Background
For nearly four decades, polyunsaturated fatty acids (PUFAs) of the omega-3 (n-3) family have been studied extensively in relation to prevention and treatment of cardiovascular disease (Reference Dyerberg, Bang and Stoffersen1–Reference Marchioli, Schweiger and Tavazzi5). The health-promoting effects of n-3 fatty acids (FAs) may be partially due to their immune-modulating and anti-inflammatory actions (Reference Das6–Reference Dawczynski, Schubert and Hein8). Although this was first described in cardiovascular disease, the potential role that inflammatory mediators play in a host of other diseases and conditions including metabolic bone diseases such as osteoporosis has caused investigators to extend studies of n-3 FAs to include skeletal outcomes(Reference Ding, Parameswaran and Udayan9–Reference Kruger, Coetzee and Haag13).
Osteoporosis is a pervasive public health problem. According to the World Health Organization, osteoporosis affects more than 75 million people in the United States, Europe and Japan. The estimated lifetime risk of hip, vertebral or wrist fractures is approaching 30-40 % in developed countries, a prevalence similar to that of coronary heart disease (14). Chronic inflammation, due in part to increased cytokine expression after menopause and with aging, is one mechanism contributing to the pathogenesis of osteoporosis. Both pro-inflammatory and anti-inflammatory cytokines and hormones interact to regulate osteoblast and osteoclast differentiation and activity. The balance in these systems is central to the pathogenesis of osteoporosis(Reference Hofbauer and Schoppet15, Reference Boyce and Xing16).
The anti-inflammatory effects of n-3 FAs are well-known. Recently, a promising association between higher n-3 FA intake and improved bone turnover markers and bone mineral density (BMD) in humans has been reported in some(Reference Coetzee, Haag and Kruger17–Reference Weiss, Barrett-Connor and von Muhlen19) but not all(Reference Macdonald, New and Golden20, Reference Trebble, Stroud and Wootton21) studies. This has led to interest in n-3 FAs as a nutritional factor that may decrease risk for osteoporotic fractures.
PUFAs have two primary physiological functions in humans. First, they are present as phospholipids in membranes and contribute to an optimum lipid bilayer structure to allow for intercellular communication and highly differentiated membrane functions. Second, they are the primary precursors of bioactive lipid mediators, including the eicosanoids, which have autocrine and paracrine actions throughout the body (Reference Stipanuk22, Reference Albertazzi and Coupland23). Alpha linolenic acid (ALA: 18:3 n-3) is the essential n-3 FA in humans (Reference Albertazzi and Coupland23). ALA can be converted to longer chain n-3 FAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in most people, but the extent of this conversion appears to be small, especially when intake of n-6 FAs is high, as is typical in Western diets (Reference Kris-Etherton, Taylor and Yu-Poth24, Reference Whelan and Rust25). Marine sources provide most of the EPA and DHA found in the diet and incorporated into blood and tissues.
Though the association of n-3 FAs to bone turnover markers appears promising, the real test of clinical significance is the impact of n-3 FAs on osteoporotic fracture. A small number of epidemiological studies investigating the relationship of fish consumption or dietary n-3 FA consumption to fracture risk have yielded mixed results. In a large cohort of over 35,000 men and women from the European Prospective Investigation into Cancer and Nutrition (EPIC-Oxford), no differences were found in risk of fracture in those who reported eating fish only compared to those consuming meat plus fish in their typical diet (Reference Appleby, Roddam and Allen26). Consumption of both n-3 FAs and n-6 FAs in relation to osteoporotic fracture risk was investigated in a case-control study of 167 Spanish men and women over 65 years of age hospitalized for fracture versus matched controls. There was no significant association of total n-3 FA intake to fragility fracture, but there was a significantly elevated risk of fracture in individuals reporting the highest quartile of n-6 FA intake (>18g/d)(Reference Martinez-Ramirez, Palma and Martinez-Gonzalez27). However, in 137,486 postmenopausal women in the Women's Health Initiative consuming small amounts of EPA+DHA, higher n-6 FAs were associated with a modest decrease in total fracture risk, but higher EPA+DHA intake was associated with a small increase in risk of fractures. Interestingly, women with the highest EPA+DHA intake in this cohort also had the lowest calcium and vitamin D intake. No association was noted between ALA or EPA+DHA intake and hip fracture risk(Reference Orchard, Cauley and Frank28).
