INTRODUCTION
The fin whale (Balaenoptera physalus) is a marine mammal belonging to the suborder of baleen whales (Cetacea, Mysticeti). It is the second-largest animal on earth after the blue whale (Balaenoptera musculus). Although fin whales are found in all major oceans and a number of seas, from polar to tropical waters, it is rare in the southern North Sea, and in most cases solitary animals are seen (Camphuysen and Peet, Reference Camphuysen and Peet2006). It feeds by filtering a large volume of seawater, enabling to harvest large zooplankton mainly Northern krill (Meganyctiphanes norvegica) but also small schooling fish such as herring (Clupea harengus), squid and crustaceans including copepods (Lambertsen, Reference Lambertsen1986; Ruys and Soulier, Reference Ruys and Soulier2013). Cetaceans serve as hosts to numerous parasites, including many nematode species such as crassicaudids (Nematoda, Spirurida) (Lambertsen, Reference Lambertsen1986). Crassicauda spp. occur under the skin or in deep tissues of the mammary glands, in cranial sinuses and the urogenital system (Geraci and St. Aubin, Reference Geraci and St. Aubin1987; Lambertsen, Reference Lambertsen1986; Zucca et al. Reference Zucca, Di Guardo, Pozzi-Mucelli, Scaravelli and Francese2004; Jabbar et al. Reference Jabbar, Beveridge and Bryant2015). Three nominal species of adult Crassicauda have been described in the urogenital system of the fin whale, namely C. crassicauda, C. boopis and C. pacifica (Lambertsen, Reference Lambertsen1985, Reference Lambertsen1986). The former is the smallest of the three, and occupies the lower urinary tract (urethra), whereas the latter two infect the kidneys and are considered as taxonomically synonymous as morphologically indistinguishable (Lambertsen, Reference Lambertsen1985).
Crassicauda boopis (Tetrameridae, Baylis, 1920) is a round worm attaining a length of approximately 1·5–2 m. The parasite shows a large anterior part (5 mm wide), which is often observed protruding in the blood flow and an attenuated posterior portion (1 mm wide) traversing the ureteric epithelium and lying free within renal ducts. Larvated eggs are observed and larvae can be found in urine of infected animals (Rees, Reference Rees1953; Lambertsen, Reference Lambertsen1985). Of several known crassicaudid infections, those caused by C. boopis are highly pathogenic. Infections with adult C. boopis can be associated with a marked thrombophlebitis of the vascular system draining the kidneys. The tissue reaction can cause multiple obstructions of the primary renal veins. The osmotic stress experienced by the whale in its naturally saline environment is then aggravated by a pathologic stress to the kidneys. Consequently, severe infections with C. boopis could reduce blood flow from the kidneys to such a level that the whale succumbs to congestive renal failure. Moreover, the finding of intravascular parasitic masses and associated thrombi supports the possibility of a thromboembolism in crassicaudiosis. This is especially true in case of pulmonary lesions with parasite fragments and mineralized lesions (Lambertsen, Reference Lambertsen1992).
The life cycle of C. boopis is not entirely known yet. It is largely hypothetical based on the pattern of lesions observed, and the frequent report of heteroxenous transmission for marine Spirurida (Lambertsen, Reference Lambertsen1986; Rhode, Reference Rhode2005). This suggests a somatic migration of C. boopis larvae from the gut after their ingestion by the whale. The primary infective route is assumed to involve penetration of the wall of the gastrointestinal tract by C. boopis larvae that are ingested by the whale and migration of these larvae up the mesenteric arteries and the aorta (Lambertsen, Reference Lambertsen1986; Díaz-Delgado et al. Reference Díaz-Delgado, Fernández, Xuriach, Sierra, Bernaldo de Quirós, Mompeo, Pérez, Andrada, Marigo, Catão-Dias, Groch, Edwards and Arbelo2016). Entry of C. boopis into the kidneys, its definitive habitat, could well involve larval movements via two different routes: from the aorta directly within the wall of the arterial system or by the arterial blood flow. Thereafter, the developing larvae would have easy access to both the urinary ductwork and the renal veins. Finally, by further developing in the venous system of the kidney, the adult stage could then extend from the urinary ducts to the caval vein. Persistent larvae migrans are suggested by lesions induced by developing C. boopis larvae that are not carried by the arterial bloodstream to the kidneys but rather lodge in the capillaries of other tissues (Lambertsen, Reference Lambertsen1992).
