Interventions targeting a combination of increased physical activity, reduced sedentary behaviour and promotion of healthy eating have been shown to be most promising in the treatment of childhood obesity(Reference Oude Luttikhuis, Baur and Jansen1). Increased treatment effect is seen in programmes where the parents are targeted as the main agents of change(Reference Kitzmann, Dalton and Stanley2), enhancing parental leadership, parenting skills and taking advantage of the parents' ability to modify the shared family environment(Reference Epstein, Paluch and Roemmich3). However, the most effective way to involve parents in these interventions remains unclear and warrants further investigation(Reference Golan, Kaufman and Shahar4, Reference Reinehr5).
Many studies on multi-component childhood obesity treatment are too short in duration to see a significant reduction in adiposity, but still focus solely on adiposity outcomes and may therefore underestimate the interventions' effectiveness(Reference Kitzmann, Dalton and Stanley2). Measuring changes in health-related behaviours, such as dietary intake, may be an alternative indicator of treatment effect(Reference Kitzmann, Dalton and Stanley2) when the duration of the study is too short to see the effects on adiposity. A systematic review of randomised trials found that interventions including dietary modification are effective in reducing the adiposity of obese children, but details of the dietary intervention or participants' dietary intake are rarely described(Reference Collins, Warren and Neve6).
The present study is a family-based child obesity trial with different parental interventions: therapist-led groups (TLG) and self-help groups (SHG). The objective of the present study was to compare the long-term (24 months) effectiveness of these interventions by assessing changes in adiposity and dietary intake, by achieving changes in lifestyle based on the families' situation at baseline. This is thus one of few trials existing with assessment beyond 1 year(Reference Collins, Okely and Morgan7) and the first to compare the long-term changes in adiposity and dietary intake accompanying different ways to involve parents in family-based obesity treatment of children. Details of the dietary intervention and participants' dietary intake are described.
Methods
Participants, study setting and ethics
Children who were referred by their general practitioner to outpatient obesity treatment at St Olav University Hospital, Trondheim, Norway, in 2005–8, were assessed for eligibility. The inclusion criteria were as follows: age 7–12 years; BMI z-scores ≥ 2; participation of at least one parent; the ability to participate in a group setting. Families were excluded if the obese child was mentally retarded, if there was an organic cause of obesity or if the child used medication that may interfere with growth or weight control. No data on puberty stage of the participating children were recorded.
The treatment was conducted in an outpatient hospital setting. Only accredited psychologists, paediatricians, clinical dietitians and physiotherapists with experience of treating children with obesity were permitted to manage patients in the trial. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving patients were approved by the Regional Ethical Committee for Medical Research. Written informed consent was obtained from all parents. All data were analysed anonymously. The registration number of the present trial is nct00842777.
Interventions
Reduced child adiposity was targeted through gradual changes that the families could manage to maintain over time, based on international and Norwegian recommendations(Reference Barlow8, 9). The focus of both the TLG and SHG interventions was to establish regular mealtimes, increase the intake of fruits, vegetables and other high-fibre food, reduce the intake of added sugar and fat, conduct at least 1 h of moderate physical activity per d and reduce sedentary behaviour gradually, towards a maximum of 2 h per d.
The main focus of the TLG sessions was to enhance the parents' competence to accomplish the targeted lifestyle changes. A detailed treatment manual was devised. A total of ten sessions were conducted with the following topics: expectancies and goal setting; communication about obesity, diet and physical activity; daily physical activity; everyday dietary habits; mastery and motivation; guidance and setting boundaries; the role of siblings and the social network; parent's history of diet and physical activity; self-concept and body image; vacations and birthday parties. In brief, each group session was led by two therapists, and each session included the following: a presentation of the topic of the session followed by a group discussion; a discussion of the homework assignment for the present session; in some sessions also a role play on the topic of the session. A series of written material, such as ‘fridge notes’, home activity sheets and goal attainment sheets, was developed.
