Recommended dietary macronutrient levels differ among teleosts(1). Optimal levels of digestible protein (DP) and digestible non-protein energy (NPE; fat or starch) are often related to the species’ trophic level or feeding habitat. Dietary protein levels for maximum growth of fish depend also on the digestible energy (DE) level or DP:DE ratio, and hence on the amount of NPE in the diet(Reference Wilson, Halver and Hardy2). Studies on the effect of dietary NPE on growth and nutrient utilisation mostly focused on total NPE levels(Reference Dias, Alvarez and Diez3–Reference Shiau and Peng8), despite clear evidence that the protein-sparing effect of digestible fat and carbohydrates is species-dependent, varying according to species’ feeding habitat (cold v. warm-water) or feeding habit (carnivorous v. omnivorous)(Reference Furuichi and Yone9–Reference Sargent, Henderson, Tocher and Halver13). For example, while rainbow trout or other salmonids efficiently utilise dietary lipid above 10 % without negative effects on growth(Reference Sargent, Henderson, Tocher and Halver13–Reference Watanabe15), other fish species such as turbot, Senegalese sole, Nile tilapia and grass carp seem to display a limited ability to use high dietary fat(Reference Chou and Shiau16–Reference Regost, Arzel and Cardinal22). On the other hand, most warm-water fish such as Nile tilapia appear to utilise higher levels (up to 40 % of diet DM) of dietary carbohydrates than cold-water fish such as rainbow trout ( ≤ 20 % of diet DM)(1, Reference Wilson10, Reference Shiau23, Reference Wang, Liu and Tian24). The limited ability of rainbow trout (carnivorous-like species) compared to, for example, Nile tilapia and common carp (omnivorous-like species) to efficiently utilise dietary carbohydrates as a NPE source is illustrated by the prolonged hyperglycaemia after oral administration of glucose or high-carbohydrate meals (>20 % of diet DM) in the former(Reference Bergot25–Reference Palmer and Ryman27). Similar induction of enzyme activity or gene expression of glucose-phosphorylating enzymes by dietary carbohydrates in the liver of rainbow trout and common carp(Reference Panserat, Médale and Blin28), however, suggests that metabolic mechanisms other than glycolysis per se are implicated in the control of dietary glucose utilisation. Yet, surprisingly few studies have compared, by using identical diet formulations, possible metabolic mechanisms underlying differences in macronutrient utilisation between different fish species.
The present study aims to highlight divergences between two teleosts, rainbow trout and Nile tilapia, in the response of intermediary metabolism to changes in dietary DP:DE ratio and in NPE source. To this purpose, four isoenergetic diets were formulated with a DP:DE ratio above (27 mg/kJ) and below (14 mg/kJ) the optimal DP:DE ratio for both tilapia(1, Reference Kaushik, Doudet and Médale29) and rainbow trout(1, Reference Dias, Corraze and Arzel30). At each dietary DP:DE ratio, a contrast was created in the NPE source by replacing fat by an isoenergetic amount of digestible starch. The diet-induced responses of rainbow trout and Nile tilapia in terms of nutrient gain and utilisation were then associated with data on circulating plasma metabolites and activity levels of key enzymes involved in hepatic intermediary metabolism.
Materials and methods
Fish, feed and growth trial
The feeding trial with rainbow trout was conducted following the Guidelines of the National Legislation on Animal Care of the French Ministry of Research (Décret no. 2001-464 of May 29, 2001). All procedures involving Nile tilapia were carried out in accordance with the Dutch law on experimental animals and were approved by the Wageningen University Animal Experimental Committee (DEC). Details on the feeding and digestibility trials of the fish used in this study have been described previously for rainbow trout(Reference Saravanan, Schrama and Figueiredo-Silva31) and tilapia(Reference Saravanan, Geurden and Figueiredo-Silva32), with specific focus on the regulation of diet-induced differences in voluntary feed intake as a function of dietary energy use. Briefly, four isoenergetic (approximately 18·5 MJ DE/kg diet DM) diets (2 × 2 factorial design) were formulated (Table 1) having a contrast in (i) the ratio of DP:DE: high (HP, approximately 27 mg/kJ) or low (LP, approximately 14 mg/kJ) and (ii) the type of NPE source: fat (F) v. starch (S) which were iso-energetically exchanged. We thus had four experimental diets that were expected to exacerbate differences in metabolic response, having a high DP:DE ratio with fat (HPF) or starch (HPS) as the NPE source or a low DP:DE ratio with fat (LPF) or starch (LPS) as the NPE source (Table 1). Inclusion of 15 % of cellulose as indigestible filler to the F-diets allowed an identical nutrient and energy density between diets. The diets were carefully fed by hand to apparent visual satiation to triplicate groups of rainbow trout (initial body mass 32 g; 17°C; INRA Donzacq experimental fish farm, France) and Nile tilapia (initial body mass 40 g; 28°C; Wageningen University, The Netherlands) for 42 and 48 d, respectively. At the end of the feeding trial, the liver (nine fish/treatment) was dissected at 7 h after the last meal in rainbow trout and 3 h after the last meal in Nile tilapia and immediately stored at − 80°C for subsequent analyses of enzyme activities. Blood samples (nine fish/treatment) were collected at 7 and 40 h after the meal in trout and at 3 and 36 h after the meal in tilapia. Postprandial sampling times were selected to represent the time at which plasma metabolites are found to peak and return to basal levels in the respective species (our own observation). Blood samples were taken from the caudal vein and collected into tubes using sodium fluoride (2 %) and potassium oxalate (4 %) as anti-coagulant. Plasma samples, obtained after centrifugation (3000 g for 15 min at 4°C), were stored at − 20°C for further analyses.
