Pseudobulbar affect (PBA) is a disorder involving episodes of involuntary or exaggerated emotional expression that do not correspond with the valence or degree of (1) the patient’s subjective emotional experience and (2) environmental stimuli.Reference Lauterbach, Cummings and Kuppuswamy 1 It has been reported with various neurological disorders including tumors of the posterior fossa, especially those involving the pons and midbrain.Reference McCormick and Lee 2 We report the first case, to our knowledge, of resolution of PBA after temporal lobectomy for epilepsy associated with an unresectable petrous apex meningioma extending along the clivus.
A 60-year-old, right-handed woman presented with medically refractory epilepsy since the age of 47 years. She had been diagnosed with an unresectable left petrous apex meningioma extending inferiorly along the clivus at the age of 37 years, which had been immediately debulked. Residual mass effect on the midbrain, pons and extension into the cavernous sinus was associated with longstanding diplopia with left partial oculomotor and abducens palsy, hypesthesia in the left V2 distribution and left-sided hearing impairment. There was no dysarthria or dysphagia. Otherwise, the remainder of her cranial nerve examination was unremarkable as was examination of tone, power, coordination, reflexes and sensation throughout. She continued to have a seizure cluster every 3 days despite being on carbamazepine, lamotrigine and clobazam.
Since the meningioma was diagnosed, the patient’s family had noted that she was increasingly impulsive and unable to await her turn to speak in conversations, with frequent interruptions. This progressively worsened, particularly over the past 5 years, during which emotional lability was also persistently present. There was no evidence on direct and collateral history to indicate a depressive, anxiety, psychotic or substance use disorder. There had been no episodes of persistent low mood or anhedonia lasting longer than 2 days. There was no past history of features meeting criteria for manic or hypomanic episodes, such as persistently elevated, expansive or irritable mood, persistent increase in activity or energy, decreased need for sleep, grandiosity and increase in goal-directed activity. There was no temporal relationship between her behavioral symptoms and seizures. There was no family history of bipolar disorder or epilepsy.
During the interview, she made frequent interruptions after which she would immediately apologize. Speech was increased in rate but not pressured. She described her mood as good but her affect was labile; she rapidly vacillated between tearfulness when talking about her psychosocial stressors and joy when talking about her daughters. The degree of her emotional expression was more than expected based on her thought content. Thought process was tangential and overinclusive of detail. Thought content and perception did not show any evidence of psychosis. Symptoms of PBA were not psychometrically quantified. Citalopram was prescribed for PBA but she stopped it within days because of reported “out-of-body” sensations.
On brain MRI (Figure 1), the size of the meningioma showed no interval change compared with 3 years earlier. It measured 6.7×3.7×3.0 cm and exerted mass effect medially as described above (with encasement of the left cavernous internal carotid artery) and laterally toward the mesial temporal region. Neuropsychological assessment revealed mild impairments in attention capacity, aspects of executive functions including response inhibition and problem-solving and moderate impairment in visual planning/organization. Verbal memory was average; although left anterior temporal lobectomy would carry a risk of significant verbal memory decline, risk of global amnesia was low. Four seizures were captured on 48-hour ambulatory electroencephalography, all with left temporal lobe origin, in keeping with the likely effect of compression from the meningioma. In discussion with the patient, the decision was made to proceed with a combination of anterior temporal lobectomy and mesial temporal resection. It was made clear to the patient that this was aimed at seizure control rather than improving PBA, although the latter was the more disabling symptom.