The SHG were based on the principle of mutual help, derived from the participants' own experiences and knowledge. A health professional attended the two first and the last meeting to organise the group and facilitate group rules, but did not offer any education or guidance regarding how to reduce adiposity.
Stratified by the age, sex and BMI of their child, parents were randomly allocated to the TLG or SHG intervention (1:1 ratio) using a computer-generated list of random numbers. Both the TLG and SHG consisted of parents from four to six families.
All children, regardless of their parents' group affiliation, participated in age-matched groups of six to twelve children led by a clinical dietitian and a physiotherapist. The aim was for the children to gain positive experiences related to physical activity and healthy eating, and the psychosocial consequences of being obese were addressed in a session led by a psychologist. All families attended five individual counselling sessions with a clinical dietitian and a physiotherapist to discuss the family's progress and to define new goals.
The design of the study was based on the findings from pilot studies from 2003 to 2005, suggesting that it was preferable to have an intensive phase of 6 months at the beginning of the intervention period followed by a longer and less intensive phase of 18 months (S Steinsbekk and R Ødegård, unpublished results). The TLG, SHG and children's groups met simultaneously every second week for ten sessions during the first 6 months. During this 6-month period, each family also met monthly for individual counselling. Over the remaining 18 months of the 24-month intervention, the groups met five times at the hospital, and four individual family counselling sessions were conducted. Each of the fifteen group sessions lasted 2 h, while each of the ten individual family counselling sessions lasted 30 min. The study was completed in February 2010.
Outcome measurements
The primary study outcomes of the present study were changes in the percentage of body fat (BF), BMI z-scores and dietary intake. Data were collected in the hospital setting by members of the treatment staff at baseline (before randomisation), and after 6 and 24 months of treatment. Apart from the health professionals performing the dual-energy X-ray absorptiometry, it was not possible to keep assessors blinded to treatment condition. A standard protocol was used to facilitate the objective and reliable measurement of height and weight.
During anthropometric assessments, children wore light clothing and no shoes. Weight was obtained by a digital scale (Seca 930; Vogel&Halke) and height was measured by a stadiometer (Hyssna Limfog AB). BMI was calculated in kg/m2, and BMI z-score was computed according to international reference values(Reference Cole, Bellizzi and Flegal10). Dual-energy X-ray absorptiometry (Hologic QDR Discovery) was used to estimate BF.
A 4 d food record (three consecutive weekdays and one weekend day) was used to estimate the children's dietary intake. Children and parents were instructed to register everything the children ate and drank during the 4 d period. To improve the participants' accuracy in reporting portion sizes, a booklet(Reference Lillegaard, Overby and Andersen11) with a photograph series of thirteen food items of known portion weights was distributed to the families for comparison instead of weighing all food. A clinical dietitian reviewed the food record together with the family at the forthcoming individual consultation. Daily energy and macronutrient intake was calculated from the reported food intakes using a Web-based dietary analysis program based on the Norwegian Food Composition Table 2006 (Mat på Data 5.1; Norwegian Food Safety Authority, the Directorate for Health and the Department of Nutrition at the University of Oslo), and was compared with Norwegian dietary recommendations(9): protein (10–20 % of energy intake (E%)); fat (25–35 E%); SFA ( ≤ 10 E%); MUFA (10–15 E%); PUFA (5–10 E%); carbohydrates (50–60 E%); added sugar ( ≤ 10 E%); dietary fibre (2–3 g/MJ). Intake of macronutrients was expressed both as grams and as E% and fibre as nutrient density (g/MJ), because children aged 7–12 years have different requirements for amount of food, whereas the same macronutrient composition is recommended for this age group(9). Energy intake was reported per kg of body weight and as a ratio of energy intake:estimated BMR (energy intake:BMR). BMR was estimated from weight and height using age- and sex-dependent Schofield equations(Reference Schofield12).