Table 1 Ingredient and analysed composition of the diets fed to rainbow trout and Nile tilapia

HPF, high protein with fat as the major non-protein energy source; HPS, high protein with starch as the major non-protein energy source; LPF, low protein with fat as the major non-protein energy source; LPS, low protein with starch as the major non-protein energy source; DP, digestible protein; DE, digestible energy.
* Supplied by Sopropêche.
† Supplied by Roquette.
‡ Supplied by Ajinomoto Eurolysine.
§ Supplied by Daudruy.
∥ Supplied by Rettenmeier et Söhne.
¶ Other (g/kg): 20 g Diamol (indigestible marker, Diamol GM; Franz Bertram); 10 g vitamin and mineral premix (INRA UPAE 78 200 Jouy en Josas) and for LPF and LPS 4 g CaCO3, 18 g Ca(HPO4)2 and 5 g Na2CO3.
** Calculated as (DE total − DE from protein − DE from lipid)/17·7, using energy values (kJ/g) of 23·7, 39·6 and 17·7 for protein, fat and carbohydrates, respectively.
Feed, whole-body and plasma metabolite analyses
Feed and whole-body analyses were made as detailed previously for rainbow trout(Reference Saravanan, Schrama and Figueiredo-Silva31) and Nile tilapia(Reference Saravanan, Geurden and Figueiredo-Silva32). Plasma glucose and TAG were determined as described previously(Reference Saravanan, Schrama and Figueiredo-Silva31). For the determination of total plasma free amino acids, samples were treated by ultra-filtration to eliminate protein, with a centrifugation at 2000 g for 2 h followed by filtration (filters Amicon-Microcon, YM 100 000 Da). Next, 500 μl of water and 300 μl of ninhydrine reagent (2 % solution; Sigma) were added to 100 μl of filtrate and left for 10 min at 100°C. Then, 1·5 ml of ethanol were added progressively to stop the reaction. After 30 min in the dark, absorbance was measured at 570 nm using a spectrophotometer (Shimadzu UV-160A; Shimadzu Scientific Instruments). Results are expressed in μmol/ml of plasma.
Enzyme activity measurement
Activities of alanine aminotransferase (ALAT, EC 2.6.1.2), aspartate aminotransferase (ASAT; EC 2.6.1.1), glutamate dehydrogenase (GDH, EC 1.4.1.2), glucose-6-phosphate dehydrogenase (G6PD, EC 1.1.1.49), malic enzyme (ME, EC 1.1.1.40), fatty acid synthase (FAS, EC 2.3.1.38), hexokinase (HK; EC 2.7.1.1) and glucokinase (GK, EC 2.7.1.2) were determined in rainbow trout and Nile tilapia liver as described previously(Reference Figueiredo-Silva, Corraze and Kaushik33). Activity of GK was corrected by measuring glucose dehydrogenase activity (EC 1.1.1.47), a moderately active microsomal enzyme in fish liver that can introduce significant bias into GK measurements on frozen tissue(Reference Panserat, Capilla and Gutierrez34). To measure glucose-6-phosphatase (G6Pase; EC 3.1.3.9) activity, the liver microsomal fraction was suspended in buffer (100 mm-NaH2PO4; 25 mm-Na2HPO4; 2mm-EDTA; 1mm-dithiothreitol (DTT), pH 7) and G6Pase assayed according to Panserat et al. (Reference Panserat, Médale and Breque35). Activity of 3-hydroxyacyl-CoA dehydrogenase (HOAD, EC 1.1.1.35) was determined as described previously(Reference Figueiredo-Silva, Kaushik and Terrier36). Enzyme activity (units IU), defined as μmol of substrate converted to product per min at assay temperature, was expressed per mg of soluble protein (specific activity). Soluble protein content (Bradford method) was determined using a protein assay kit (Bio-Rad) and bovine serum albumin as a standard.
Statistical analysis
Statistical analyses were performed using the STATISTICS 7.0 package (StatSoft, Inc.). All data were tested for normality and homogeneity of variances by Kolmogorov–Smirnov and Bartlett tests, and then submitted to a two-way ANOVA, using dietary DP:DE and NPE as independent factors. When the interaction between DP:DE and NPE was significant (P< 0·05), individual means were compared using the Tukey honest significant difference test. Pearson correlation coefficients (R) were obtained by the Pearson product moment correlation distribution and considered significant when P< 0·05.
Results
Table 2 summarises the data on food intake and growth performances for both rainbow trout and Nile tilapia. Data on total food intake, initial and final body mass and efficiency of nutrient retention have been presented before for trout(Reference Saravanan, Schrama and Figueiredo-Silva31) and for tilapia(Reference Saravanan, Geurden and Figueiredo-Silva32). At the end of the 42- and 48-d feeding trial, trout exhibited a 2- to 3-fold and tilapia a 5- to 6-fold increase in body mass (Table 2). The thermal growth coefficients obtained with the high-DP:DE diets were similar for tilapia (1·9–2·1) and trout (2·0–2·1). Thermal growth coefficients of trout were affected by both the DP:DE ratio and NPE source, with a significant interaction between both; replacement of fat by digestible starch significantly reduced thermal growth coefficients of trout at low but not at high DP:DE (Table 2). Thermal growth coefficients of tilapia were reduced by the replacement of fat by digestible starch, but not by the dietary DP:DE ratio (Table 2).
Table 2 Growth performance, food intake, digestible nutrient intake, nutrient gain and retention in rainbow trout and Nile tilapia fed diets with different digestible protein-to-energy (DP:DE) ratio and non-protein energy source (NPE, fat v. starch) for 6 weeks (Mean values and standard deviations, n 3)

HPF, high protein with fat as the major NPE; HPS, high protein with starch as the major NPE; LPF, low protein with fat as the major NPE; LPS, low protein with starch as the major NPE; TGC, thermal growth coefficients; MBW, metabolic body weight.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different and showed a significant interaction between the two tested factors (DP:DE v. NPE; P< 0·05 two-way ANOVA).