Recently, the relation of various types of n-3 FAs to hip fracture risk was examined in 522 postmenopausal women and 352 men in the Framingham Osteoporosis Study. Higher ALA consumption was associated with lower hip fracture risk in women, but not men. No relationship was observed between EPA+DHA intake and hip fracture(Reference Farina, Kiel and Roubenoff29). Likewise, researchers in the Cardiovascular Health Study reported that neither fish intake nor EPA+DHA intake were associated with risk for hip fracture in 5045 participants aged 65 or older, and the LA intake of the background diet did not modify this relationship(Reference Virtanen, Mozaffarian and Cauley30). Suzuki et al. examined the impact of diet and lifestyle factors on hip fracture risk in a case-control study of 4573 Japanese elderly. Fish consumption 3–4 times per week was associated with a decreased risk of hip fracture when compared to the referent of < 2 fish meals per week. However, eating more than 4 fish meals per week did not improve relative risk(Reference Suzuki, Yoshida and Hashimoto31). Thus, epidemiological data regarding PUFAs and risk of total fracture or hip fracture remains equivocal.
Observational studies yield valuable information regarding associations of exposure to disease in free-living populations, but they do not allow strong conclusions to be drawn regarding cause and effect. Therefore, in order to examine the effect of n-3 FAs on prevention and/or treatment of osteoporosis, we undertook a systematic review of randomized controlled trials (RCTs) reported in the literature and cataloged electronically from 1946-April, 2011 to determine the effect of n-3 FA supplementation on BMD and fractures, and secondarily on regulation of bone turnover.
Materials and Methods
Criteria for considering studies for this review
This review was restricted to English language reports of RCTs with skeletal outcomes that were conducted in adults or children (> = 1 year old) using n-3 FA fortified foods, diets or n-3 FA supplements alone or in combination with other vitamins/minerals, compared to placebo. The primary skeletal outcomes of interest were incident fracture at any site and BMD. Secondary skeletal outcomes included bone formation markers [bone specific alkaline phosphatase (BSAP) and osteocalcin (OC)], bone resorption markers [N-terminal telopeptide (NTx), C- terminal telopeptide (CTx), urinary pyridinoline (u-pyr) and urinary deoxypyridinoline (u-dpyr)] and regulators of bone turnover [osteoprotegerin (OPG), receptor activator of NFkB ligand (RANKL), OPG/RANKL].
Studies in which subjects had co-morbidities were included with the exception of co-morbidities that could directly impact both fatty acid absorption and skeletal outcomes (i.e. Crohn's disease, irritable bowel syndrome, ulcerative colitis, celiac disease, cystic fibrosis). There were no restrictions based on gender of participants or race/ethnicity. If allocation of treatment and placebo were not random, studies were excluded.
Search methods for identification of studies
PubMed® database, including MEDLINE and OLDMEDLINE, was searched on April 14, 2011 and EMBASE database (containing references from 1974 – present) and EMBASE Classic (containing references from 1947-1973) was searched on April 23, 2011. MEDLINE is the primary component of PubMed® and time coverage is generally 1966 to the present. OLDMEDLINE is a subset of PubMed® that contains over 2 million citations from two print indexes: Cumulated Index Medicus and the Current List of Medical Literature from the years 1946 through 1965(32). We used the following search strategy: Omega 3 fatty acids or n-3 fatty acids or essential fatty acids or eicosapentaenoic fatty acids or EPA or ethyl-EPA or ethyl eicosapentaenoic fatty acid or docosahexaenoic acid or DHA or docosapentaenoic acid or DPA or alpha linolenic acid or ALA or LNA or polyunsaturated fatty acids, including the MeSH terms: “Fatty Acids, Omega-3”[MeSH] OR “Fatty Acids, Essential”[MeSH]OR “Fatty Acids, Unsaturated”[MeSH] OR “Fish Oils”[MeSH])OR “Fats, Unsaturated”[MeSH] AND bone disease or osteoporosis or osteopenia or fracture, including MeSH terms: “Bone Diseases, Metabolic”[MeSH]OR bone diseases OR osteoporosis OR fracture. Additionally, bibliographies from retrieved papers were checked and reviewers were asked to contribute any other references for consideration.
