Gangliosides are negatively charged glycosphingolipids that consist of a hydrophobic ceramide and a hydrophilic oligosaccharide chain bearing one or more sialic acid residues in addition to a number of sugars, namely glucose, galactose, N-acetylglucosamine and N-acetylgalactosamineReference Wiegandt, Agranof and Aprison1. Ceramide is an N-acylsphingosine in which the acyl residue is usually a saturated fatty acid with a chain length of more than 14 carbons. Svennerholm developed the classical and most frequently used nomenclature for gangliosides, which is based on two letters and one subscript numberReference Svennerholm2. The first letter indicates the “series”, which is different depending on the carbohydrate core and metabolic pathway (for example, G = ganglio series). The second letter indicates the number of sialic acid residues (M, D, T, Q, P, H or S, corresponding to one, two, three, four, five, or exceptionally, six or seven residues). Finally, the subscript corresponds to five minus the number of neutral monosaccharides residues present in the molecule. Attempts to develop more systematic approaches to naming gangliosides, such as that established by the Commission of the International Union of BiochemistryReference Wiegandt, Agranof and Aprison1 have not gained popularity, because of their complexity. From the molecular standpoint, a ganglioside presents one aspect that is exposed to the external face of a cell or a membrane, and a lipid soluble hydrophobic tail. The exposed carbohydrate moiety is normally the portion that functions as receptor, antigen and/or ligand in biological functions. Fig. 1 shows a diagram of the biosynthesis pathway of gangliosides.

Fig. 1 Biosynthetic pathways for gangliosides. (a) Biosynthesis of lactosylceramide. (b) Biosynthesis of gangliosides from lactosylceramide. Taken from Ref. 20.
Although gangliosides were initially isolated from brain and are especially abundant in neural tissues, they are widely distributed in most vertebrate tissues and fluids. Gangliosides are usually isolated from these sources by total lipid extraction and solvent partition. Total gangliosides are usually quantified as lipid bound sialic acid (LBSA) by colorimetric determination. To know the actual concentration of gangliosides in these tissues or fluids, it is necessary either to know the distribution of individual gangliosides and their molecular weight to get it from the LBSA content, or to use alternative techniques such as high performance liquid chromatography or mass spectroscopy and nuclear magnetic resonance combined with proper standards.
From a nutritional point of view the presence of gangliosides in milk, as well as the changes in content and individual profile that occur during lactation, could have special relevance.
Milk gangliosides
Milk gangliosides are almost exclusively associated with the membrane fraction of the fat globule, which is derived mainly from the apical plasma membrane of the apocrine secretory cells in the lactating mammary glandReference Keenan3. Milk gangliosides were initially studied in bovine milkReference Bushway and Keenan4, GD3 being the major ganglioside and GM3 the next most abundant. Other gangliosides amounted to no more than 20 % of the total ganglioside content. Results from several studies carried out on milk from cows, goats and ewes have recently been reported describing changes between species and during lactation, as well as seasonal variations in the concentration of gangliosidesReference Puente, García-Pardo and Hueso5–Reference Puente, García-Pardo, Rueda, Gil and Hueso8. Changes in ceramide moiety in bovine milk gangliosides with stage of lactation has also been observedReference Martín, Martín-Sosa and Hueso9.
The content and distribution of gangliosides in human milk have been reported by several authorsReference Laegreid, Otnaess and Fuglesang10–Reference Pan and Izumi14. The last three studies investigated samples from different periods of lactation, and, in addition, observed a selective change in the relative concentrations of GM3 and GD3 between colostrum (days 1-5) and mature milk. The most abundant ganglioside in human milk at the beginning of lactation was GD3, while at the end of this period GM3 was the major ganglioside. A major finding in the study from our groupReference Rueda, Puente, Hueso, Maldonado and Gil13 was the detection of previously unreported highly polar gangliosides in human milk. Because of their high polarity they might be polysialogangliosides or complex gangliosides with branched oligosaccharide chains. In a different study, four gangliosides, in addition to GD3 and GM3, have been detected and assumed to tentatively be gangliosides of the c-seriesReference Pan and Izumi14.
Differences in the relative concentration of individual gangliosides in human milk from mothers delivering preterm and term infants have been reportedReference Rueda, García-Salmerón, Maldonado and Gil15. In addition, changes in fatty acid composition of human milk gangliosides through lactation have been describedReference Martin-Sosa, Martin, Castro, Cabezas and Hueso16 and differences in fatty acid composition between bovine and human milk gangliosides have been detailedReference Bode, Beermann, Mank, Kohn and Boehm17. These studies are of interest because the lipid component of gangliosides is frequently neglected in structural characterisation studies.