* TGC = 1000 × ((final body mass)1/3− (initial body mass)1/3)/(temperature (°C) × number of d).
† Expressed as g/kg MBW per d. MBW is calculated as the geometric mean body mass at the power of 0·8, i.e. √(initial body mass × final body mass/1000)0·8.
‡ Expressed as g/kg MBW per day-degree. Day-degrees at constant temperature are calculated as temperature (°C) × number of d.
In both species, diet-induced differences in daily protein gains generally associated well with growth, with the exception of the low protein gains at low DP:DE in tilapia (Table 2). Protein and lipid retention efficiencies (Table 2) were expressed per unit digestible nutrient intakes in order to correct for the possible effects of cellulose on nutrient uptakes. Feeding diets with a low v. high DP:DE ratio almost doubled protein utilisation efficiency (protein gain per unit DP intake) in tilapia (from 37 % to over 59 %), independent of the NPE source. Protein retention efficiency in trout fed the low-DP:DE diets was lower than in tilapia and was only improved with fat and not starch as the major NPE source (44 and 34 %, respectively, Table 2).
The amount of lipid deposited per unit protein accretion was generally higher in tilapia than in trout (Table 2). The reduction of the DP:DE ratio (from approximately 27 to approximately 14 mg/kJ) and the addition of fat as the NPE source increased lipid deposition in both species (Table 2). Diet-induced changes in lipid retention efficiency (lipid gain per unit digestible lipid intake), being highly enhanced by the replacement of fat by starch, were comparable between both species at high DP:DE (>200 %, Table 2). In contrast, both species displayed a distinct response when fed the low-DP:DE diets. Here, the replacement of fat by starch enhanced lipid retention in tilapia (150 v. 98 %, with diet LPS v. LPF) but not in trout (67–68 % with both diets LPS and LPF, Table 2).
Table 3 compares the effect of dietary macronutrient composition on plasma levels of free amino acids, glucose and TAG in trout (7 and 40 h) and tilapia (3 and 36 h). Postprandial plasma free amino acid levels reflected DP levels in both species without significant effect of NPE source. Postprandial plasma glucose levels increased in both species following the reduction of DP:DE and the replacement of fat by starch. This effect was more pronounced in trout than in tilapia and was still visible in 40 h feed-deprived trout but not in 36 h feed-deprived tilapia. In trout, postprandial glycaemia showed a significant interaction between DP:DE and NPE, being markedly higher with diet LPS. In tilapia, no interaction was noted between both factors on glycaemia. Diets containing fat as the NPE increased plasma TAG levels in both species, this effect being highly pronounced in tilapia fed the LPF diet, with an interaction between both dietary factors.
Table 3 Levels of plasma metabolites in rainbow trout and Nile tilapia fed diets with different digestible protein-to-energy (DP:DE) ratio and non-protein energy source (NPE, fat v. starch) for 6 weeks (Mean values and standard deviations, n 9)

HPF, high protein with fat as the major NPE; HPS, high protein with starch as the major NPE; LPF, low protein with fat as the major NPE; LPS, low protein with starch as the major NPE; FAA, free amino acids.
a,b,c Mean values within a row with unlike superscript letters were significantly different and showed a significant interaction between the two tested factors (DP:DE v. NPE; P< 0·05 two-way ANOVA).
The activity of the selected key enzymes of amino acid catabolism (Fig. 1), lipogenesis and β-oxidation (Fig. 2), glycolysis and gluconeogenesis (Fig. 3) was analysed in the liver of trout and tilapia, 7 and 3 h after the meal, respectively. Except for the activity of ASAT, which was higher in tilapia than in trout liver, overall activity levels of ALAT and GDH were similar between both species. The activities of ALAT, ASAT and GDH varied according to differences in DP intakes in both species (R>0·70; P< 0·05). In addition, ALAT and GDH activities were reduced in tilapia (Fig. 1(B) and (F)) by the replacement of fat by starch and were negatively correlated with digestible carbohydrate intake (R − 0·80; P< 0·05). No such effect of the source of NPE on hepatic transdeamination activities was detected in trout.
