Introduction
Enterococcal species are significant pathogens in bloodstream infections (BSIs) and account in the USA, for approximately 4% and 9% of community-acquired and nosocomial bacteraemia, respectively, and are the second most common cause of nosocomial BSIs [Reference Wisplinghoff1–Reference Kollef4]. Similarly, in Europe, the prevalence of enterococcal BSIs appears to be increasing [Reference Buetti5]. In Japan, enterococci are the third most common pathogen in nosocomial BSIs and the crude mortality rate of enterococcal bacteraemia is the second worst in Japanese university hospitals [Reference Nagao6]. Worldwide, reported mortality rates for enterococcal BSI range from 14% to 48% [Reference Noskin, Peterson and Warren7–Reference Dahl11]. The treatment of enterococcal bacteraemia is complicated by antimicrobial resistance as all Enterococcus species are inherently resistant to cephalosporins and acquired resistance to penicillins, aminoglycosides and glycopeptides is increasingly common [12]. Although the prevalence of vancomycin-resistant enterococci (VRE) is gradually increasing in North America and parts of Europe [Reference Buetti5, 12], the prevalence of vancomycin-susceptible isolates remains high in many European countries and Japan [Reference Hamada13]. Indeed, survey reports suggest that VRE isolates are relatively less common in enterococcal bacteraemia [Reference Hamada13–Reference Fortún18], while the ampicillin-susceptible species, e.g. E. faecalis [Reference Dahl and Bruun19], are more frequently isolated from blood cultures than their ampicillin-resistant counterparts, such as E. faecium. Ampicillin is not recommended as the first choice for empirical therapy to treat enterococcal bacteraemia in a setting with the frequent isolation of E. faecium. In regions where the prevalence of VRE is low, the susceptibility or resistance to ampicillin of isolates is the major concern when selecting initial antibiotics for treatment. In such a context, the aim of this study was to identify the predictive and prognostic factors associated with ampicillin-resistant enterococcal bacteraemia.
Methods
Study design and population
All patients aged 16 years or older with a first episode of enterococcal bacteraemia attending Kyoto University Hospital, a 1121-bed tertiary care hospital in Kyoto, Japan, were identified from a laboratory database of all positive blood cultures from 1 January 2009 to 31 December 2015. Patients were excluded who had more than one enterococcal species in blood cultures and if they had another bacteraemic episode with an enterococcal species different from that in the first episode during the study period. Study approval was obtained from the Institutional Review Board at Kyoto University and informed consent was waived due to the anonymous nature of the data.
Patient data
Electronic medical charts of all patients with enterococcal bacteraemia were reviewed for demographic (age and sex), microbiological (enterococcal species isolated from blood, antimicrobial susceptibility, the presence of mixed flora) and clinical data. Minimum inhibitory concentrations were determined by a broth microdilution test and interpreted according to standard criteria [20].
Clinical data included the acquisition site (nosocomial, community or intensive care unit (ICU)-acquired), underlying diseases, a prior operation within 6 months, presence of indwelling devices, immunosuppression, prior antibiotic exposure within 30 days, number of days from admission to onset, prior hospitalisation or ICU admission within 1 year, the isolation of enterococci from body sites other than blood within 1 year of the index culture, the source of bacteraemia and exclusion of contamination and 30-day mortality. An infection was designated as nosocomial if onset occurred more than 48 h after admission to hospital [Reference Garner21]. Likewise, an infection was considered as healthcare-associated community-onset if onset of bacteraemia occurred within 48 h and patients fulfilled any of the following criteria prior to the onset of bacteraemia: (1) received intravenous treatment at home, wound care, home-based nursing care or self-administered intravenous medical therapy within the previous 30 days; (2) visited a hospital or haemodialysis clinic or received intravenous chemotherapy within the prior 30 days (3) had been admitted to an acute care hospital for at least 2 days within the prior 90 days; or (4) had been admitted to a nursing home or long-term care facility [Reference Friedman22]. A community-acquired infection was designated if onset was within 48 h of admission but did not meet the criteria for healthcare-associated community-onset infection. Likewise, an ICU-acquired infection was one where bacteraemia was diagnosed after 48 h following admission to ICU. A prior operation included any skin incisions and excluding puncture procedures. Neutropaenia was defined as an absolute neutrophil count <500/μl and severity of bacteraemia was based on the Pitt bacteraemia score (PBS) [Reference Korvick23]. The degree of comorbidity was evaluated by the Charlson comorbidity index (CCI) [Reference Charlson24].
