Diabetic nephropathy (DN) is one of the most serious and common microvascular complications of diabetes mellitus, and is a major cause of end-stage renal disease( Reference Baylis, Atzpodien and Freshour 1 ). Mesangial cells play a vital role in the pathogenesis of DN. Mesangial cell proliferation and hypertrophy have been identified to be early events in the progress of diabetic kidney disease( Reference Sheu, Ho and Chao 2 ). However, mesangial cell apoptosis, which has been observed in both experimental and human glomerular diseases including DN, becomes an important event as the disease progresses( Reference Shimizu, Kitamura and Masuda 3 – Reference Ying, Wang and Sanders 5 ). It has been demonstrated that mesangial cell apoptosis is related to an increase in albuminuria( Reference Mishra, Emancipator and Kern 6 ), and may play a pathological role in progressive glomerulosclerosis by reducing the number of mesangial cells( Reference Sugiyama, Kashihara and Makino 4 ). Therefore, seeking effective methods for inhibiting mesangial cell apoptosis may be of great clinical importance in the treatment of DN.
Oxidative stress is considered to be a trigger of mesangial cell apoptosis(
Reference Jiao, Xu and Yue
7
). Reactive oxygen species (ROS), such as
![]() $$O _{2}^{ - \z.rad } $$
, H2O2,
$$O _{2}^{ - \z.rad } $$
, H2O2,
![]() $$HO _{2}^{\z.rad } $$
and ∙OH, are generated when stimulated by pro-inflammatory factors. Excess ROS generation leads to increased metabolite accumulation in diabetes(
Reference Radeke, Meier and Topley
8
,
Reference Forbes, Coughlan and Cooper
9
). There is mounting evidence that ROS initiate mesangial cell apoptosis in vitro
(
Reference Sugiyama, Kashihara and Makino
10
,
Reference Moreno-Manzano, Ishikawa and Lucio-Cazana
11
). Mitochondria are the major source of ROS. Mitochondrial dysfunction could lead to more ROS generation and result in further oxidative stress and increased apoptosis. It has been reported that mitochondrial dysfunction resulting from the decrease in mitochondrial DNA (mtDNA) is associated with the pathogenesis of diabetes mellitus and its complications including DN(
Reference Santos, Hunakova and Chen
12
). Therefore, the inhibition of mitochondrial dysfunction may be an effective way to suppress apoptosis and slow down the progression of DN.
$$HO _{2}^{\z.rad } $$
and ∙OH, are generated when stimulated by pro-inflammatory factors. Excess ROS generation leads to increased metabolite accumulation in diabetes(
Reference Radeke, Meier and Topley
8
,
Reference Forbes, Coughlan and Cooper
9
). There is mounting evidence that ROS initiate mesangial cell apoptosis in vitro
(
Reference Sugiyama, Kashihara and Makino
10
,
Reference Moreno-Manzano, Ishikawa and Lucio-Cazana
11
). Mitochondria are the major source of ROS. Mitochondrial dysfunction could lead to more ROS generation and result in further oxidative stress and increased apoptosis. It has been reported that mitochondrial dysfunction resulting from the decrease in mitochondrial DNA (mtDNA) is associated with the pathogenesis of diabetes mellitus and its complications including DN(
Reference Santos, Hunakova and Chen
12
). Therefore, the inhibition of mitochondrial dysfunction may be an effective way to suppress apoptosis and slow down the progression of DN.
PPARγ co-activator-1α (PGC-1α), a transcriptional co-activator, is highly expressed in tissues with high-capacity mitochondrial systems such as brown adipose tissue, skeletal muscle, heart and kidneys( Reference Puigserver, Wu and Park 13 , Reference Lin, Puigserver and Donovan 14 ). The overexpression of the transcriptional co-activator PPAR (PGC-1α) can prevent aldosterone-induced mitochondrial dysfunction in podocytes and regulate mitochondrial oxidative stress( Reference Yuan, Huang and Wang 15 , Reference Olmos, Valle and Borniquel 16 ). AMP-activated protein kinase (AMPK) and silent information regulator T1 (SIRT1) have been found to be capable of regulating PGC-1α activity and energy metabolism of mitochondria( Reference Arany, Foo and Ma 17 ). It has been suggested that high glucose-induced mesangial cell damage lowered the activity of the AMPK–SIRT1–PGC-1α axis and exacerbated oxidative stress as well as apoptotic cell damage in diabetes( Reference Kim, Lim and Youn 18 ). Therefore, targeting the activation of the AMPK–SIRT1–PGC-1α axis may offer new approaches for the treatment of mesangial cell apoptosis and DN.