Identifying specific gangliosides in complex mixtures is laborious, and requires well established validated assays or well characterised reagents, such as monoclonal antibodies or lectins. Usually, ganglioside content is estimated by means of lipid bound sialic acid (LBSA) as a surrogate parameter. For this reason it is relevant to use studies that actually measure individual ganglioside profiles, in addition to LBSA measurements, when estimating the actual concentration of gangliosides in a particular source, fluid or tissueReference Takamizawa, Iwamori, Mutai and Nagai12–Reference Pan and Izumi14. From these studies, if the percentage of each individual ganglioside, as well as its molecular weight, are known, it is possible to calculate the actual concentration of GD3 plus GM3, the two main individual gangliosides, in human milk. Table 1 shows the average content of total gangliosides and of GD3 plus GM3, expressed as LBSA and actual concentration, for different periods of lactation, and for each one of the studies referred to above. The average value of GD3 plus GM3 in human milk, weighted according to the number of samples, would be of 13·2 mg/L considering colostrum, transitional and mature human milk, or 11·1 mg/L, when only mature human milk is consideredReference Takamizawa, Iwamori, Mutai and Nagai12–Reference Pan and Izumi14.
Table 1 Average content of total gangliosides and of GM3 and GD3, expressed as mg LBSA/l and as mg gangliosides/l, at different stages of lactation

Molecular weights: Sialic acid = 309; Milk GM3 = 1198·5; Milk GD3 = 1577.
Colostrum: 1–5 days; Transitional milk: 6–17 days; Mature milk: more than 18 days.
Several authors have also studied the composition of gangliosides and other sialoglycoconjugates in infant formulasReference Sánchez-Díaz, Ruano, Lorente and Hueso18, Reference Pan and Izumi19. These studies have confirmed that both the pattern and content of gangliosides in human milk and infant formulas differed markedly - GD3 is the main species detected in infant formulas and their total ganglioside content is significantly lower than that of human milk.
The role of dietary gangliosides on immunity and the prevention of infection
The role of gangliosides in human milk continues to be a subject of research and discussion in the fields of paediatric nutrition and glycobiology. As described above, the distribution profile of milk gangliosides selectively changes during lactation, suggesting that gangliosides may participate in physiological processes that take place in the early development of infantsReference Rueda, Gil, Huang and Sinclair20, Reference Rueda, Maldonado, Narbona and Gil21.
Most of the functions in which gangliosides are implicated involve cell-cell recognition during normal differentiation or tissue targeting. It is also well known that gangliosides can be “kidnapped” by pathogenic agents and toxins that use them as unintended receptors, and that this is the main mechanism by which gangliosides can prevent infection. Human-milk gangliosides have been involved in the inhibition of Escherichia coli and Vibrio cholerae enterotoxinsReference Otnaess, Laegreid and Ertresrag22. This inhibitory action was later attributed to the monosialoganglioside GM1, which has been identified as the unintended mammalian cell surface receptor for these enterotoxinsReference Laegreid and Otnaess23. More recently, the free milk oligosaccharide sialyllactose, which is the oligosaccharide present in GM1, has been identified as an inhibitor of cholera toxin adhesion to target tissuesReference Idota, Kawakami, Murakami and Sugawara24. GM1 is found in human milk in very low concentrations, and immunological methods are necessary to detect it on high performance thin layer chromatography platesReference Laegreid and Otnaess23.
Several studiesReference Teneberg, Willemsen, de Graaf and Karlsson25–Reference Idota and Kawakami27support the notion that cell surface gangliosides function as “unintended” target receptors for bacterial adhesion in specific tissues; dietary gangliosides are putative decoys that interfere with pathogenic binding. Such compounds could modify the intestinal microflora in the neonate and reduce the infectious capacity of pathogenic bacteria. Other findings also describe that sialylated compounds have growth-promoting effects on bifidobacteria and lactobacilliReference Idota, Kawakami and Nakajima28, Reference Nakano, Sugawara and Kawakami29.
Only one clinical study has been published to date testing the role of dietary gangliosides in humansReference Rueda, Sabatel, Maldonado, Molina and Gil30. In this study, the performance of a formula containing gangliosides was compared to that of a control formula. To assess their impact on faecal flora, gangliosides were fed to pre-term infants, at a concentration of 1·43 mg/100 kcal, which was close to that detected in human milk by the same authorsReference Rueda, Puente, Hueso, Maldonado and Gil13. The molecular identity of the gangliosides to those of human milk was not the issue since, as discussed above, the most important consideration of glycoconjugates as pathogenic adhesion inhibitors is poly-valence. However, we sought to replicate human milk in terms of total ganglioside content as opposed to mimicking the average of its molecular composition and its effect on intestinal microflora. The particular impact of gangliosides on E. coli and on Bifidobacteria is shown in Fig. 2. The faecal E. coli counts in preterm infants fed the ganglioside-supplemented formula was lower than that observed in infants fed the standard formula for the first month of life. Furthermore, dietary gangliosides were also able to increase Bifidobacteria counts in faeces, which supports a prebiotic role for these glycolipids. It is difficult to pinpoint the structural features of gangliosides responsible for specific biological effects; however, it is important to keep in mind that their carbohydrate portions either resemble or are identical to those of free oligosaccharides with proven prebiotic capabilities.