Epidemiological characteristics
The proportions of healthcare-associated community-onset and nosocomial enterococcal bacteraemias each year were determined as well as the trend of the rate for ampicillin-resistant strains in all enterococcal bacteraemias. Similarly, we examined the trend of the antimicrobial use density (AUD) for penicillins, carbapenems, cephalosporins, aminoglycosides, fluoroquinolones and anti-methicillin-resistant Staphylococcus aureus (MRSA) antibiotics in the wider hospital and compared the results with the detection rate of ampicillin-resistant enterococcal strains in nosocomial bacteraemia.
Statistical analysis
The Cochran-Armitage test was used to determine trends in the proportion of healthcare-associated community-onset enterococcal bacteraemia cases and the isolation rate of ampicillin-resistant strains. Spearman's rank correlation analysis was used to test for association between antibiotic consumption and the rate of ampicillin-resistant strains. In the comparison of ampicillin-resistant and ampicillin-sensitive cases, categorical variables were compared using the Fisher exact test and continuous variables by the Mann–Whitney U test. The threshold for significance was a P-value <0.05. Factors with a P-value <0.10 in a single-variable analysis were entered into a logistic regression analysis. We conducted a backward stepwise procedure so that only significant variables were left in the model. In the comparison of 30-day survivors and non-survivors cases, categorical variables were compared using the Kaplan–Meier method and compared with a log-rank test and continuous variables by the Cox Proportional Hazards analysis. The threshold for significance was a P-value <0.05. For 30-day mortality, the CCI and factors with a P-value <0.10 in a single-variable analysis were entered into the Cox Proportional Hazards analysis. We also conducted a backward stepwise procedure so that only significant variables were left in the model. All statistical analyses were conducted using EZR version 1.33 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) [Reference Kanda25].
Results
A total of 255 patients with 272 episodes of positive blood cultures for enterococci were identified during the study period; five cases were considered as pseudobacteraemia on clinical review. There were 32 episodes where the same blood culture harboured different enterococcal species or multiple blood cultures with different enterococci in 15 patients. Therefore, 235 patients with single bacteraemic episodes were included in the study.
In total, 113 (48.1%), 98 (41.7%) and 24 (10.2%) episodes were caused by E. faecium, E. faecalis and other enterococci, respectively. Their antibiotic susceptibilities are shown in Supplemental Table S1. There were 124 (52.8%) ampicillin-susceptible and 111 (47.2%) ampicillin-resistant episodes of enterococcal bacteraemia.
All E. faecalis isolates were susceptible to ampicillin and as very few ampicillin-resistant non-faecium isolates were identified, we focused only on E. faecium cases for further analysis. The great majority of E. faecium bacteraemias (101; 89.4%) were designated as nosocomially acquired and the remainder as healthcare-associated community-onset cases. Figure 1 shows that over the study period the number of nosocomial E. faecium bacteraemias decreased from 25 to 6 episodes and the proportion of healthcare-associated community-onset episodes increased from 3.8% to 25%. The detection rate for ampicillin-resistant strains among all cases and nosocomial bacteraemias exceeded 81% and 87%, respectively. Healthcare-associated community-onset cases due to ampicillin-resistant strains were first identified at our hospital in 2012 and since 2014, all such isolates have been ampicillin resistant; significant increases were evident in the proportion of healthcare-associated community-onset cases (P = 0.009) and the isolation rate for ampicillin-resistant strains from bacteraemias (P = 0.005). The AUD trajectory for each antibiotic is shown in Supplemental Figure S1. Spearman's rank correlation coefficients for penicillins, carbapenems, cephalosporins, aminoglycosides, fluoroquinolones and anti-MRSA agents were 0.185 (P = 0.691), 0.482 (P = 0.274), −0.074 (P = 0.875), 0.259 (P = 0.574), −0.185 (P = 0.691) and −0.445 (P = 0.317), respectively. No correlation was observed between the amounts of β-lactams, aminoglycosides, fluoroquinolones and anti-MRSA agents prescribed and the detection rate for ampicillin-resistant E. faecium.