Proanthocyanidin extracts, derived from grape seed, have been reported to possess a wild range of potent biological activities including free radical scavenging, antioxidant, anti-inflammatory and anti-tumour activities( Reference Houde, Grenier and Chandad 19 , Reference Li, Li and Gao 20 ). Grape seed procyanidin B2 (GSPB2) is one of the main components of proanthocyanidin extracts from grape seed, and accounts for 6·4 % in grape seed( Reference Yamakoshi, Saito and Kataoka 21 ). GSPB2 is composed of two molecules of flavan-3-ol ( − )-epicatechin linked by a 4b → 8 bond. It has more powerful bioactivity than other water-soluble polyphenols. Previous studies have found that GSPB2 could prevent ROS generation, inhibit endothelial cell apoptosis, and has protective effects against DN( Reference Li, Li and Cai 22 , Reference Zhang, Li and Li 23 ). However, most of the studies investigating the effects of GSPB2 on DN have focused on animal models, and there is little information available about the benefits of GSPB2 on DN in in vitro systems.
The aim of the present study was to investigate the potential protective effect of GSPB2 on apoptosis and mitochondrial dysfunction in rat mesangial cells treated with high-dose glucosamine (GlcN), and to explore whether this protective effect was, at least in part, due to the activation of the AMPK–SIRT1–PGC-1α axis.
Materials and methods
Chemicals and reagents
GSPB2 was purchased from Jianfeng Inc. (>95 % pure, batch no. 20080915). Minimum essential medium (MEM) and fetal bovine serum (FBS) were obtained from Gibco Invitrogen Corporation. Trypsin, dimethyl sulfoxide (DMSO), GlcN and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were all purchased from Sigma. The terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphates (dUTP) nick-end labelling (TUNEL) detection kit was obtained from KeyGen Biotech. The Annexin-V-fluorescein (FLUOS) Staining Kit was obtained from Roche Diagnostics.
Cell culture
Rat mesangial cells (HBZY-1) were obtained from the Center of Type Culture Collection, Chinese Academy of Medical Sciences. The cells were cultured in MEM supplemented with 10 % FBS and incubated at 37°C under a humidified atmosphere of 5 % CO2. The cells were used at the fifth passage. All experiments were performed in the logarithmic phase of cell growth. The cells were incubated for 24 h in serum-deprived (0·2 % FBS) MEM. Then, mesangial cells were cultured in MEM and 10 % FBS with or without 15 mm-GlcN and two concentrations of GSPB2 (2·5 and 10 μg/ml) for 24 h. The highest concentration of GSPB2 in plasma was 14 μg/ml after being taken for 2 h, so these two concentrations of GSPB2 can be reached in vivo. Cells treated with 15 mm-mannitol instead of GlcN were used as osmolarity control.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
MTT assay was used to measure cell viability. Mesangial cells (104 cells/well) were incubated in ninety-six-well plates. MEM was removed and replaced with serum-deprived (0·2 % FBS) medium, and incubated for 24 h before the experiment started. The cells were then treated with high-dose GlcN (15 mm) with or without the two concentrations of GSPB2 (2·5 and 10 μg/ml) or mannitol (15 mm) for another 24 h. Then, 100 μl MTT (0·5 mg/ml) were added. After continued incubation at 37°C for an additional 4 h, the medium was carefully removed. The cells were dissolved in 100 μl DMSO in each well, and the optical density of eight wells for each group was read at 570 nm using a microplate reader. Values were normalised to those of cells in the control group, which were considered 100 % viable.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling assays and flow cytometry
Apoptosis analysis was performed by TUNEL and flow cytometry. Mesangial cells were seeded into six-well plates at a density of 1 × 105 cells/ml. Then, the cells were subjected to TUNEL assays according to the manufacturer's protocol. After various treatments, the cells were fixed in 4 % paraformaldehyde for 30 min under ambient temperature. Then, the cells were washed three times with PBS and permeabilised with 1 % Triton X-100 for 3–5 min. After being washed with PBS and blocked with 3 % H2O2, the samples were incubated with 50 μl of terminal deoxynucleotidyl transferase enzyme reaction solution (45 μl equilibration buffer, 1·0 μl tetramethyl rhodamine isothiocyanate (TRITC)-5-dUTP and 4·0 μl terminal deoxynucleotidyl transferase enzyme) for 1 h at 37°C, and then analysed at a detection wavelength of 573 nm and an excitation wavelength of 543 nm by fluorescence microscopy (Nikon Eclipse TE2000-S).