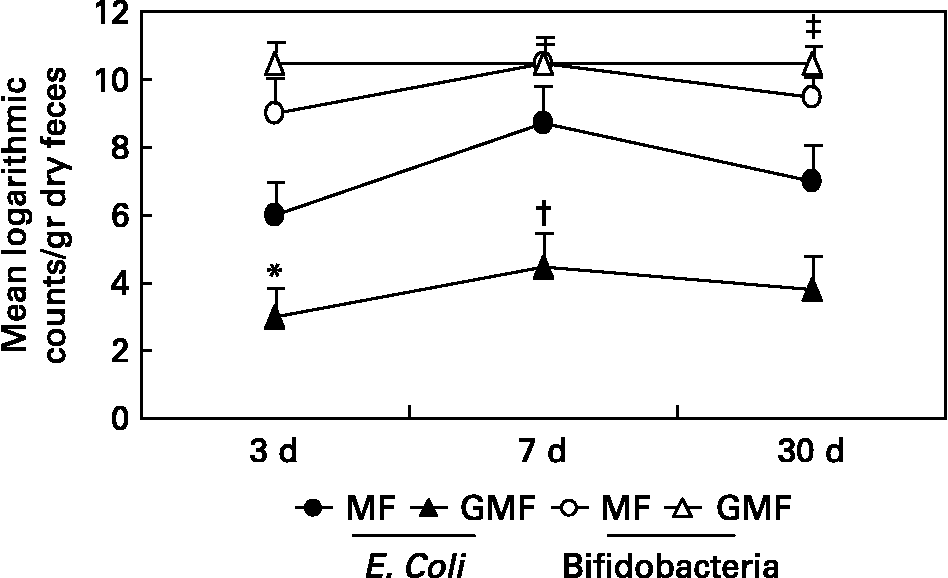
Fig. 2 Logarithmic microbial counts in dry faeces of preterm newborn infants fed on milk formula (MF) and milk formula supplemented with gangliosides, at 1·43 mg/100 kcal (GMF). Results are means ± SEM. Twenty samples were analised for each feeding group. Samples were collected at 3, 7 and 30 days of life. Kruskal-Wallis and Friedman nonparametric tests were used to determine the effects of diet and postnatal age as sources of variation. *P < 0·01(versus MF group); †P < 0·001 (versus MF group); ‡P < 0·05. Data are taken from Ref. 30.
Another notable aspect is the ability of gangliosides to modulate the development or behaviour of cells of the immune system. Since some studies suggest that gangliosides could be involved in the activation of T cellsReference Yuasa, Scheinberg and Houghton31 and in the differentiation of different lymphocyte subpopulationsReference Ebel, Scmitt, Peter-Katalinic, Kniep and Mühlradt32, Reference Taga, Tetaud, Mangeney, Tursz and Wiels33, human milk gangliosides or gangliosides added to infant formulas might substantially contribute to the processes of proliferation, activation and differentiation of immune cells, especially of those from the intestine, in the neonate. Our group reported some findings related to the effect of dietary gangliosides on intestinal immunity in mice at weaning. Animals fed with gangliosides showed an earlier development of cytokine-secreting cells, and a higher number of Th1 and Th2 cytokine-secreting lymphocytes in lamina propria and Peyer's patch lymphocytes after 4 weeks of feedingReference Vázquez, Gil and Rueda34. Another important finding was that dietary gangliosides increase the number of intestinal IgA-secreting cellsReference Vázquez, Gil, García-Olivares and Rueda35 and the luminal content of secretory IgA in weanling miceReference Vázquez, Gil and Rueda36. According to these results, dietary gangliosides positively modulate the production and secretion of IgA at intestinal level, which constitutes the main mechanism of defense against microorganisms entering through the gastrointestinal tract. Fig. 3 shows the effect of dietary gangliosides on the luminal content of intestinal IgA in mice at weaning. Ganglioside influences on in vitro intestinal lymphocyte proliferation were also reportedReference Vázquez, Gil and Rueda37. Gangliosides elicited differential effects on intestinal lymphocyte proliferation depending on the presence and concentration of specific structures, suggesting that dietary gangliosides may influence the development of intestinal immunity by stimulating or inhibiting proliferative or inhibitory responses in intestinal lymphocytes during early infancy.