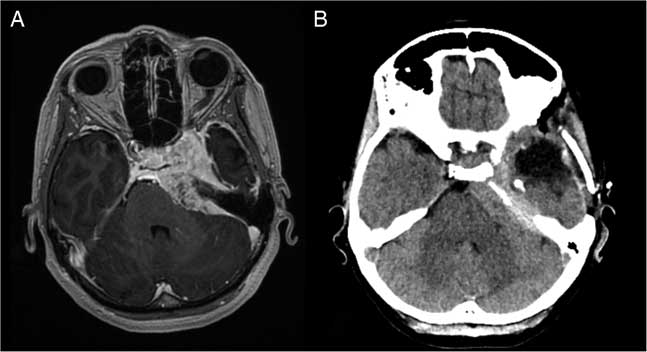
Figure 1 Comparison of axial images before and after left temporal lobectomy. (A) Pre-operative 1.5T T1-weighted post-gadolinium MRI; avidly enhancing left petrous apex meningioma extends along the clivus with mass effect on the left anterolateral aspect of the pons, as well as the inferomedial margin of the left temporal lobe. (B) Post-operative non-contrast CT; hypodense fluid collection in the middle cranial fossa following temporal lobectomy and partially calcified, hyperdense meningioma with mass effect on the adjacent pons.
At 2 weeks post-operatively, the PBA had essentially subsided, along with the rapid speech and tangentiality. On postoperative CT (Figure 1), the meningioma appeared to exert less mass effect on the brainstem. At 2 months post-operatively, the patient remained seizure free. She noted mild deterioration in verbal memory but was pleased with the outcome in terms of seizure control, affective improvement and quality of life.
On the basis of the model proposed by Lauterbach et al,Reference Lauterbach, Cummings and Kuppuswamy 1 PBA in our patient appears to have arisen owing to compression of the meningioma on the “voluntary” frontopontine pathway, which normally inhibits a network that regulates “involuntary” emotional expression including the amygdala-hypothalamus-periaqueductal gray-dorsal tegmentum complex. Several case reports have described PBA with extra-axial tumors of the posterior fossa.Reference McCormick and Lee 2 McCormick and LeeReference McCormick and Lee 2 highlight the reversibility of this presentation in two cases after the resection of a petroclival meningioma.
In our patient, although resection of the meningioma was not possible, PBA resolved on resection of adjacent temporal lobe structures. We hypothesize that the resolution of PBA was the result of two possible mechanisms. The removal of the adjacent temporal lobe structures might have allowed the tumor to exert more mass effect laterally, with some relief of medial mass effect on the brainstem. In addition, the PBA could have been abolished owing to the resection interrupting the involuntary network described above, including the amygdala, and thus inhibiting emotional expression. This possibility is supported by reports of diminished emotional response to fear-conditioning after temporal lobectomy.Reference Weike, Hamm, Schupp, Runge, Schroeder and Kessler 3 Decreased emotional expression has also been described with Kluver–Bucy syndrome, typically involving lesions to the amygdala bilaterally but occasionally described in partial form with unilateral lesions.Reference Morcos and Guirgis 4 , Reference Salim, Kim, Kimbrell, Petrone, Roldan and Asensio 5
This latter hypothesis is particularly interesting given that it opens the possibility of surgical intervention for PBA. Apart from resection, this could also involve deep brain stimulation (DBS), for example targeting the basolateral amygdala. Stimulation on the right side in rats and bilaterally in few human case series has been shown to inhibit behavioral activation in response to stressful cues.Reference Stidd, Vogelsang, Krahl, Langevin and Fellous 6 , Reference Koek, Langevin and Krahl 7 A pilot randomized controlled trial of basolateral amygdala DBS in post-traumatic stress disorder is underway.Reference Koek, Langevin and Krahl 7
In conclusion, this case illustrates the reversibility of chronic PBA from a petrous meningioma after temporal lobe resection. We encourage further studies exploring the potential for surgical intervention and DBS for PBA.
Statement of Authorship
All authors contributed to the clinical care of this patient, and to preparation and editing of the manuscript.
Disclosures
Dr. Moien Afshari reports personal fees from UCB Pharma lecture honorarium and Sunovion lecture honorarium, outside the submitted work. Drs. Hassan, Sahjpaul, and Tan have no conflicts of interest to declare.