Statistical methods
The distribution of the data was found to be normally distributed, and comparisons of baseline study characteristics between the groups, and between-group differences for the mean change in outcomes from baseline to 6 and 24 months were analysed with independent-samples t tests, using PASW 19.0 software (IBM Corporation). Within-group parameter changes were analysed using a paired t test. Statistical significance was set at P< 0·05. To correct for multiple comparisons to control the expected proportion of incorrectly rejected null hypotheses, the calculated P values were corrected for multiple testing by a false discovery rate using the Benjamini–Hochberg method(Reference Benjamini and Hochberg13).
Results
Participants
The flow of participants throughout the trial and the number of participants in each group from whom anthropometrical and dietary data were obtained at baseline, 6 and 24 months are described in Fig. 1. Of the 123 families assessed for eligibility, ninety-nine (80·5 %) consented (forty-eight girls, fifty-one boys) and were randomly assigned to the TLG (n 47) or SHG (n 52) intervention. The retention rate was 90 % after 6 months and 81 % after 24 months, and there were no differences in retention rates between the groups.
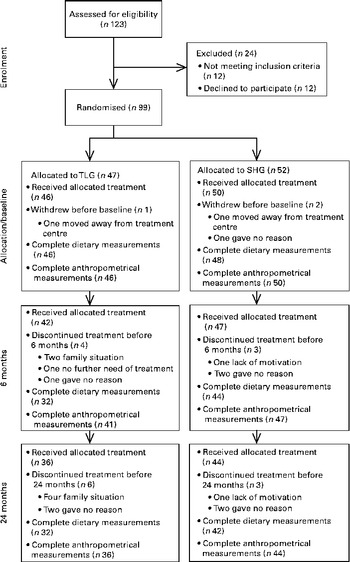
Fig. 1 Flow diagram of the participants' course throughout the study, randomly allocated to the parallel therapist-led group (TLG) or self-help group (SHG).
Not all children handed in their food record forms at all visits, and it was of interest to see whether there were any differences between the children who handed in their forms and those who did not. As shown in Fig. 1, most children in both intervention groups did hand in their food record forms at baseline (TLG, 100 %; SHG, 96 %), and after 6 months (TLG, 76 %; SHG, 94 %) and 24 months (TLG, 89 %; SHG, 95 %). Regarding age, sex, BF, BMI z-score and energy intake at baseline, there were no differences between participants who handed in food record forms at 6 and 24 months, compared with those who did not (data not shown).
At baseline, the mean E% from SFA in both intervention groups was above the recommended amount ( ≤ 10 E%), while the TLG's mean E% from added sugar was above the recommended amount ( ≤ 10 E%) and the SHG's mean E% from carbohydrates was below the recommended amount (50–60 E%). The mean E% of the remaining variables was in accordance with Norwegian recommendations(9).
There were no statistically significant differences between the two groups at baseline, regarding child and parent anthropometry, child age, sex, energy intake and BMR (Table 1). All but two of the participating children were Caucasian; one child was of African origin and another was of Latin American origin.
Table 1 Baseline characteristics of obese children (n 83) and their parents, participating in a randomised controlled trial by treatment group and total group* (Mean values and standard deviations)
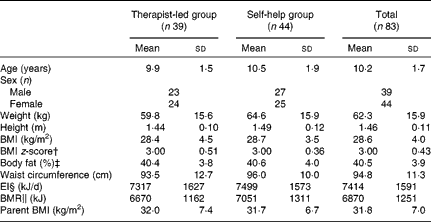
EI, energy intake.
* Mean values were not significantly different between the intervention groups (P>0·05).
† BMI z-score was calculated according to Cole et al. (Reference Cole, Bellizzi and Flegal10).
‡ Body fat was measured by dual-energy X-ray absorptiometry.
§ EI was estimated from 4 d food records.
∥ BMR was calculated with Schofield equations(Reference Schofield12).
Changes during the treatment
Except for the change in E% from added sugar from baseline to 6 months (TLG, − 2·8 (sd 4·4); SHG, 0·9 (sd 7·2); P< 0·05), there were no significant between-group differences for the change in BF, BMI z-scores or dietary intake from baseline to 6 and 24 months (data not shown).