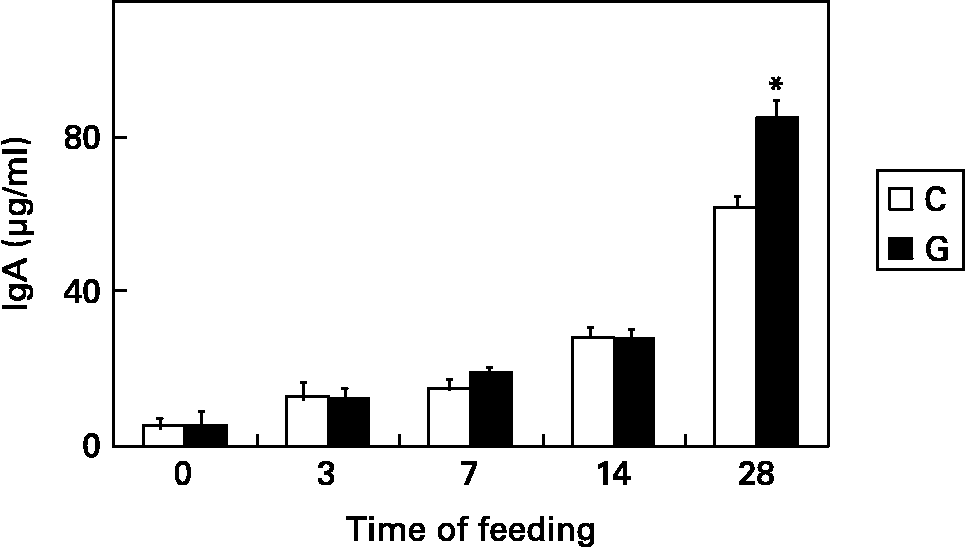
Fig. 3 Concentration of IgA, normalised by intestine length and mouse weight, at the intestinal lumen of mice fed on a control diet (C) or the same diet supplemented with gangliosides, at 47 mg/kg (G). Samples were collected at 3, 7, 14 and 28 days of feeding. Results are means ± SEM. Two-way ANOVA and Bonferroni test were used to determine the effects of diet and time of feeding as sources of variation *P < 0·05. Data are taken from Ref. 36.
On the other hand, a recent publication describes the role of milk gangliosides, especially GD3 at early lactation (that is colostrum and transitional milk), in modulating immunityReference Bronnum, Seested, Hellgren, Brix and Frokiaer38. This publication describes how GM3 and GD3 differentially inhibit dendritic cell maturation and effector functionalities, reflecting the same compositional changes found in human milk. It is important to keep in mind that developing and modulating immunity includes not only promoting the defense against external aggression (for example infection), but also the promotion of adequate tolerance against non-aggressive antigens, which is also equally important in early infancy, and dendritic cells have a prominent role in the regulation of this process. In summary, this publication concludes that because GD3 inhibits dendritic cell functionalities overall more than GM3, we may conclude that the immune modulating effect of the ganglioside fraction of breast milk might be more prominent in the commencement of lactation during which the milk contains the most GD3.
A study has also suggested a role of gangliosides in the prevention of infection by parasitesReference Suh, Belosevic and Clandinin39. Results from this study showed that dietary gangliosides had a protective effect against Giardia muris infection in vivo and affected the survival of Giardia lamblia trophozoites in vitro. Several recent reports from the same group describing the effects of dietary gangliosides on neonatal rats suggest yet another role for gangliosides. One of these reportsReference Park, Suh, Ramanujam, Steiner, Begg and Clandinin40 shows that dietary gangliosides are absorbed in the small intestine and transported to different sites, altering ganglioside levels in the intestinal mucosa, plasma and brain, and thus possibly having the potential to change developing enterocyte function. Another reportReference Birecki, Drozdowski, Suh, Park, Clandinin and Thomson41 describes that dietary gangliosides enhance in vitro lipid uptake in weanling rats, probably by a modification in the physical properties of the brush border membrane, and dietary gangliosides have been noted to increase the content and composition of phospholipids containing PUFA in the weanling rat intestineReference Park, Suh, Thomson, Ramanujam and Clandinin42. These recent reports suggest that dietary gangliosides might have an effect on developing enterocyte function, but it is not known if they are also related to modulation of intestinal immunity and prevention of infection.
In conclusion, dietary gangliosides may have an important role during early infancy in modifying intestinal microflora and promoting the development of intestinal immunity and oral tolerance in the neonate, and as a consequence preventing infections during early infancy. However, further studies are required to clarify the mechanisms involved in these actions and the relevance of this finding to clinical outcomes in neonates.
Conflict of interest statement
RR is an employee from Abbott Laboratories. Part of the work described in this article has been funded by this company.