The finding of larvae and larvated eggs in the urine suggest that the primary portal of exit is the urinary tract. The presence of eggs in the pulmonary airspace is also compatible with low levels of shedding via the whale's blow (Lambertsen, Reference Lambertsen1986).
Krill, such as M. norvegica, which is the principal prey of fin whales in the northern Atlantic Ocean, could potentially play a role as an intermediate host (Lambertsen, Reference Lambertsen1985; Sigurjonsson and Vikingsson, Reference Sigurjonsson and Vikingsson1995; William et al. Reference William, Bernd and Thewissen2009).
The finding of young calves infected with C. boopis suggests either a transplacental transmission or the urinary contamination of the environment followed by an oral infection of the calf before being able to feed by itself. Finally, galactogenic transmission cannot entirely be disregarded (Díaz-Delgado et al. Reference Díaz-Delgado, Fernández, Xuriach, Sierra, Bernaldo de Quirós, Mompeo, Pérez, Andrada, Marigo, Catão-Dias, Groch, Edwards and Arbelo2016).
This paper aims at describing a rarely reported case of C. boopis in fin whale and its molecular screening for parasite identification improvement.
MATERIALS AND METHODS
On 9 November 2015, a fin whale was observed on the bulb of a vessel in the Channel Terneuzen – Ghent (The Netherlands – Belgium). According to the captain of the vessel, the whale had not been present on the bulb 2 days prior to the observation (Jan Haelters, personal communication). The ship (204 m long, cruising speed of 18–20 kts) had sailed from Porto de Santos (Brazil) to Ghent in 12 days. The fin whale was removed from the bulb in the port of Ghent and transported to shore for necropsy (Fig. 1) following a standardized procedure (Jauniaux et al. Reference Jauniaux, Garcia Hartmann, Haelters, Tavernier and Coignoul2002).

Fig. 1. Fin whale (Balaenoptera physalus) on the necropsy site.
Parasites from veins and kidneys were preserved in 80% alcohol for morphological and molecular identification. Cephalic and genital regions of the parasite were microscopically investigated after clearing in lactophenol. Renal tissue was preserved in 10% buffered formaline and embedded in paraffin wax. Sections were stained with haematoxylin and eosin for histopathological examination.
Molecular identification
Genomic DNA was isolated from 10 worms using DNeasy Blood & tissue kit (Qiagen, Germany) following the manufacturer's recommendations. The 18S rRNA gene was amplified from genomic DNA by PCR using G18S4-F (5′-GCTTGTCTCAAAGATTAAGCC-3′) and reverse 136-R (5′ TGATCCTTCTGCAGGTTCACCTAC-3′) primers (Nadler et al. Reference Nadler, Carreno, Mejía-Madrid, Ullberg, Pagan, Houston and Hugot2007; Jabbar et al. Reference Jabbar, Beveridge and Bryant2015).
PCRs were conducted in 25 µL volumes containing Taq polymerase master mix (Qiagen, Germany). PCR cycling conditions were 94 °C for 5 min, then 35 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 80 s, followed by 72 °C for 7 min. Amplicons were observed on 2% agarose gel. A PCR amplifying internal portion of the 18S rRNA gene was designed using primers Crassic1160R (5′-CAGGTGAGTTTTCCCGTGTT-3′) and reverse Crassic352F (5′-AAACGGCTACCACATCCAAG-3′) in order to facilitate the sequencing. Examination of PCR amplicons was done on a 2% agarose gel. PCR products were subsequently purified using MinElute PCR purification kit (Qiagen, Germany) and subjected to automated DNA sequencing in both directions (Giga genomic platform, University of Liège). Sequences were aligned using Clustal Omega (http://www.ebi.ac.uk) and a consensus was submitted to Blastn (http://blast.ncbi.nlm.nih.gov).