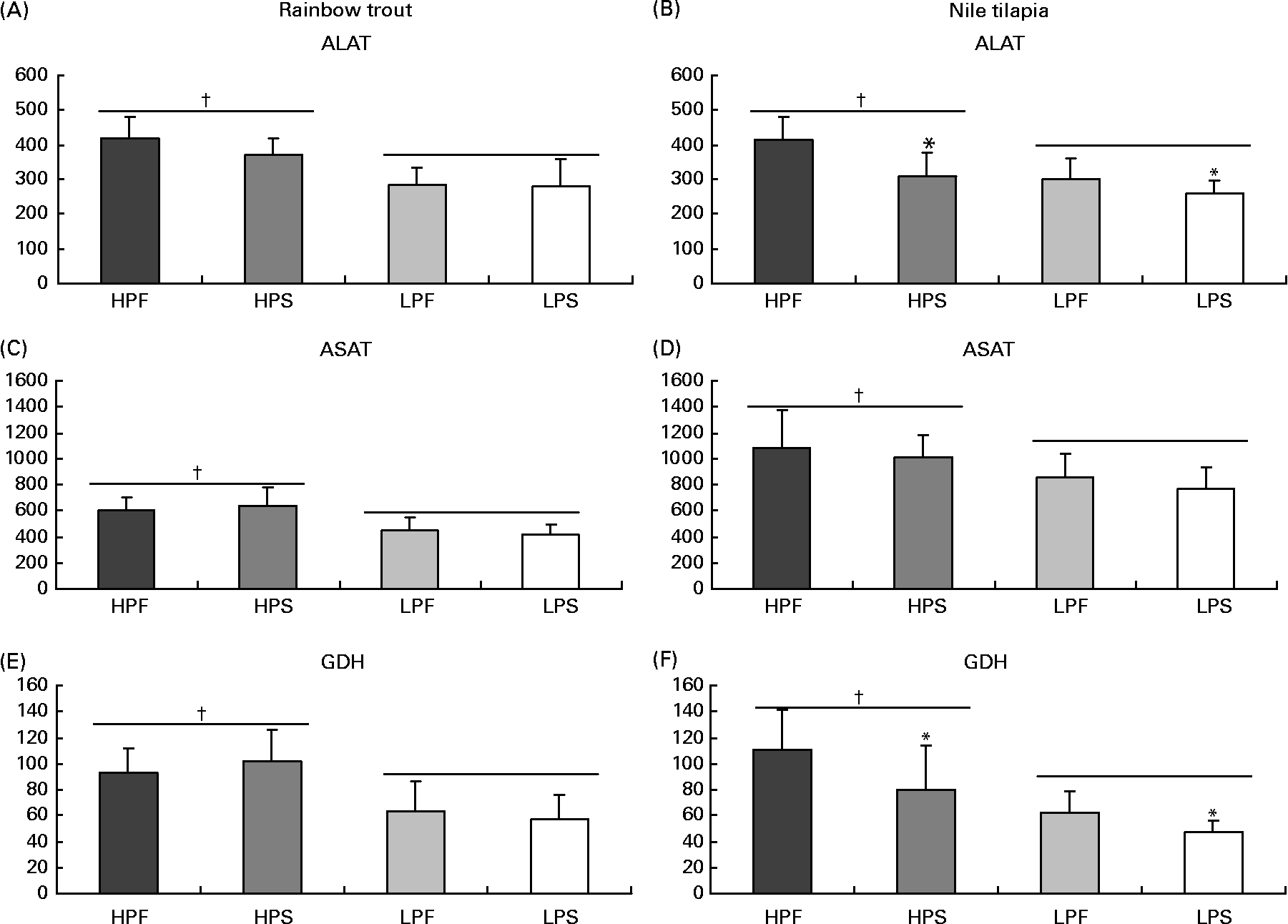
Fig. 1 Specific activities (mIU/mg protein) of two aminotransferases (alanine aminotransferase (ALAT) and aspartate aminotransferase (ASAT)) and of glutamate dehydrogenase (GDH) deaminating enzyme in the liver of rainbow trout (7 h) and tilapia (3 h) fed with different digestible protein-to-energy (DP:DE) ratio and non-protein energy source (NPE, fat v. starch). HPF, high protein with fat as the major NPE; HPS, high protein with starch as the major NPE; LPF, low protein with fat as the major NPE; LPS, low protein with starch as the major NPE. Values are means with standard deviations represented by vertical bars (n 9). Statistical significance for the two independent factors, DP:DE and NPE, and their interaction are as follows: (A) DP:DE, P< 0·01; NPE, P= 0·25; DP:DE × NPE, P= 0·27; (B) DP:DE, P< 0·01; NPE, P< 0·01; DP:DE × NPE, P= 0·13; (C) DP:DE, P< 0·01; NPE, P= 0·95; DP:DE × NPE, P= 0·32; (D) DP:DE, P< 0·01; NPE, P= 0·25; DP:DE × NPE, P= 0·95; (E) DP:DE, P< 0·01; NPE, P= 0·86; DP:DE × NPE, P= 0·30; (F) DP:DE, P< 0·01; NPE, P< 0·05; DP:DE × NPE, P= 0·41. * Starch-rich diets were significantly different (P< 0·05) from fat-rich diets. † High-DP:DE diets differ significantly (P< 0·05) from low-DP:DE diets.
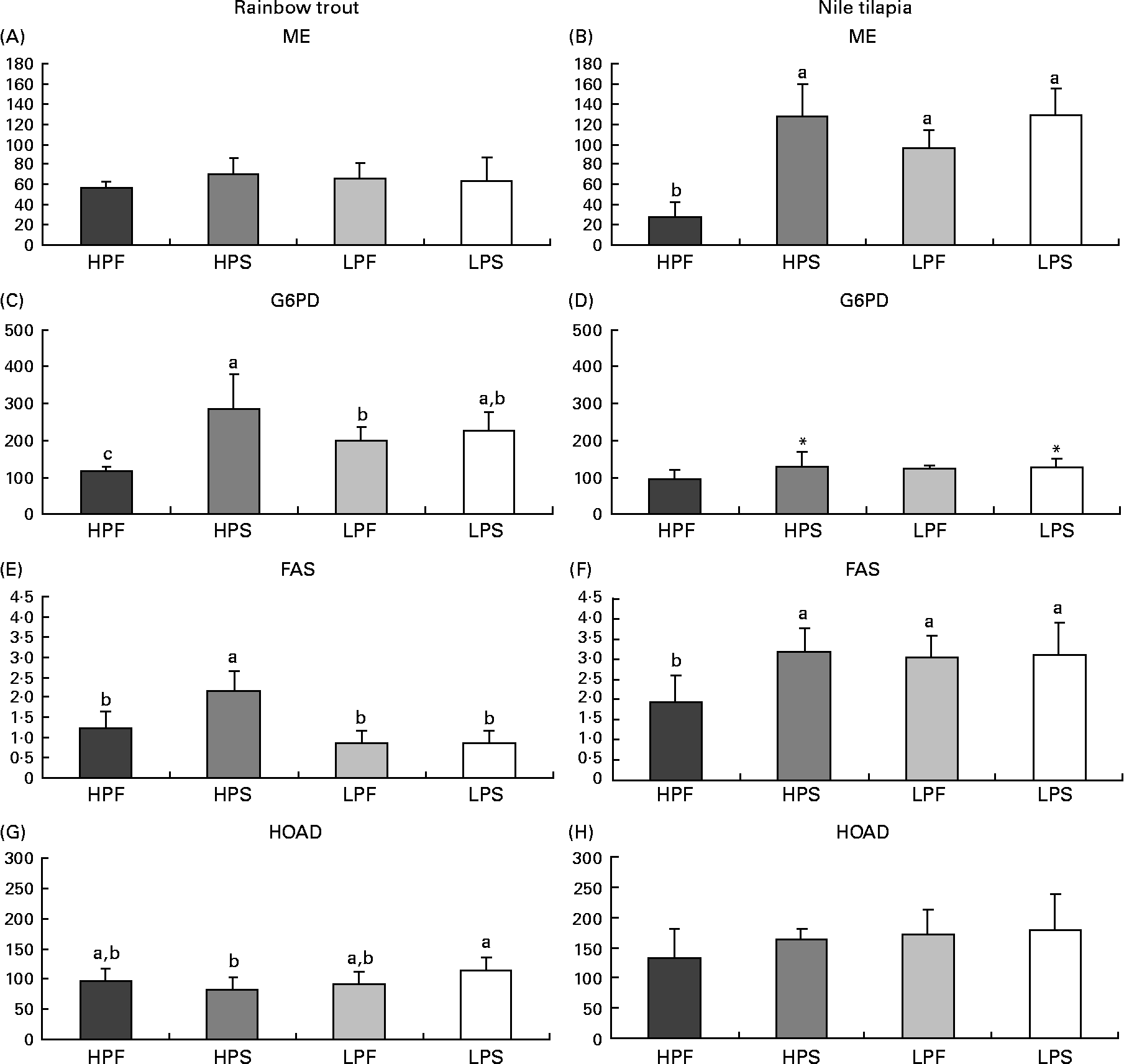
Fig. 2 Specific activities (mIU/mg protein) of lipogenic enzymes (malic enzyme (ME); glucose-6-phosphate dehydrogenase (G6PD) and fatty acid synthase (FAS)) and of 3-hydroxyacyl-CoA dehydrogenase (HOAD) in the liver of rainbow trout (7 h) and tilapia (3 h) fed with different digestible protein-to-energy (DP:DE) ratio and non-protein energy source (NPE, fat v. starch). HPF, high protein with fat as the major NPE; HPS, high protein with starch as the major NPE; LPF, low protein with fat as the major NPE; LPS, low protein with starch as the major NPE. Values are means with standard deviations represented by vertical bars (n 9). Statistical significance for the two independent factors, DP:DE and NPE, and their interaction are as follows: (A) DP:DE, P= 0·84; NPE, P= 0·28; DP:DE × NPE, P= 0·21; (B) DP:DE, P< 0·01; NPE, P< 0·01; DP:DE × NPE, P< 0·01; (C) DP:DE, P= 