For flow cytometry, we used an Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide double-staining kit (Roche Diagnostics), following the manufacturer's instructions. Briefly, 106 cells were collected after being trypsinised, washed with cold PBS, and then centrifuged at 200 g for 5 min, and the cells were resuspended in 100 μl of Annexin-V-FLUOS and propidium iodide mixture, and incubated for 15 min at 25°C in the dark. The samples were analysed by flow cytometry (BD FACSCalibur).
Colorimetric method
The colorimetric method was applied to detect the levels of glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and malondialdehyde (MDA), which were used to reflect the severity of oxidative stress. Mesangial cells (1 × 107 in 15 ml medium) were seeded in 75 cm2 tissue culture flasks. The results were calculated with the standard curve, according to the manufacturer's instructions (Beyotime Biotechnology).
Quantitative real-time PCR and analyses of mitochondrial DNA content
Total RNA and DNA from 106 mesangial cells were isolated by TRIzol reagent (Invitrogen) and the DNeasy Tissue Kit (Qiagen Sciences), respectively. The sequences are shown in Table 1. The expression level of target genes, nuclear respiratory factor 1 (NRF-1) and mitochondrial transcription factor A (TFAM), was determined by real-time RT-PCR, and the relative mtDNA content was detected using quantitative real-time PCR. Reverse transcription was performed using a reaction kit according to the manufacture's protocol (Promega Reverse Transcriptase System). The ABI 7300 Real-time PCR Detection System was used to perform real-time PCR. mtDNA and β-actin were used for the amplification of nuclear and mtDNA, respectively. Cycling conditions were as follows: denaturation programme (95°C for 10 min), and amplification and quantification by forty cycles of 95°C for 15 s and 60°C for 30 s. The values were determined relative to the control sample after normalising to the values of β-actin as the control gene, and calculated by the comparative cycle threshold (ΔΔC t) method.
Table 1 Primer sequences used for real-time PCR
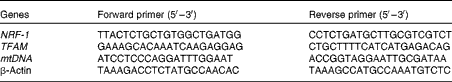
NRF-1, nuclear respiratory factor 1; TFAM, mitochondrial transcription factor A; mtDNA, mitochondrial DNA.
Western blot analysis
Cells from each group were collected and lysed for Western blotting. The whole process of operation was completed on ice. Protein concentrations were determined using a BCA protein assay kit (Beyotime Biotechnology), following the manufacturer's instructions. Equal amounts of protein were separated by 10 % SDS–PAGE and transferred to polyvinylidene fluoride membranes. After being blocked with 5 % non-fat milk or 5 % bovine serum albumin for 1 h, the membranes were incubated overnight at 4°C with the following primary antibodies: phospho-AMPK Thr172 (1:200; Santa Cruz Biotechnology), SIRT1 (1:200; Santa Cruz Biotechnology), PGC-1α (1:1000; Cell Signaling Technology), poly(ADP-ribose) polymerase (PARP, 1:1000; Cell Signaling Technology), glyceraldehyde 3-phosphate dehydrogenase (GAPDH, 1:500; ZSGB-BIO), receptor for advanced glycation end products (RAGE, 1:200; Santa Cruz Biotechnology) and β-actin (1:1000; Cell Signaling Technology). Goat anti-rabbit or anti-mouse IgG (1:4000; ZSGB-BIO) was used as the secondary antibody. Then, immune complexes were detected by enhanced chemiluminescence with Super ECL Plus Detection Reagent (Applygen) and analysed using Image Pro Plus 6.0 Software (Media Cybernetics). Values were corrected with those of β-actin used as the control.