Quality of evidence and statistical analysis
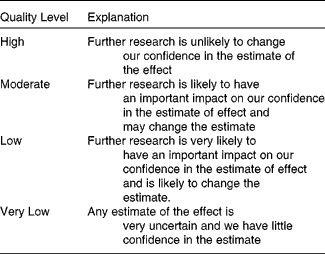
Studies meeting inclusion criteria were analyzed separately by two reviewers to evaluate quality of evidence across five categories:(1) risk of bias due to limitations in study design and/or execution such as no allocation concealment, no blinding, selective outcome reporting, intention to treat violation, or large loss to follow-up, (2) inconsistency of results considering variation in effect sizes, (3) indirectness of evidence due to differences in populations, interventions and outcomes, (4) publication bias, and (5) imprecision of results due to small number of events or wide confidence intervals. This type of assessment is based on the GRADE system(Reference Balshem, Helfand and Schunemann33, Reference Terracciano, Brozek and Compalati34), but because of heterogeneity of populations, treatments and endpoint measures, studies were not pooled for analysis. After reviewing each study in relation to the five categories, a quality of evidence level was assigned as explained in Table 1. Any discrepancies were resolved by a third independent reviewer.
Table 1 Quality of evidence levels
To compare the magnitude of the effect of n-3 FAs on various endpoint measures, we used effect size and its corresponding 95 % confidence interval(Reference Cohen35–Reference Hedges LaO37) to summarize the differences between treatment and the control. Effect size is the difference between the means of the two groups (M1 - M2) divided by the within-group standard deviation (S): i.e. (M1 - M2)/S. The accuracy of the estimated effect size is quantified by its confidence interval, which is influenced by the sample size. To make conclusions, if the confidence interval of the estimated effect size includes zero, then no statistically significant difference between the two groups was found for that particular endpoint. The absolute magnitude of the effect size indicates its strength: 0·2 suggests a small effect, whereas an effect size of 1·0 indicates a large effect.
Results
Results of the PubMed® and EMBASE search yielded 122 and 188 papers respectively, of which 24 studies were flagged for potential inclusion based on title and abstract. Of these, nine RCTs met our inclusion criteria. Additionally, a RCT published soon after the initial search was submitted by one of the reviewers and added to the included studies. The 10 RCTs selected for full analysis of quality and effect size are summarized in Table 2. Relevant aspects of study design, including whether skeletal outcomes were analyzed as part of an ancillary study within a larger study or as secondary endpoint measures, are detailed for the reader. Effect size estimates with 95 % confidence intervals and the quality of evidence level with footnoted explanations are also included in the table.
Table 2 Summary of randomized controlled trials included in systematic review organized by primary outcome measures

*BMD - Bone mineral density; OC - Osteocalcin; BSAP - Bone specific alkaline phosphatase; Dpyr - deoxypyridinoline; uPyr - urinary pyridinoline; NTX- N-terminal telopeptide; CTX - C-terminal telopeptide; Cr - Creatinine; OPG - osteoprotegerin; RANKL-receptor activator of nuclear factor κβ
** The effect sizes for these measures were based on the data estimated from the figures in the paper.
1 No intention to treat analysis, indirect evidence regarding effect of ALA related to resistance training, short duration of treatment for endpoint measured.
2 Indirect evidence regarding effect of ALA related to multiple components of flaxseed and wheat germ.
3 Unclear if double blinded, indirect evidence regarding EPA+DHA related to multiple components of supplement.
4 Large loss to follow up, small sample size in each age category, no intention to treat analysis.
5 Large loss to follow up and/or exclusion, no intention to treat analysis, only one secondary endpoint measure, ancillary data analysis.