0·50; NPE, P< 0·01; DP:DE × NPE, P< 0·01; (D) DP:DE, P= 0·22; NPE, P< 0·05; DP:DE × NPE, P= 0·16; (E) DP:DE, P< 0·01; NPE, P< 0·01; DP:DE × NPE, P< 0·01; (F) DP:DE, P< 0·05; NPE, P< 0·05 DP:DE × NPE, P< 0·05; (G) DP:DE, P= 0·09; NPE, P= 0·59; DP:DE × NPE, P< 0·05; (H) DP:DE, P= 0·08; NPE, P= 0·22; DP:DE × NPE, P= 0·38. * Starch-rich diets were significantly different (P< 0·05) from fat-rich diets. In case of a significant interaction (DP:DE × NPE, P< 0·05), mean values lacking a common letter differ significantly.
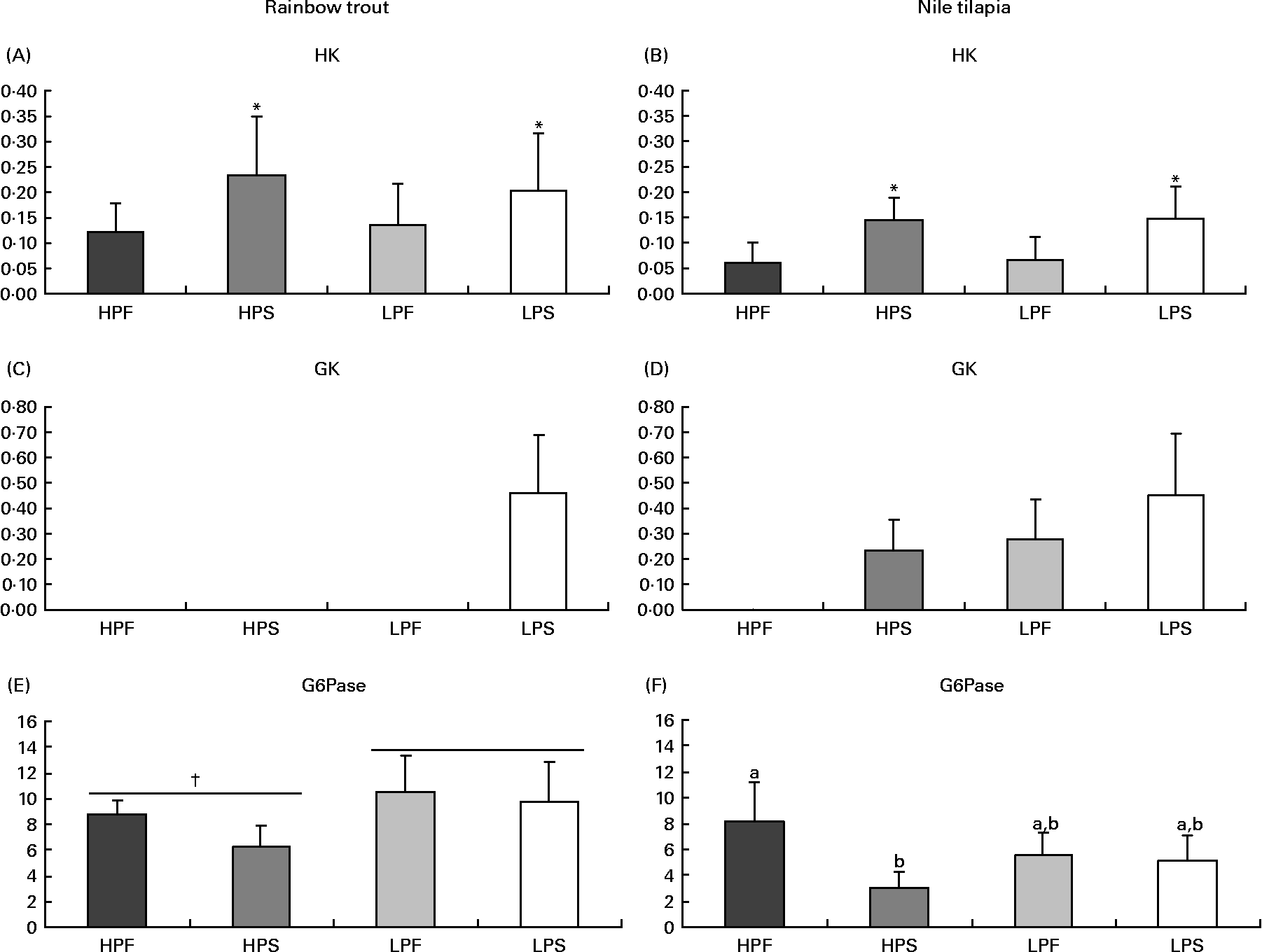
Fig. 3 Specific activities (mIU/mg protein) of glycolytic (hexokinase (HK) and glucokinase (GK)) and gluconeogenic (glucose-6-phosphatase (G6Pase)) enzymes in the liver of rainbow trout (7 h) and tilapia (3 h) fed with different digestible protein-to-energy (DP:DE) ratio and non-protein energy source (NPE, fat v. starch). HPF, high protein with fat as the major NPE; HPS, high protein with starch as the major NPE; LPF, low protein with fat as the major NPE; LPS, low protein with starch as the major NPE. Values are means with standard deviations represented by vertical bars (n 9). Statistical significance for the two independent factors, DP:DE and NPE, and their interaction are as follows: (A) DP:DE, P= 0·80; NPE, P< 0·05; DP:DE × NPE, P= 0·53; (B) DP:DE, P= 0·86; NPE, P< 0·01; DP:DE × NPE, P= 0·92; (C) no statistical test; (D) no statistical test; (E) DP:DE, P< 0·01; NPE, P= 0·05; DP:DE × NPE, P= 0·25; (F) DP:DE, P= 0·73; NPE, P< 0·01; DP:DE × NPE, P< 0·01. * Starch-rich diets were significantly different (P< 0·05) from fat-rich diets. † High DP:DE diets differ significantly (P< 0·05) from low-DP:DE diets. In case of a significant interaction (DP:DE × NPE, P< 0·05), mean values lacking a common letter differ significantly.
The changes in dietary macronutrient composition differently affected hepatic lipogenic enzyme (FAS, ME, G6PD) activities (Fig. 2). In the liver of both species, a significant interaction between DP:DE ratio and NPE source was noted on FAS activities. In trout, FAS activity was higher with diet HPS than with diet HPF and the low-DP:DE diets (Fig. 3(E)). Tilapia also