In both intervention groups, BF, BMI z-scores and energy intake significantly decreased from baseline to 6 months and from baseline to 24 months (Table 2; see Fig. 1 for the flow of the participants). In the TLG and SHG, respectively, a reduction in BMI z-scores of 0·22 and 0·19 units was observed from baseline to 6 months, and a reduction of 0·18 and 0·17 units was seen from baseline to 24 months. Concomitant with this, a 4·7 and 4·4 % reduction in BF was seen from baseline to 6 months, and a 4·8 and 5 % reduction in BF from baseline to 24 months in the TLG and SHG, respectively.
Table 2 Measures of adiposity and dietary intake at baseline, 6 and 24 months of the study participants by treatment group, and changes from baseline to 6 and 24 months§∥ (Mean values and standard deviations)
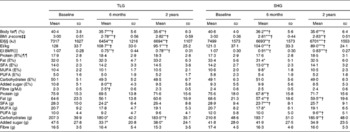
TLG, therapist-led group; SHG, self-help group; EI, energy intake; E%, percentage of energy intake.
* Within-group change from baseline to 6 months or baseline to 24 months calculated with paired-samples t tests: *P< 0·05, **P< 0·01, ***P< 0·001.
† Significant when adjusted for multiplicity(Reference Benjamini and Hochberg13).
§ There were no differences between the two groups at baseline, 6 months or 24 months calculated with independent-samples t tests.
∥ Except for the change in E% from added sugar from baseline to 6 months, there were no between-group differences in change from baseline to 6 and 24 months calculated with independent-samples t tests.
¶ Body fat was measured by dual-energy X-ray absorptiometry.
‡‡ BMI z-score was calculated according to Cole et al. (Reference Cole, Bellizzi and Flegal10).
§§ EI was estimated from 4 d food records.
∥∥ BMR was calculated with Schofield equations(Reference Schofield12).
¶¶ Percentage of daily EI.
The dietary macronutrient composition changed, with mean E% from added sugar decreasing (P< 0·01) and mean g fibre/MJ increasing (P< 0·05) from baseline to 6 months in the TLG and mean E% from SFA decreasing from baseline to 6 months (P< 0·01) in the SHG, but these changes were not sustained after 24 months. In both groups, the reported mean E% from SFA was 3·0–4·5 % above Norwegian recommendations at all assessment points, whereas mean E% from MUFA was 0·2 % below the recommended level at 6 months in the SHG and mean E% from added sugar was 0·8 % above the recommended level at baseline in the TLG. The mean E% from carbohydrates were 0·7 and 1·5 % below the recommended level at 6 and 24 months, respectively, in the TLG, and 0·9 and 1·0 % below the recommended level at baseline and 24 months, respectively, in the SHG. The mean E% of the remaining macronutrients was in line with Norwegian recommendations. Apart from a significant decrease in energy intake/kg in both intervention groups, no significant changes were found from 6 to 24 months for BF, BMI z-scores or dietary intake in either group.
Discussion
To our knowledge, the present study is the first to compare the long-term changes in adiposity and dietary intake accompanying different ways to involve parents in family-based child obesity treatment. The key study findings were that no significant differences were detected for the change in adiposity and dietary intake between children of parents in the TLG and SHG. In both groups, the children achieved a significant reduction in BF and BMI z-scores after 6 months, which persisted throughout 24 months of treatment. Also, both groups achieved a significant reduction in energy intake from baseline to 6 months, which was sustained after 24 months, with an even further reduction in energy intake/kg from 6 to 24 months in both groups. In contrast to many child obesity treatment studies included in systematic reviews(Reference Oude Luttikhuis, Baur and Jansen1, Reference Collins, Warren and Neve6), the present study had generalisable recruitment methods, true randomisation, long-term treatment and high retention rates, and a short-term as well as a long-term assessment was conducted. Using BF measured by dual-energy X-ray absorptiometry as an outcome further strengthens the findings, as this is a more specific measure of obesity which is more accurate and sensitive to change compared with measures of only height and weight in children(Reference Helba and Binkovitz14).