6 Large loss to follow up, small sample size, unclear if double blinded, placebo not identified.
7 Large loss to follow up and/or exclusion, no intention to treat analysis, small sample size, indirect evidence regarding effect of ALA, EPA+DHA related to multiple components of fortified foods.
8 Small sample size, ancillary data analysis.
9 Moderate sample size, ancillary data analysis, indirect evidence regarding effect of EPA+DHA related to multiple components of fortified food.
10 Insufficient data to calculate effect size, placebo not identified, wide standard deviations of endpoint measures, incomplete description of statistical methods.
Four(Reference Dawczynski, Schubert and Hein8, Reference Martin-Bautista, Muà ± oz-Torres and Fonolla38–Reference Kruger, Coetzer and de Winter40) of the 10 RCTs had significant positive effect size estimates with 95 % confidence intervals greater than zero, indicative of a positive effect of n-3 FA treatment on the endpoint measured compared to control. Five studies(Reference Appleton, Fraser and Rogers41–Reference Bassey, Littlewood and Rothwell45) showed no significant difference in effect size of treatment versus control, and one study(Reference Kolahi, Ghorbanihaghjo and Alizadeh46) did not provide sufficient data for effect size calculation. A moderate quality of evidence level was given to three of the four significant RCTs (See Table 2). A low quality of evidence level was assigned to one(Reference Dawczynski, Schubert and Hein8) of the four RCTs because of a serious risk of bias associated with drop out or exclusion of >50 % of participants and a small number of final events/participants (n 21).
Discussion
This systematic review of RCTs of n-3 FAs and bone disease yielded 10 studies for inclusion which reflected heterogeneous populations, treatments and endpoint measures. No RCTs addressing fracture as an outcome were identified. Four studies investigated BMD as a primary outcome, with only one(Reference Kruger, Coetzer and de Winter40) showing significant improvement or maintenance of BMD in a sample of elderly Caucasian South African women with osteopenia or osteoporosis treated with a combination of evening primrose oil (high in linoleic and gamma linolenic acid), fish oil and calcium versus a placebo of coconut oil and calcium for 18 months. The effect size for femoral neck BMD in the trial by Kruger et al. was very large (2·16; 95 % CI 1·52, 2·79), with effect on lumbar BMD in the moderate range (0·75; 95 % CI 0·22, 1·27). The very large effect size seen with femoral neck BMD is likely due in part to the relatively small sample size (n 60 completed study); but it is also possible that the combination of n-6 FAs, n-3 FAs and 600 mg of calcium carbonate may preferentially impact hip BMD, especially in frail, elderly women. In the Women's Health Initiative, hip BMD, but not total spine BMD, was significantly higher in postmenopausal women taking 1000 mg calcium carbonate and 400 IU of vitamin D daily versus placebo over 9 years of follow-up(Reference Jackson, LaCroix and Gass47).
Several factors could have contributed to the beneficial effects observed in the trial by Kruger et al. First, participants may have been especially responsive to the intervention because of their advanced age and nutritional status. BMD decreases with age and age is the most powerful predictor of fracture(Reference Looker, Melton and Borrud48, Reference Robbins, Aragaki and Kooperberg49). Positive changes in BMD related to n-3 FA supplementation may be more easily detected in vulnerable groups of individuals such as this elderly cohort. Additionally, these women were consuming less than the Recommended Dietary Allowance at the time for calcium, magnesium and vitamin D, and according to the researchers, could have been marginally deficient in essential fatty acids prior to study entry. Compromised nutritional status may have contributed to bone frailty and made this cohort more likely to respond to nutritional intervention. Second, participants were initially treated for 18 months. By comparison, other RCTs examining BMD treated participants for 3-12 months (Bassey et al., 2000; Dodin et al., 2005; Cornish & Chilibeck, 2009), which may not be enough time to see changes in BMD at some skeletal sites, especially in a much younger, healthier population. Finally, including n-3 FAs with n-6 FAs and calcium carbonate may have promoted a synergistic effect on bone.