displayed high FAS (Fig. 2(F)) and ME (Fig. 2(B)) activities with diet HPS, which in this species were not different from those found in fish fed the low-DP:DE diets. ME activities were not affected by diet composition in trout (Fig. 2(A)), whereas those in tilapia (Fig. 2(B)) paralleled FAS activities (Fig. 2(F)). The response of G6PD to changes in macronutrient composition was also species-specific and its activities overall lower in tilapia than in trout (Fig. 2(C) and (D)). The replacement of fat by starch increased G6PD in tilapia (Fig. 2(D)), whereas in trout there was a higher G6PD activity only with diet HPS (Fig. 2(C), interaction between DP:DE and NPE). Species-specific differences in the response of the lipogenic enzyme activities to NPE source were further evidenced by the positive correlation between carbohydrate intake and ME, G6PD and FAS in tilapia (R>0·75; P< 0·05). This correlation was only seen in trout for G6PD (R 0·76; P< 0·05) and not for ME (R − 0·16; P>0·05) or FAS (R 0·20; P>0·05). Interestingly, FAS activities were positively correlated with DP intakes in trout (R 0·66; P< 0·05), but not in tilapia (R − 0·54; P>0·05) and negatively correlated with digestible lipid intakes in trout (R − 0·77; P< 0·05) and not in tilapia (R − 0·15, P>0·05). Finally, HOAD activity, a marker for fatty acid oxidation, was higher in trout (Fig. 2(G)) fed LPS compared to HPS diet, whereas no nutritional regulation of HOAD was seen in tilapia (Fig. 2(H)).
Regarding glucose phosphorylating enzymes (Fig. 3), starch-rich diets induced an increase in hepatic HK activity in both species (Fig. 3(A) and (B)). GK activity was only detected in trout (Fig. 3(C)) fed the starch-rich LPS diet and in all tilapia groups, except for those fed the low-starch/high-fat diet HPF (Fig. 3(D)). In tilapia, the hepatic activity of G6Pase, a marker of glucose production, was the lowest with diet HPS (Fig. 3(F)), and did not correlate with the changing macronutrient intakes (P>0·05). In trout (Fig. 3(E)), G6Pase activity was significantly higher when fed low-compared to high-DP:DE diets and not affected by increasing amounts of dietary starch (R − 0·15, P>0·05).