Statistical analysis
Data were assessed by SPSS 13.0 (SPSS Inc.). Results are presented as means and standard deviations. Homogeneity of the data was first tested by selecting the option ‘Homogeneity of variance’ in SPSS software. If variances were equal, data were evaluated by means of a one-way ANOVA with Bonferroni correction; otherwise, Tamhane's T2 test was performed. P <0·05 was considered as statistically significant.
Results
Effects of grape seed procyanidin B2 on the viability of mesangial cells treated with high-dose glucosamine
The number of mesangial cells cultured under normal glucose concentrations was not altered by GSPB2 treatment (Fig. 1(a)). In contrast, GlcN significantly suppressed cell viability compared with the control group (P <0·05). However, cell viability was significantly improved in the GSPB2 (10 μg/ml)-treated group (P <0·05; Fig. 1(b)).

Fig. 1 Effects of grape seed procyanidin B2 (GSPB2) on the viability of mesangial cells cultured under (a) normal glucose (NG) concentrations assessed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and (b) high-dose glucosamine assessed by the MTT assay. Values are means for at least three independent experiments, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P <0·05). † Mean value was significantly different from that of the glucosamine (GlcN)-treated group (P <0·05).
Effects of grape seed procyanidin B2 on the apoptosis of mesangial cells treated with high-dose glucosamine
Similar results were demonstrated using the TUNEL staining (Fig. 2(a)) and flow cytometry (Fig. 2(b)) methods. There were no differences observed between the control and mannitol-treated groups. GlcN significantly increased the percentage of apoptotic cells compared with the control group (P <0·05). Interestingly, the treatment with GSPB2 protected against GlcN-induced cell apoptosis (P <0·05).

Fig. 2 Effects of grape seed procyanidin B2 (GSPB2) on the apoptosis of mesangial cells cultured under high-dose glucosamine. (a) Representative terminal deoxynucleotidyl transferase-mediated dUTP nick-end labelling (TUNEL)-positive mesangial cells (original magnification × 400) and the quantitative analyses of the results are shown. HPF, high power field. (b) Annexin V-fluorescein isothiocyanate (FITC-A)/propidium iodide (PI-A) staining of mesangial cells in each group is shown. Values are means from three independent experiments, with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P <0·05). † Mean value was significantly different from that of the glucosamine (GlcN)-treated group (P <0·05). (A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn)
Effects of grape seed procyanidin B2 on oxidative stress in mesangial cells treated with high-dose glucosamine
As shown in Table 2, the activities of GSH-Px and SOD and the content of MDA in the mannitol-treated and control groups were similar. High-dose GlcN-induced oxidative stress in mesangial cells was demonstrated by the decrease in the activities of GSH-Px and SOD in the GlcN-treated group, but an increase in the content of MDA (P <0·05 or P <0·01) compared with the control group. On the contrary, the activities of GSH-Px and SOD were improved in the GSPB2 (10 μg/ml)-treated group (P <0·05 and P <0·01, respectively). Although the content of MDA was slightly decreased in the GSPB2-treated group, it did not differ significantly from the control group.
Table 2 Effects of grape seed procyanidin B2 (GSPB2) on glutathione peroxidase (GSH-Px), superoxide dismutase (SOD) and malondialdehyde (MDA) levels in mesangial cells cultured under high-dose glucosamine (Mean values and standard deviations, n 6 per group)
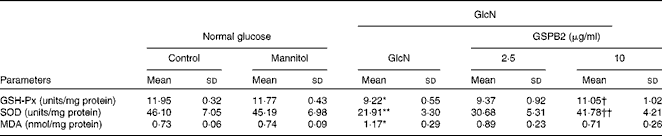
GlcN, glucosamine.
Mean value was significantly different from that of the control group: * P <0·05, ** P <0·01.
Mean value was significantly different from that of the GlcN-treated group: † P <0·05, †† P <0·01.
Effects of grape seed procyanidin B2 on poly(ADP-ribose) polymerase cleavage and the expression of glyceraldehyde 3-phosphate dehydrogenase and receptor for advanced glycation end products in mesangial cells treated with high-dose glucosamine
ROS-induced cellular damage in mesangial cells was evaluated by assaying the protein expression levels of PARP, GAPDH and RAGE, as shown in Fig. 3. There were no differences observed between the control and mannitol-treated groups with respect to these parameters. The ratio of full-length:cleaved PARP and the expression of GAPDH were reduced in the GlcN-treated group compared with the control group, while the expression of RAGE was increased (P <0·05 for each). The treatment with GSPB2 (especially the 10 μg/ml dose) reversed these parameters significantly (P <0·05 for each), which suggested that GSPB2 could protect against cellular damage, thereby inhibiting apoptosis in mesangial cells treated with high-dose GlcN.