RESULTS
Clinical presentation and post-mortem findings
The fin whale was a juvenile male of 11·60 m length showing a slight emaciation with a blubber thickness of 50 mm (dorsal), 35 mm (median) and 35 mm (ventral) (Fig. 1). The eyes were clear, with a transparent lens and the presence of gas bubbles, which suggested, together with observation reported by the captain, that the time of the death was about 2–3 days ago. External observations of the body showed an impact zone with cutaneous lacerations of about 1·65 m wide. When the abdominal cavity was opened, an associated free haemorrhagic and fibrinous liquid was present. An intestinal haematoma of 40 cm in length was observed at the impact zone. A thrombus was observed in the right lung and parasite fragments were present in the pulmonary vessels (Fig. 2). A severe parasitosis was present in the lumens of the right heart ventricle and the caudal caval vein (Fig 2). These parasites (approximately 50) were white to reddish round worms of approximately 1 m long and 5 mm in diameter. A renal thrombosis was also observed, with the presence of round worms of 20 cm in length and 1 mm in diameter (Fig. 2).
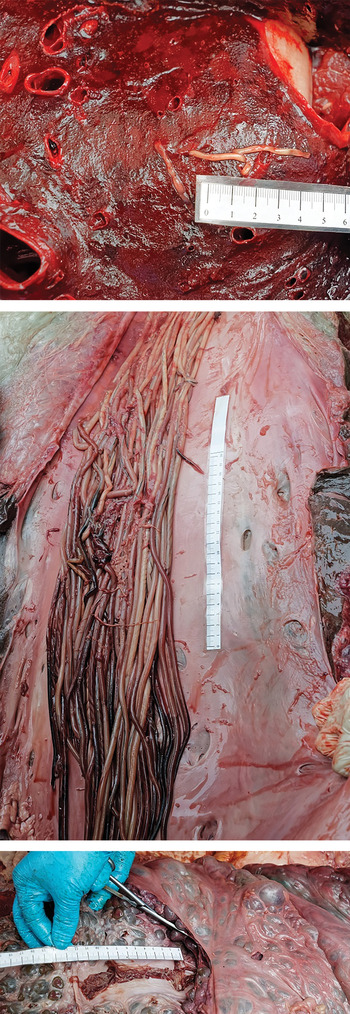
Fig. 2. Nematode segments found in lungs (top), the caudal caval vein (middle) and the renal vein (bottom) of the fin whale.
Microscopic observations
Several parasites found in the caudal caval vein were observed microscopically. A cephalic extremity was found on a large striated worm portion of 5 mm in diameter with a tapering apex (Fig. 3). The head was located at the very tip of this tapering section and exhibited a constriction; the mouth bore two lateral lips (Fig. 3). Worms found in the renal veins showed a typical posterior extremity on an attenuated worm section (1 mm in diameter). The posterior extremity of female worms was distinguished by the presence of a constriction before a rectangular end (Fig. 3). No male posterior extremity was observed. Larvated eggs measuring 50 µ m in length were found on the parasite cuticle (Fig. 4). These observations allowed us to identify two fragments most probably belonging to C. boopis. Parasite fragments found in the lungs were not preserved.
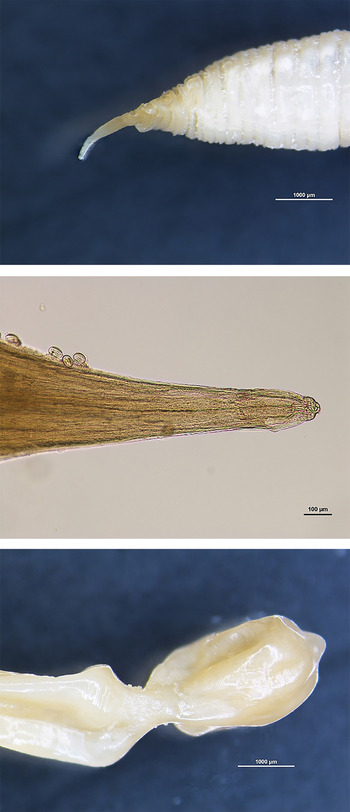
Fig. 3. Anterior extremity (20×) (top), (400×) (middle) and posterior extremity of female Crassicauda boopis (20×) (bottom).