A beneficial interaction between calcium and n-3 FAs is plausible based on work done mainly in animal and in vitro models suggesting up-regulation of duodenal calcium absorption and decreased calcium excretion with treatment of n-3 FAs(Reference Hay, Hassam and Crawford50–Reference Sun, Tamaki and Ishimaru52). Three(Reference Kruger, Coetzer and de Winter40)(Reference Dawczynski, Schubert and Hein8, Reference Martin-Bautista, Muà ± oz-Torres and Fonolla38) of the four RCTs in this review with significant positive effects on skeletal outcomes lend support to this hypothesis. As previously discussed, Kruger et al. used a calcium supplement in addition to n-6 plus n-3 FAs, while two additional RCTs delivered n-3 FAs in fortified dairy products also high in calcium, vitamins and minerals. Martin-Bautista et al. reported increased OPG, RANKL, OPG/RANKL and osteocalcin in hyperlipidemic patients receiving milk fortified with fish oil, oleic acid and vitamins A, B6, D, E and folic acid(Reference Martin-Bautista, Muà ± oz-Torres and Fonolla38). Our effect size estimates for OPG and OC endpoints from this study were very large (2·92; 95 % CI 2·26, 3·59 and 4·34; 95 % CI 3·5, 5·19, respectively), with a moderate effect size estimate (0·71; 95 % CI 0·23, 1·19) for RANKL. There were also a moderate number of participants in this study (n 72) which may inflate effect size estimates.
Differences in race/ethnicity and culture between South African, French Canadian, American, Northern European, Southern European and Middle Eastern subjects may also have contributed to varying results of these RCTs. Race/ethnicity impacts BMD(Reference Looker, Melton and Borrud48) and is a known risk factor for fractures. For example, white women have about a 60 % higher risk for hip fractures than black women and about a 75 % higher risk than Asian/Pacific Island women(Reference Robbins, Aragaki and Kooperberg49). One of the limitations of most of the reviewed RCTs is the failure to report the racial/ethnic background of the participants. Future studies investigating n-3 FAs and skeletal health need to address the paucity of data in multi-ethnic cohorts.
The source of n-3 FAs may also have a significant impact on skeletal health. When EPA+DHA were given alone (excluding concomitant administration with calcium and vitamins), there was no beneficial effect noted in RCTs in this review. In contrast, a diet high in ALA from addition of walnuts, walnut oil and flaxseed oil compared to an average American diet pattern decreased NTx, a marker of bone resorption(Reference Griel, Kris-Etherton and Hilpert39). Additionally, a mixture of plant and marine sources of n-3 FAs, with ALA in highest quantity, was used to fortify dairy products with the resultant decrease in u-Dpyr, a urinary marker of bone resorption(Reference Dawczynski, Schubert and Hein8). Recent evidence from observational studies links higher ALA intake(Reference Farina, Kiel and Roubenoff29) and ALA in red blood cells (unpublished data, Orchard, T.) with reduced risk of hip fracture. It is possible that ALA, EPA and DHA have differential effects on bone turnover. It is also important to consider the oils or foods used as placebos for n-3 FA trials. Saturated, monounsaturated and n-6 polyunsaturated fatty acids found in various ratios in olive oil, corn oil, coconut oil and wheat germ (all placebos used in RCTs in this review) may impact inflammatory pathways, calcium absorption, bone turnover and BMD differentially, making the detection of an effect from n-3 FA intervention more or less likely depending on the choice of placebo. Two of the reviewed RCTs with positive outcomes used standard dairy products as a placebo(Reference Dawczynski, Schubert and Hein8) one used coconut oil plus calcium(Reference Kruger, Coetzer and de Winter40) and one used a controlled feeding cross-over design comparing three diets(Reference Griel, Kris-Etherton and Hilpert39).
Of the several potential mechanisms whereby n-3 FAs may impact bone, two of the most well-defined involve decreasing pro-inflammatory cytokines critical to regulation of bone turnover and modulating calcium balance. Cytokines are key regulators of the osteoprotegerin/receptor activator of NFKB ligand (OPG/RANKL) ratio in bone(Reference Hofbauer and Schoppet15). RANKL is expressed in osteoblasts and activates its receptor, RANK, which is expressed on osteoclasts, thus promoting osteoclast formation and activation, as well as suppressing apoptosis of osteoclasts. Osteoprotegerin (OPG) is a secretory glycoprotein expressed by osteoblasts which blocks RANKL from activating RANK. The ratio of OPG/RANKL is critical in the pathogenesis of resorptive bone disease, with a higher ratio indicating less bone resorption(Reference Hofbauer and Schoppet15).