Fig. 3 Effects of grape seed procyanidin B2 (GSPB2) on the protein expression of poly(ADP-ribose) polymerase (PARP), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and receptor for advanced glycation end products (RAGE) in mesangial cells cultured under high-dose glucosamine. Values were normalised to those of β-actin as the control. Values are means (n 4), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P <0·05). † Mean value was significantly different from that of the glucosamine (GlcN)-treated group (P <0·05).
Effects of grape seed proanthocyanidin extracts on the mRNA expression of nuclear respiratory factor 1, mitochondrial transcription factor A and mitochondrial DNA content in mesangial cells treated with high-dose glucosamine
Real-time PCR analysis showed that the mRNA expression of NRF-1 and TFAM, two essential mitochondrial biogenesis factors for gene expression in mammals, was significantly decreased in the GlcN-treated group compared with the control and mannitol-treated groups (P <0·05 for each; Fig. 4(a)), and the mtDNA copy number was also significantly reduced (P <0·05 for each; Fig. 4(b)). Interestingly, these changes were markedly reversed after the treatment with GSPB2 (P< 0·05 for each). These findings suggest that mitochondrial dysfunction in mesangial cells treated with high-dose GlcN could be ameliorated by GSPB2 treatment.

Fig. 4 Effects of grape seed procyanidin B2 (GSPB2) on mitochondrial dysfunction in mesangial cells cultured under high-dose glucosamine as determined by real-time PCR analysis. Effects of GSPB2 on (a) the mRNA expression of nuclear respiratory factor 1 (NRF-1; ![]() ) and mitochondrial transcription factor A (TFAM;
) and mitochondrial transcription factor A (TFAM; ![]() ), and (b) mitochondrial DNA (mtDNA) copy number. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P <0·05). † Mean value was significantly different from that of the glucosamine (GlcN)-treated group (P <0·05).
), and (b) mitochondrial DNA (mtDNA) copy number. Values are means (n 6), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P <0·05). † Mean value was significantly different from that of the glucosamine (GlcN)-treated group (P <0·05).
Effects of grape seed proanthocyanidin extracts on the AMP-activated protein kinase–silent mating type information regulation 2 homologue 1–PPARγ co-activator 1α axis in mesangial cells treated with high-dose glucosamine
The protein expression of the AMPK–SIRT1–PGC-1α axis in the samples of each group was detected. As shown in Fig. 5, Western blot analysis revealed no significant differences in the protein expression levels of phospho-AMPK, SIRT1 and PGC-1α between the control and mannitol-treated groups. However, these protein levels were markedly decreased in the GlcN-treated group (P <0·05 for each). In contrast, the treatment with GSPB2 at the dose of 10 μg/ml resulted in increased protein expression levels of phospho-AMPK, SIRT1 and PGC-1α when compared with the GlcN-treated group (P <0·05 for each).

Fig. 5 Effects of grape seed procyanidin B2 (GSPB2) on the protein expression of the phosphorylated AMP-activated protein kinase (p-AMPK)–silent mating type information regulation 2 homologue 1 (SIRT1)–PPARγ co-activator-1α (PGC-1α) axis in mesangial cells cultured under high-dose glucosamine. Values were normalised to those of β-actin as the control. Values are means (n 4), with standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P <0·05). † Mean value was significantly different from that of the glucosamine (GlcN)-treated group (P <0·05).
Discussion
To the best of our knowledge, the present study was the first to report that GSPB2 could inhibit high-dose GlcN-induced apoptosis of mesangial cells in vitro. This protective effect induced by GSPB2 could be due in part to the suppression of oxidative stress, the amelioration of mitochondrial dysfunction and the activation of the AMPK–SIRT1–PGC-1α axis in mesangial cells.