Fig. 4. Larvated eggs of Crassicauda boopis (400×) (top); histopathological examination of renal tissue: nematode with larvated eggs in an arteriole. The subacute arteritis and periarteritis is characterized by infiltration of lymphocytes and macrophages (bottom).
Microscopically, significant lesions were located in the kidneys. In the renal thrombosis, a nematode with larvated eggs was observed in an arteriole (Fig. 4). A subacute arteritis and periarteritis was characterized by the infiltration of lymphocytes and macrophages in and around the blood vessel. In the renal interstitial tissue, worms were surrounded by an area of lymphocytes and macrophages with the presence of a multinucleate giant cell (Fig. 4).
Molecular identification
Amplicons of about 1700 bp and an internal portion of 808 bp were visualized on 2% agarose gel. A consensus sequence (GenBank KY263809) was made based on sequences obtained from these PCR products and was submitted to Blastn (http://blast.ncbi.nlm.nih.gov) showing 100% similarity with Crassicauda magna (GenBank KM233410).
DISCUSSION
Crassicaudiosis is one of the main parasitic diseases of fin whales (Lambertsen, Reference Lambertsen1986). The difficulties in field sampling, limited possibilities for experimental work and difficulties to observe these specimens in the marine environment make information on C. boopis scarce (Lambertsen, Reference Lambertsen1985; Balbuena and Simpkin, Reference Balbuena and Simpkin2014; Jabbar et al. Reference Jabbar, Beveridge and Bryant2015). The life cycle is not yet entirely elucidated, and little is known about potential intermediate hosts. Moreover, transmission to calves, which are regularly and heavily infested (Lambertsen, Reference Lambertsen1992), is suspected to be transplacental, whereas galactogenic transmission has to be considered even though up to now no larvae were found in milk or mammary tissue (Díaz-Delgado et al. Reference Díaz-Delgado, Fernández, Xuriach, Sierra, Bernaldo de Quirós, Mompeo, Pérez, Andrada, Marigo, Catão-Dias, Groch, Edwards and Arbelo2016). Direct transmission to calves by urine contamination was evoked (Lambertsen, Reference Lambertsen1986), which suggests emission of infective larvae in the environment and therefore a bypass in the need of an intermediate host. This suggestion seems unlikely because of the obvious thick shells of the eggs of C. boopis usually encountered in environmentally resistant eggs and the fact that habronematoids are known to use arthropod intermediate hosts consistently (Anderson, Reference Anderson2000).
In this present case, the size of the juvenile fin whale corresponds to a recently weaned fin whale, in which weaning occurs at an age of 6–7 months and a body size of 11–13 m (William et al. Reference William, Bernd and Thewissen2009). Therefore, the present report supports the theory that infestation could occur transplacentally or during lactation.
Crassicaudiosis due to C. boopis is typically a chronic disease and has proven to be potentially lethal, mainly due to congestive kidney failure, or to cause substantial morbidity in those animals which survive (Lambertsen, Reference Lambertsen1985, Reference Lambertsen1992; Jauniaux et al. Reference Jauniaux, Charlier, Desmecht, Haelters, Jacques, Losson, Van Gompel, Tavernier and Coignoul2000; Díaz-Delgado et al. Reference Díaz-Delgado, Fernández, Xuriach, Sierra, Bernaldo de Quirós, Mompeo, Pérez, Andrada, Marigo, Catão-Dias, Groch, Edwards and Arbelo2016). In some individuals, a more acute inflammatory reaction surrounds the parasites with exudate or pus. Lesions associated with C. boopis occur less commonly in the lungs, which can contain necrotic fragments of nematodes, as described in this present case, with mineral deposits (Lambertsen, Reference Lambertsen1992). Although numerous C. boopis and lesions similar to those described by Lambertsen (Reference Lambertsen1986) and Jauniaux et al. (Reference Jauniaux, Charlier, Desmecht, Haelters, Jacques, Losson, Van Gompel, Tavernier and Coignoul2000) were found in the fin whale case presented here, the cause of death could be established as the collision with the ship on the basis of the presence of large haematoma at the impact zone and the absence of evidence of renal failure. The young age of this whale and the absence of severe reaction may suggest that the infestation was not at an advanced chronic stage yet.