In the present study, no between-group differences in the effectiveness of the interventions were found. It is therefore possible that the support received by the parents in the SHG may have been as effective as the education parents received in the TLG, or the reverse, parent groups were not the most effective part of the intervention. Similar reductions in adiposity after child obesity treatment conducted under a wide range of conditions were found in a recent meta-analysis(Reference Kitzmann, Dalton and Stanley2), and studies using wait-listed controls have found significant improvements in adiposity in the control condition(Reference Golley, Magarey and Baur15). These findings indicate that when some main components are present, interventions may be efficient in the short term, regardless of how treatment is delivered, and parent readiness to change has been suggested as one such key predictor of treatment success(Reference Barlow8). The detection of obesity through recruitment and baseline assessment may be sufficient, by making families aware of the child's obesity and motivating them to change behaviour(Reference Hughes, Stewart and Chapple16). It is therefore possible that the comparable improvement in children's adiposity seen in both intervention groups was a result of the parents' readiness to make lifestyle changes.
Children with parents in the TLG and SHG achieved a significant reduction in BMI z-scores of 0·22 and 0·19 units from baseline to 6 months, whereas others have found a 0·31 unit reduction(Reference Okely, Collins and Morgan17) and a 0·10 unit reduction(Reference Hughes, Stewart and Chapple16), both reported at the end of 6-month family-based interventions. Few child obesity treatment studies have reported a change in BF, but one high-intensity, family-based 12-month programme including exercise, dietary and behaviour modification has reported a 4·0 % reduction in BF after 12 months(Reference Savoye, Shaw and Dziura18). The 4·8 and 5 % reduction in BF after 24 months found in the TLG and SHG, respectively, of the present study is thus an interesting finding, as the SHG of the present study is estimated to be much less resource-intensive compared with the aforementioned high-intensity programme. The substantial reduction in BF and the modest reduction in BMI z-score may indicate that the children acquired increased muscle mass. To our knowledge, no study comparable with the present study has been published on child obesity treatment with 24 months duration. The clinical significance of adiposity reduction found in the present study may be questioned, as one study(Reference Ford, Hunt and Cooper19) has suggested that a reduction in BMI z-scores of at least 0·25 is required to improve adiposity and metabolic health in obese adolescents. A recent study, however, has found that even a modest reduction in BMI z-score of < 0·1 is associated with improvement in several cardiovascular risk factors(Reference Kolsgaard, Joner and Brunborg20), which would make the results of the present study clinically significant.
The present study focused on gradual changes that the families were expected to be able to maintain over time, as emphasised by an expert committee on obesity treatment in children(Reference Barlow8). An intervention period of 24 months should be of adequate duration to see substantial reductions in adiposity, whereas other benefits of behavioural obesity treatment (e.g. weight-related behaviours such as dietary habits) may be more noticeable in such a short term(Reference Barlow8). In the present study, a reduction in adiposity and energy intake persisted from 6 to 24 months of treatment, i.e. 18 months after the intensive part of the intervention in both TLG and SHG. These findings are of great interest, as they could imply that the participating families acquired important changes in eating habits independent of the parental intervention. In the long run, these beneficial eating habits could contribute to a reduced risk of childhood obesity tracking into adult life(Reference Reilly, Methven and McDowell21) for the participating children, particularly if the children's muscle mass was increased, thereby increasing the proportion of metabolically active tissue and hence BMR. Changes in dietary intake in the present study are consistent with similar findings from the few child obesity interventions reporting this outcome(Reference Alexy, Reinehr and Sichert-Hellert22–Reference Waling, Lind and Hernell24), namely a decrease in energy intake, concomitant with minimal changes in macronutrient composition.
