final revisions. The authors thank very particularly the investigators and the data entry operators for their devotion.
Author contributions
TG and AC designed the project. ASD collected the field data. AK, TG and AC analysed the data and interpreted the results. AK, TG and AC drafted the manuscript. DD and YL revised the manuscript. All the authors read and approved the final manuscript.
Conflict of interest
None.
Ethical standards
According to the current Guinean legislation, a retrospective study that does not change routine management of patients does not require the opinion of an Ethics Committee.
Availability of data and materials
Data and materials are available on request by e-mail. However, each request will be processed in accordance with the Guinean legislation on the availability of research data.