Crassicauda genus is represented by large size nematodes with C. boopis as the second longest nematode in whales. Consequently, obtaining intact specimens from host tissues is a real challenge and the species is frequently only described from fragments, leading to uncertainty in their identification. Therefore, molecular biology could be useful in the identification of these specimens. Consensus sequence of 18S rRNA gene obtained from these present samples was similar to the sequence obtained from parasites found in subcutaneous tissues of a pygmy sperm whale (Kogia breviceps) and identified as C. magna (Jabbar et al. Reference Jabbar, Beveridge and Bryant2015). Additionally, this sequence was similar to the partial sequence of a worm located in the kidney of a Cuvier's beaked whale (Ziphius cavirostris) (Díaz-Delgado et al. Reference Díaz-Delgado, Fernández, Xuriach, Sierra, Bernaldo de Quirós, Mompeo, Pérez, Andrada, Marigo, Catão-Dias, Groch, Edwards and Arbelo2016). While adults of C. boopis and C. magna are morphologically distinct and found in different body sites, molecular analysis of the 18S rRNA gene seems insufficient for reliable species identification. This could be a major restriction mainly for larvae identification.
It is noteworthy that all morphological classifications to date have included Crassicauda spp. in the order Spirurida, superfamily Habronematoidea, family Tetrameridae and subfamily Crassicaudinae (Lambertsen, Reference Lambertsen1985; Jabbar et al. Reference Jabbar, Beveridge and Bryant2015), while recent phylogenetic analysis based on the 18S rRNA gene showed C. magna as a member of the Acuarioidea superfamily (Jabbar et al. Reference Jabbar, Beveridge and Bryant2015). The 18S rRNA gene shows highly conserved regions, and although this gene is often used for deep phylogenetic relationship investigation, multigene analysis should be performed to refine this phylogenetic analysis.
This fin whale arrived in Belgium probably after a ship-strike in the Bay of Biscay or in the Western English Channel, given the track of the ship and the estimation of period of death. Fin whales are large, long-distance swimmers that often occur far offshore, and not many causes of natural mortality are known, such as predation or starvation which are probably of minor significance. However, decline of major zooplankton populations (Steinberg and Landry, Reference Steinberg and Landry2017) could lead to poor nutrition and therefore to an increase in the severity of endemic parasitism by compromising disease resistance (Lambertsen, Reference Lambertsen1992). For this reason, Lambertsen (Reference Lambertsen1986) suggested that diseases like crassicaudiosis may be the most significant mortality factor in large balaenopterid whales (Raga et al. Reference Raga, Balbuena, Aznar and Fernández1997). Further research should be able to collect information on animals infected by parasites especially C. boopis in order to implement a model including the effect of parasitism in calf and adult whale populations.
Conclusion
Crassicaudiosis is potentially the most significant mortality factor in large balaenopterid and reported cases are scarce. Further research is surely needed in order to elucidate the life cycle of the parasite, its taxonomy and impact on whale populations. However, the present report supports the theory that infestation could occur transplacentally or during lactation.
The species is frequently only described from fragments, leading sometimes to uncertainty in their identification. Therefore, molecular biology could be useful in the identification of these specimens with multigene analysis helping to refine identification and phylogenetic analysis.
ACKNOWLEDGEMENTS
The authors are very grateful to Allison Balin, Jurgen Decraene, Bart De Pauw, Françoise Maréchal, Michael Sarlet and Patrick Vervaet. The authors also thank the volunteers of the veterinary Faculty of Ghent University and the veterinary Faculty of Liege University. They are grateful for the excellent cooperation with the crew of the Premium do Brasil, the local authorities of the city of Ghent, port authorities, the Federal public service health, food chain safety and environment, Citrosuco (and in particular Jean-Pierre Vandecasteele), the Civil Protection Unit, and many others for their role in securing the animal and making the scientific investigation possible.
FINANCIAL SUPPORT
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors. The work was carried out in the framework of the Marine Animals Research and Intervention Network (MARIN), coordinated by the RBINS.
CONFLICT OF INTEREST
None.