The effect of n-3 FAs on nuclear factor-kappaB (NF-κB) has been examined in vitro. Pre-treatment of osteoclasts with EPA decreased tumor necrosis factor α (TNF α)-induced NF-κB protein expression and activation in a dose-dependent manner(Reference Zwart, Pierson and Mehta53). The n-3 FAs have been shown to decrease production of the n-6 FA derived eicosanoid, prostaglandin E2 (PGE2), (Reference Watkins, Li and Seifert54, Reference Baggio, Budakovic and Nassuato55) and increase bone formation markers, alkaline phosphatase and osteocalcin(Reference Watkins, Lippman and Le Bouteiller11). High PGE2 decreases OPG production and increases RANKL expression(Reference Hofbauer and Schoppet15). DHA added to osteoblastic cell cultures does not stimulate RANKL, but the n-6 FA, arachidonic acid, stimulates RANKL and inhibits OPG secretion by 25-30 % thus reducing the OPG/RANKL ratio(Reference Coetzee, Haag and Kruger17). There was a relatively large positive effect on OPG and osteocalcin in subjects consuming milk fortified with n-3 FAs, oleic acid and vitamins (Martin-Bautista et al., 2010). These data suggest that there is an optimum balance of n-3 and n-6 FAs needed to promote a less inflammatory cytokine environment favorable to bone remodeling.
A second possible mechanism by which n-3 FAs may influence bone is related to up-regulation of intestinal calcium absorption. Essential FAs are necessary for maximal vitamin D-dependent calcium absorption(Reference Hay, Hassam and Crawford50). DHA and EPA decrease urinary calcium excretion in a rat model of osteoporosis exacerbated by restricted food intake(Reference Sun, Tamaki and Ishimaru52) and in patients with idiopathic calcium nephrolithiasis, fish oil decreases urinary calcium excretion and returned the high level of calcium absorption toward normal(Reference Baggio, Budakovic and Nassuato55). In vitro and animal studies show up-regulation of duodenal calcium absorption by DHA via modulating Calcium-ATPase when calcium levels are low(Reference Haag, Magada and Claassen51, Reference Haag and Kruger56). In ovariectomized rats, calcium-ATPase activity at the intestinal basolateral membrane increases significantly following supplementation with essential FAs in a ratio of 1/3, GLA/EPA+DHA.(Reference Leonard, Haag and Kruger57). Thus, the essential n-3 and n-6 FAs may act to increase calcium absorption and decrease calcium excretion, especially when dietary calcium intake is low.
Conclusions
In summary, an n-3 and n-6 FA mixture combined with calcium, n-3 FA fortified dairy products, and a high ALA diet resulted in statistically significant positive effects on bone-related outcomes in diverse individuals in four RCTs in this review. However, five RCTs showed no significant effects of n-3 FA intervention. Due to the small number of RCTs and the heterogeneity of the studies, we are unable to make strong conclusions regarding supplementation of n-3 FAs and skeletal health. Although there is insufficient evidence to support a positive relation between n-3 FA and prevention or treatment of osteoporosis at this time, it appears that any benefit might be enhanced by inclusion of n-3 FAs in foods high in calcium, vitamins and minerals, or in concentrated oil mixtures with other PUFAs and calcium. To definitively address the role of both plant-based and marine sources of n-3 FAs for reducing risk for osteoporosis, there is a need for further large scale investigation of the differential effects of various n-3 FAs in relation to skeletal outcomes, particularly fracture.
Acknowledgements
Contribution of Authors: TO and FC searched databases. TO and RJ determined studies for inclusion, reviewed literature, graded evidence, prepared manuscript. TO and XP extracted data. XP performed statistical analysis. SI arbitrated graded evidence and reviewed manuscript. FC reviewed manuscript. There is no conflict of interest. Source of Funding: The project described was supported by Award Number UL1RR025755 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221.