Procyanidin absorption depends on the degree of polymerisation. Deprez et al. ( Reference Deprez, Brezillon and Rabot 24 ) showed that procyanidin dimers and trimers are absorbed through the human intestinal cell line Caco-2, but procyanidins with an average polymerisation degree of 7 are unabsorbed. Procyanidin polymers are believed to be fermented by intestinal microflora to produce more smaller molecular-weight phenolic acids and some other unknown metabolites of colonic origin( Reference Ferruzzi, Lobo and Janle 25 ). In a study investigating the bioavailability of GSPB2 in plasma, rats were orally administered with procyanidin B2( Reference Baba, Osakabe and Natsume 26 ). The study found that procyanidin B2, epicatechin and 3′-O-methyl-epicatechin were present in the plasma and urine of rats, and procyanidin B2 reached a maximum plasma concentration at 30–60 min after its consumption. The peak concentration of procyanidin B2 in plasma was 0·5 μm after the administration of procyanidin B2 (50 mg/kg body weight). However, further studies are still needed to clarify the mechanisms of the absorption of GSPB2. Additionally, GSPB2 and its metabolites are specifically accumulated in different organs such as liver, adipose tissue and muscle.
A high concentration of glucose (30 mm) is used to induce the apoptosis of mesangial cells. It has been observed that exposure to high levels of GlcN could cause apoptosis as well( Reference Fiordaliso, Leri and Cesselli 27 , Reference Liu, Paterson and Chin 28 ). Previous studies have proved that protracted exposure to a high dose of GlcN leads to the apoptosis of mesangial cells in a manner that is similar to that observed in high-glucose conditions( Reference James, Le and Scholey 29 ). In the present study, we observed that mesangial cell apoptosis was induced by the treatment with 15 mm-GlcN. In addition, since the dose of mannitol, as osmolarity control, was much lower than the high concentration of glucose (30 mm), the influence of osmotic pressure on the results was minimised. Therefore, the 15 mm-GlcN dose was used in the subsequent experiments. As shown previously, GSPB2 could exert protective effects against DN by inhibiting advanced glycation end product-induced apoptosis of endothelial cells( Reference Li, Li and Cai 22 , Reference Zhang, Li and Li 23 ). The present results showed that after the treatment with GSPB2 (especially at a dose of 10 μg/ml) for 24 h, there was a significant inhibition of mesangial cell apoptosis. These data suggest that GSPB2 plays a role in the prevention of GlcN-induced apoptosis, and GSPB2 therapy can be adopted as an effective approach to resist mesangial cell apoptosis in DN.
Oxidative stress plays a pivotal role in the induction of mesangial cell apoptosis( Reference Baker, Mooney and Hughes 30 ). SOD is an enzyme responsible for catalysing the conversion of superoxide anion radical into peroxides, which are then converted into water by GSH-Px, and MDA is the most abundant by-product of lipid peroxidation( Reference Arivazhagan, Thilakavathy and Panneerselvam 31 ). It has been demonstrated that GSPB2 is a powerful antioxidant, and its antioxidative activity is much stronger than that of vitamins C and E( Reference Bagchi, Ray and Bagchi 32 , Reference Bagchi, Garg and Krohn 33 ). Consistently, the present results showed that the activities of GSH-Px and SOD were markedly increased after the oral administration of GSPB2. In addition, the production of ROS was increased under the condition of oxidative stress. High levels of ROS induce DNA strand breaks, thereby activating PARP. The enzyme PARP is involved in a number of cellular processes including DNA repair and programmed cell death. During apoptosis, PARP is rapidly cleaved, and thus the detection of cleaved PARP is a diagnostic test for apoptosis in cells( Reference Zhang, Wu and Wang 34 ). Subsequently, the activity of GAPDH that is inhibited by cleaved PARP results in the increased expression of RAGE, which ultimately leads to cellular damage( Reference Giacco and Brownlee 35 ). In the present study, we observed ROS-induced cellular damage in mesangial cells treated with high-dose GlcN. However, GSPB2 inhibited the cleavage of PARP, improved the activity of GAPDH and down-regulated the expression of RAGE, which provided more evidence on its protective effects on mesangial cells against cellular damage.