Discussion
Rainbow trout and Nile tilapia have a similar optimal dietary DP:DE ratio of approximately 17–19 mg/kJ(1, Reference Dias, Corraze and Arzel30) despite their different feeding habits (carnivorous v. omnivorous) and thermal preferendum (cold v. warm-water). In order to highlight possible metabolic mechanisms underlying the discrepancy in the response between both species to changes in macronutrient intakes, we kept the dietary DP:DE ratios well above (27 mg/kJ) or below (14 mg/kJ) both species’ optimal DP:DE ratio. Tilapia and trout displayed a number of divergent responses to the dietary changes in DP:DE ratio and NPE source. These were related to growth, nutrient gains, nutrient utilisation efficiency (nutrient gain per unit digestible nutrient intake), plasma metabolite levels and with the activity of key enzymes involved in the intermediary metabolism in the liver of both species. The choice of sampling times (3 and 7 h after the meal, respectively, for tilapia and trout) was based on postprandial peak levels of metabolites in both species.
In both species, the replacement of fat by starch as the NPE source reduced the growth rate, although to a lesser extent in tilapia than in trout. This agrees with the literature that warm-water fish use more efficiently greater amounts of digestible carbohydrates (up to 40 %) than cold-water fish such as rainbow trout(Reference Wilson10, Reference Shiau23, Reference Wang, Liu and Tian24). The high digestible fat level (20 %) in diet LPF did not negatively affect growth or protein utilisation in tilapia differing from reports of reduced growth in Nile tilapia fed dietary fat above 14 %(Reference Ali and Al-Asgah19), but in line with the protein-sparing effect of dietary fat up to 18 % in hybrid tilapia (Oreochromis mossambicus× Oreochromis niloticus)(Reference De Silva, Gunasekera and Shim37). Our data on the response of tilapia to the two DP:DE levels show that tilapia handles well the storage/oxidation interplay of the high NPE load when fed low-protein diets containing approximately 280 g DP/kg. A comparison of the efficiency of protein retention (per unit DP intake) between both species shows that this was improved at low relative to high DP:DE only when fat was used as the major NPE source in trout(Reference Saravanan, Schrama and Figueiredo-Silva31) and independently of NPE source in tilapia(Reference Saravanan, Geurden and Figueiredo-Silva32). This emphasises the importance of considering the type and not only the level of NPE in rainbow trout fed low-protein diets. Protein retention efficiency at high DP:DE was similar in both species and, moreover, independent of the NPE source. That NPE-induced changes in protein sparing are not discernible at protein intakes above requirement has been documented in several fish species, including rainbow trout(Reference Geurden, Gondouin and Rimbach38) and tilapia(Reference Kaushik, Doudet and Médale29).
To our knowledge, this study is the first to undertake a comparative analysis of the enzymatic responses to changes in dietary DP:DE and NPE source in both species fed the same diets. Data on the relationship between diet-induced changes in protein utilisation and those in the hepatic activity level of enzymes involved in amino acid catabolism are contradictory. Previous studies reported that salmonids have little or no adaptation in activity of hepatic amino acid catabolising enzymes to variations in the amount of dietary protein supply(Reference Cowey, Walton and Halver39, Reference Kirchner, Kaushik and Panserat40). In contrast, some other studies have shown a positive relationship between dietary protein intake and activity levels of transdeamination enzymes in tilapia(Reference Gaye-Siessegger, Focken and Becker41) as well as in rainbow trout(Reference Lupianez, Sanchezlozano and Garciarejon42). Except for ASAT, the activity of which was higher in tilapia than in trout, we did not find any difference in ALAT and GDH specific activity levels between the two species. Moreover, reducing the protein supply (low v. high DP:DE) reduced the activity of the transdeamination enzymes in the liver of both species in a similar manner. These findings hence do not allow to explain the lower protein retention efficiency in trout (34–44 %) compared to that in tilapia (58–59 %) at low DP:DE. Further, the response of the studied enzymes to changes in NPE source did not reflect the observed variations in protein retention efficiency. That fat constitutes a more efficient NPE source than starch in trout at low DP:DE (LPF v. LPS) was not reflected in differences in hepatic HOAD activity, a marker of mitochondrial fatty acid β-oxidation. The absence of nutritional regulation of HOAD activities in tilapia favours the idea that the energy provided by the supplementary lipid is preferentially directed towards storage in this species(Reference Chou and Shiau16, Reference Hanley18, Reference Ali and Al-Asgah19) as reflected by the higher body fat deposition per unit protein accretion than in trout. The activity of FAS, used as a marker of lipogenesis, also showed a species-specific response to changes in dietary lipogenic substrates. At low DP:DE, hepatic FAS activity was low in trout and high in tilapia, suggesting a difference in the utilisation of the NPE source between both species at low protein intakes. Besides, the finding that the high FAS activity in tilapia was unaffected by the NPE source indicates either (i) the absence of a retro-inhibitory effect of the high fat content of diet (LPF) on lipogenic enzymes which contrasts with the inhibitory role of dietary fat on lipogenesis in tilapia(Reference Gaye-Siessegger, Focken and Abel43) as in other fish(Reference Dias, Alvarez and Diez3, Reference Gélineau, Corraze and Boujard44, Reference Likimani and Wilson45), or (ii) an enhanced lipogenesis by the very high carbohydrate level (45 %) with diet LPS, in line with findings in other teleosts(Reference Dias, Alvarez and Diez3, Reference Fernández, Miquel and Cordoba4) including rainbow trout(Reference Brauge, Corraze and Médale46), or (iii) a combination of both, as diet LPF was rich in fat and starch. The enhanced lipogenic response of both species following the replacement of fat by starch at high DP:DE (diet HPS v. HPF) does not help to clarify the confounding role of lipid and carbohydrate on lipogenesis. Interestingly, the current data indicate species-specific differences in the dietary regulation of the pentose phosphate pathway (G6PD) and pyruvate-malate cycle (ME) as providers of NADPH reducing equivalents for lipogenesis. ME activity in trout liver was fairly low as already observed(Reference Dias, Corraze and Arzel30, Reference Barroso, García-Salguero and Peragon47, Reference Walzem, Storebakken and Hung48) and unaffected by dietary factors, whereas G6PD activity, highly responsive to changes in macronutrient supply, paralleled that of FAS. In tilapia, the relative importance of G6PD and of ME activity varied considerably with diet composition, the latter being generally higher than that of G6PD. This does not fully comply with the overall higher activity of G6PD to that of ME reported in hybrid tilapia O. niloticus× Oreochromis aureus fed starch-rich diets(Reference Lin and Shiau49), which in our study was only true with diet HPF.