The present study has some limitations. We did not include a no-treatment control group in the present study and thus the change in BMI z-score may just reflect the natural course of the children's growth. However, it is reasonable to assume that both intervention groups would have been successful relative to a no-intervention control group, as findings from several studies(Reference Golley, Magarey and Baur15, Reference Wright, Emmett and Ness25, Reference Wilfley, Tibbs and Van Buren26) indicate that adiposity continues to increase in obese children not receiving treatment. There are ethical issues associated with having a long-term no-intervention group in child obesity studies(Reference Warren, Golley and Collins27), and some have argued that because an intervention is established to be more effective than the passage of time, there is little to be learned from repeated comparisons with no-treatment or wait-listed controls(Reference Gilles, Cassano and Shepherd28). Also, no information was available regarding the puberty stage of the children, meaning that we had an unknown ratio of pre-pubertal to pubertal children in the present study. The puberty stage may have affected the children's level of physical activity; however, we have recently published that the physical activity of these children was in fact decreased after 6 and 24 months(Reference Steinsbekk, Wichstrom and Odegard29), supporting the belief that reduced adiposity in the children in the present study was mainly due to reduced energy intake. Also, the inherent difficulties of measuring dietary intake in children are well recognised(Reference Collins, Watson and Burrows30, Reference Magarey, Watson and Golley31), being prone to reporting error, mostly through under-reporting(Reference Collins, Watson and Burrows30), which increases with children's age(Reference Bandini, Must and Cyr32) and with increasing adiposity(Reference Fisher, Johnson and Lindquist33), thus the present findings should be interpreted in the context of some limitations. It is nevertheless important to report dietary intakes in children to evaluate which eating habits are amenable to change(Reference Collins, Watson and Burrows30), and using the same dietary assessment method throughout the study increases the likelihood of systematic rather than random errors. The estimated food record is considered an accurate dietary assessment tool(Reference Biro, Hulshof and Ovesen34), with the ability to provide an estimate of energy and macronutrient intake when reported for 3–10 d, including week and weekend days. Comparing foods with photographs has been recognised as potentially improving estimates of dietary intake in a paediatric population(Reference Higgins, LaSalle and Zhaoxing35) and is less burdensome for the participants than a weighed food record(Reference Higgins, LaSalle and Zhaoxing35). Despite the limitations, we believe that the relative reduction in reported energy intake is real, based on the observed reduction in adiposity during the treatment period.
There is little doubt that cost-effectiveness will become an increasingly important consideration in future health decisions. As the two intervention groups in the present study were equally effective, a logical inference is that the least expensive intervention is the more cost-effective. The potential resource savings of the SHG, instead of the TLG, have important health cost implications. The SHG might be the solution to the scarcity of health professionals in primary health care. However, it is yet to be determined whether the SHG are equally effective as other types of lifestyle interventions. Based on these findings, we recommend that clinicians should focus on the child's energy intake and explore the possibilities for reducing the child's total energy intake together with the family, focusing on both type and the amount of food eaten.
In conclusion, the present randomised parallel-group trial demonstrated that obese children participating in two family-based interventions including TLG and SHG for parents achieve equally positive, persistent improvements in adiposity and dietary intake. Improvements in adiposity and dietary intake were sustained from 6 to 24 months of treatment, i.e. 18 months after the intensive part of the intervention. This could imply that the children acquired favourable changes in their eating habits that combined with a reduced BF mass and a possibly increased muscle mass may contribute to a reduced risk for childhood obesity tracking into adult life. Considerable work remains to determine the optimal way to involve parents to achieve persistent, cost-effective reduction in the adiposity of obese children. Further research should be performed to clarify whether the SHG should be preferred to parental group treatment for similar children with obesity.
Acknowledgements
We thank all children and parents who have contributed to the present study. We are also grateful to our colleagues at St Olav University Hospital. The present study was supported by the Liaison Committee for Central Norway Regional Health Authority; the National Council of Mental Health/Health and Rehabilitation, NTNU; St Olav University Hospital; the Bergen Medical Research Foundation and the Meltzer Foundation. S. S., R. Ø. and L. W. contributed to the study design. S. S. and R. Ø. contributed to the conduction of the study. All authors contributed to the analysis and interpretation of the data. H. T. H., S. S., L. W. and O. A. G. performed the statistical analysis. H. T. H. drafted the manuscript. All authors critically revised the final manuscript. The authors declare no conflicts of interest.