Mitochondrial dysfunction, involved in the pathogenesis of DN, is the terminal event in the process involving oxidative stress-induced programmed cell death in mesangial cells( Reference Kang, Frencher and Reddy 36 ). Mitochondria are not only ‘the power plant’ of the cell, but also the major source of ROS generated as the by-products of oxygen metabolism. mtDNA is a primary target of attack by ROS because it is deficient in DNA repair capacity, lacks histone-like coverage, and is localised close to the electron transport chain( Reference Bohr 37 ). ROS may injure mtDNA and impair the electron transport chain, which leads to the production of high levels of ROS( Reference Al-Kafaji and Golbahar 38 ). GSPB2 has the capacity to scavenge free radical and reduce the production of ROS due to its powerful antioxidative activity, which provides the possibility for the improvement of mitochondrial dysfunction. To evaluate mitochondrial dysfunction, we selected the following indicators: NRF-1; TFAM; mtDNA copy number. NRF-1 is a positive regulator of TFAM, which plays a role in mitochondrial transcription and regulates the transcription and replication of mtDNA( Reference Parisi and Clayton 39 ). The mtDNA copy number was identified as a surrogate marker of mitochondrial function( Reference Cho, Park and Lee 40 ). Studies have found that PGC-1α, a major regulator of oxidative metabolism and mitochondrial biogenesis, could bind to and co-activate the transcriptional function of NRF-1 on the promoter for TFAM, which, in turn, promotes mtDNA transcription and replication( Reference Karthikeyan, Sarala Bai and Niranjali Devaraj 41 , Reference Cheng, Gao and Xu 42 ). The activation of PGC-1α improves mitochondrial biogenesis following oxidant injury( Reference Wu, Puigserver and Andersson 43 ). Therefore, PGC-1α plays a key role in mitochondrial dysfunction and oxidative stress. Although there have been few studies describing the effect of GSPB2 on the expression of PGC-1α, some have showed that polyphenols (e.g. resveratrol) enhance the expression of PGC-1α( Reference Yuan, Huang and Wang 15 , Reference Rasbach and Schnellmann 44 ), indicating that GSPB2, which is also one of the polyphenols, might have a role in promoting the expression of PGC-1α. Moreover, it has been found that GSPB2 activates AMPK and increases the protein levels of SIRT1, which are the regulators of PGC-1α activity and energy metabolism of mitochondria, in human umbilical vessel cells( Reference Wang, Moustaid-Moussa and Chen 45 ). It has been reported that the activity of the AMPK–SIRT1–PGC-1α axis is lowered in high glucose-induced mesangial cells, leading to cell damage, exacerbated oxidative stress as well as apoptotic cell death in diabetes( Reference Kim, Lim and Youn 18 ). In the present study, the inactivation of the AMPK–SIRT1–PGC-1α axis was found in mesangial cells treated with high-dose GlcN. Interestingly, our data showed that GSPB2 could restore the protein levels of phospho-AMPK, SIRT1 and PGC-1α, suggesting the activation of the AMPK–SIRT1–PGC-1α axis by GSPB2. Furthermore, the present data showed that the activation of the AMPK–SIRT1–PGC-1α axis increased the expression levels of the transcription factors NRF-1 and TFAM, and, subsequently, activated the transcription and replication of mtDNA. These protective effects of GSPB2 on mitochondrial function have also been reported in in vivo studies( Reference Karthikeyan, Sarala Bai and Niranjali Devaraj 41 , Reference Cui, Liu and Feng 46 ).
In conclusion, the present study suggested that GSPB2 could inhibit high-dose GlcN-induced mesangial cell apoptosis in vitro, which may be due to the improvement of oxidative stress, mitochondrial dysfunction and the activity of the AMPK–SIRT1–PGC-1α axis. The AMPK–SIRT1–PGC-1α axis could be one of the molecular mechanisms involved in the renal protective effects of GSPB2. These results provide further evidence that GSPB2 may be a potential therapeutic agent for the treatment of DN.
Acknowledgements
The present study was supported by the research grants from the National Natural Science Foundation of China (81102116 and 81072293) and the Research Fund for the Doctoral Program of Higher Education (20110001120026). The funders contributed to the study design, but had no role in the analysis of the study or the writing of this article.
The authors’ contributions are as follows: Y. L. and Z. Z. formulated the research question and designed the study; L. B. and X. C. performed the analysis and analysed the data; L. B. wrote the paper; X. C. made some modifications in the manuscript.
The authors have no conflicts of interest to disclose.