Between-species comparison of plasma glucose in fish fed the low-DP:DE diets confirms the poor control of postprandial glycaemia in rainbow trout after oral administration of glucose or high-carbohydrate meals(Reference Bergot25–Reference Palmer and Ryman27). This is illustrated by the 2- to 3-fold higher plasma glucose in trout (1·9–4·6 g/l) compared to tilapia (0·9–1·1 g/l) when fed diets with a low DP:DE ratio. Supplying different amounts of carbohydrates altered the activities of glucose-phosphorylating enzymes in a similar manner in both species, with GK (HK IV) activity being strongly enhanced by high carbohydrate intakes. A similar response and comparable activity levels in trout and tilapia were also seen for low-Km HK (HK I–III), reported as being little regulated by dietary factors in fish(Reference Enes, Panserat and Kaushik12). These similarities between species in the hepatic response and activity levels of GK and HK to changes in diet composition agree with findings from a previous study comparing glycolytic responses of trout and carp(Reference Panserat, Médale and Blin28). These data comply with the idea that the hepatic glycolytic potential itself does not explain the poor control of postprandial glycaemia in trout fed carbohydrate-rich diets(Reference Panserat, Médale and Blin28, Reference Panserat, Perrin and Kaushik50). The high glycaemia and low FAS activity levels observed here in trout (LPF v. LPS) is in conformity with our own recent data where we observed that hyperglycaemia in trout fed a diet rich in both starch and fat was associated with reduced hepatic lipogenesis(Reference Figueiredo-Silva, Panserat and Kaushik51). Differences in hepatic lipogenesis have also been suggested to explain differences in glucose use among rainbow trout lines divergently selected for muscle fat content(Reference Skiba-Cassy, Lansard and Panserat52). Likewise, the higher lipogenic capacity in tilapia compared to that in trout at low protein intakes suggests that, at similar hepatic glycolytic rates, the directing of carbohydrates towards lipogenesis in tilapia favoured the control of glycaemia in this species. At the same time, tilapia fed the low v. high DP:DE diets had reduced G6Pase activity, reflecting reduced gluconeogenesis. Persistent gluconeogenesis in trout fed starch-rich diets, reported to exacerbate hyperglycemia, has been attributed to the relatively high amount of protein in trout diets(Reference Panserat, Capilla and Gutierrez34, Reference Panserat, Médale and Breque35), providing gluconeogenic (amino acids) substrates and/or promoting insulin resistance abolishing G6Pase inhibition as with mammalian type 2 diabetes(Reference Tremblay, Lavigne and Jacques53). However, our data of increased G6Pase activity in trout at low instead of high DP:DE do not support this hypothesis, requiring further consideration. Taken together, the hyperglycaemia in trout fed low-protein diets, as observed here and in some previous studies(Reference Kirchner, Kaushik and Panserat40, Reference Figueiredo-Silva, Panserat and Kaushik51), probably results from the inability of rainbow trout to direct glucose towards lipogenesis and to reduce gluconeogenesis which probably relates with the low growth and low protein accretion in this species at suboptimal DP:DE.
In summary, the feeding of diets with varying DP:DE ratio and NPE source to both trout and tilapia shows species-specific differences in dietary macronutrient use, particularly evident at low DP:DE. The finding that only fat and not digestible starch improved protein utilisation efficiency at low DP:DE in trout further underlines the greater importance of the NPE source in this species than in tilapia. This study also reveals species-specific responses of enzymes involved in hepatic intermediary metabolism. The high lipogenic and reduced gluconeogenic capacity seem to contribute to the better use of carbohydrates and to the improved glycaemia control in tilapia compared with rainbow trout at low dietary DP:DE ratios.
Acknowledgements
The present study was funded by institutional INRA funds. A. C. F.-S. acknowledges the post-doctoral INRA fellowship (MRI, Package INRA-WUR Aquaculture Platform, Crédits CDD 9C). There are no conflicts of interest. The authors thank F. Terrier, F. Sandres and Y. Hontang (INRA Donzacq experimental fish farm), P. Aguirre and Y. Mercier (INRA St Pée sur Nivelle experimental rearing unit), E. Eding and M. ter Veld (Wageningen University, The Netherlands) for their technical assistance. The authors also thank A. Surget for the analyses of plasma total free amino acids. I. G., S. K., J. W. S., S. S. and A. C. F.-S. designed the research; S. S. conducted tilapia feeding trial and analysed growth and whole-body composition data; A. C. F.-S. performed the biochemical analyses, interpreted the results and wrote the manuscript together with I. G.; S. K., J. W. S. and S. P. contributed to the interpretation and presentation of the experimental data. All authors contributed to and approved the manuscript.