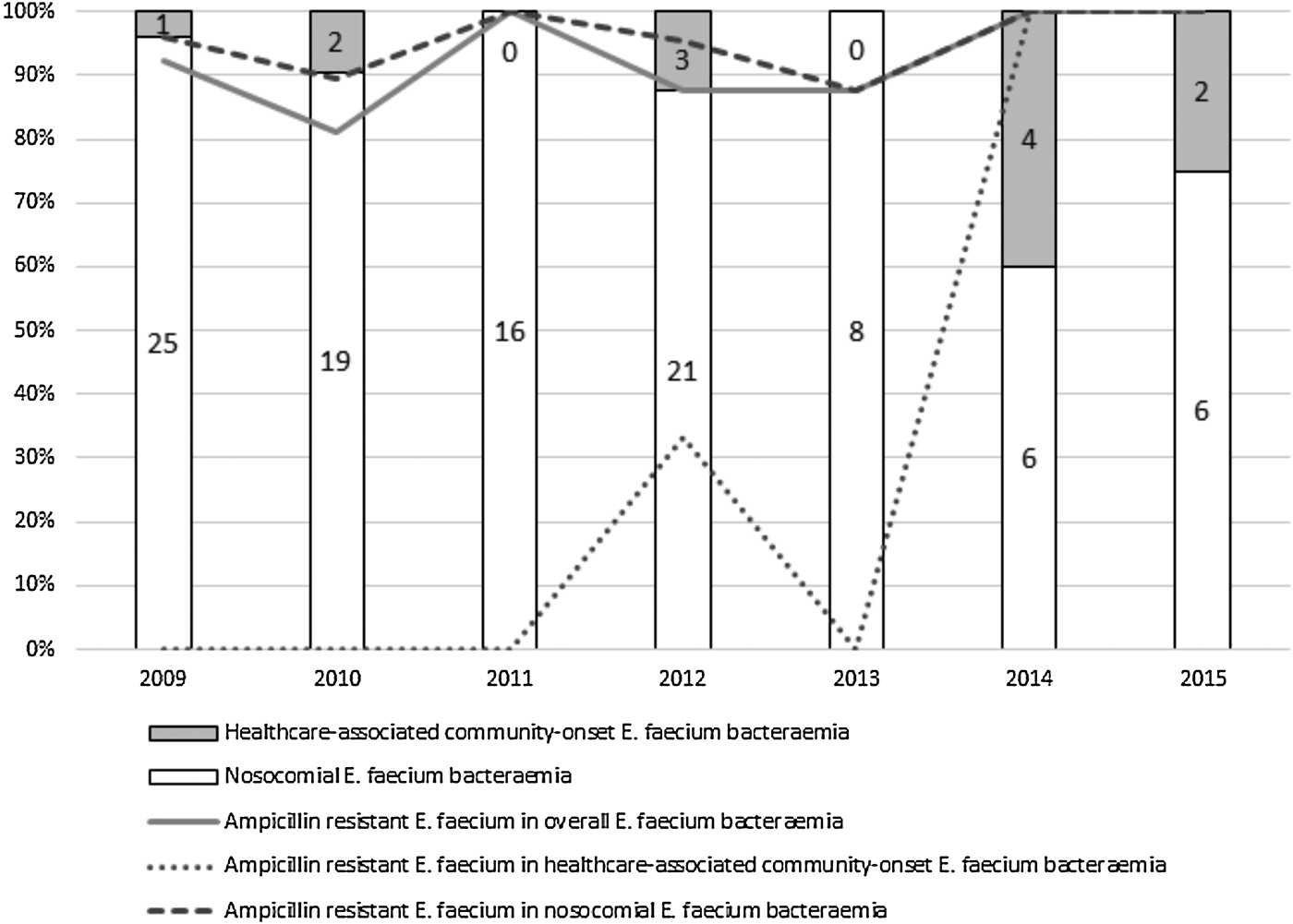
Fig. 1. Proportion of nosocomial and community-acquired E. faecium bacteraemia cases and the detection rate of ampicillin-resistant isolates.
Table 1 compares the patients from whom ampicillin-resistant and ampicillin-susceptible E. faecium were isolated and shows that the factors most associated with ampicillin resistant cases in a single-variable analysis were nosocomial acquisition, comorbid liver disease, prior surgery, solid organ transplantation, administration of immunosuppressants, prior exposure to penicillins, carbapenems and sulfamethoxazole/trimethoprim and the duration from admission to onset as well as prior ICU hospitalisation. Conversely, only patient age and prior hospitalisation were significantly associated with ampicillin-susceptible E. faecium bacteraemia cases. By multivariable logistic regression analysis only nosocomial acquisition (odds ratio (OR), 13.6; 95% confidence interval (CI) 3.16–58.3, P < 0.001) was significantly associated with ampicillin-resistant E. faecium.
Table 1. Comparison of the baseline demographics and clinical characteristics of patients with ampicillin-susceptible and ampicillin-resistant Enterococcus faecium bacteraemia
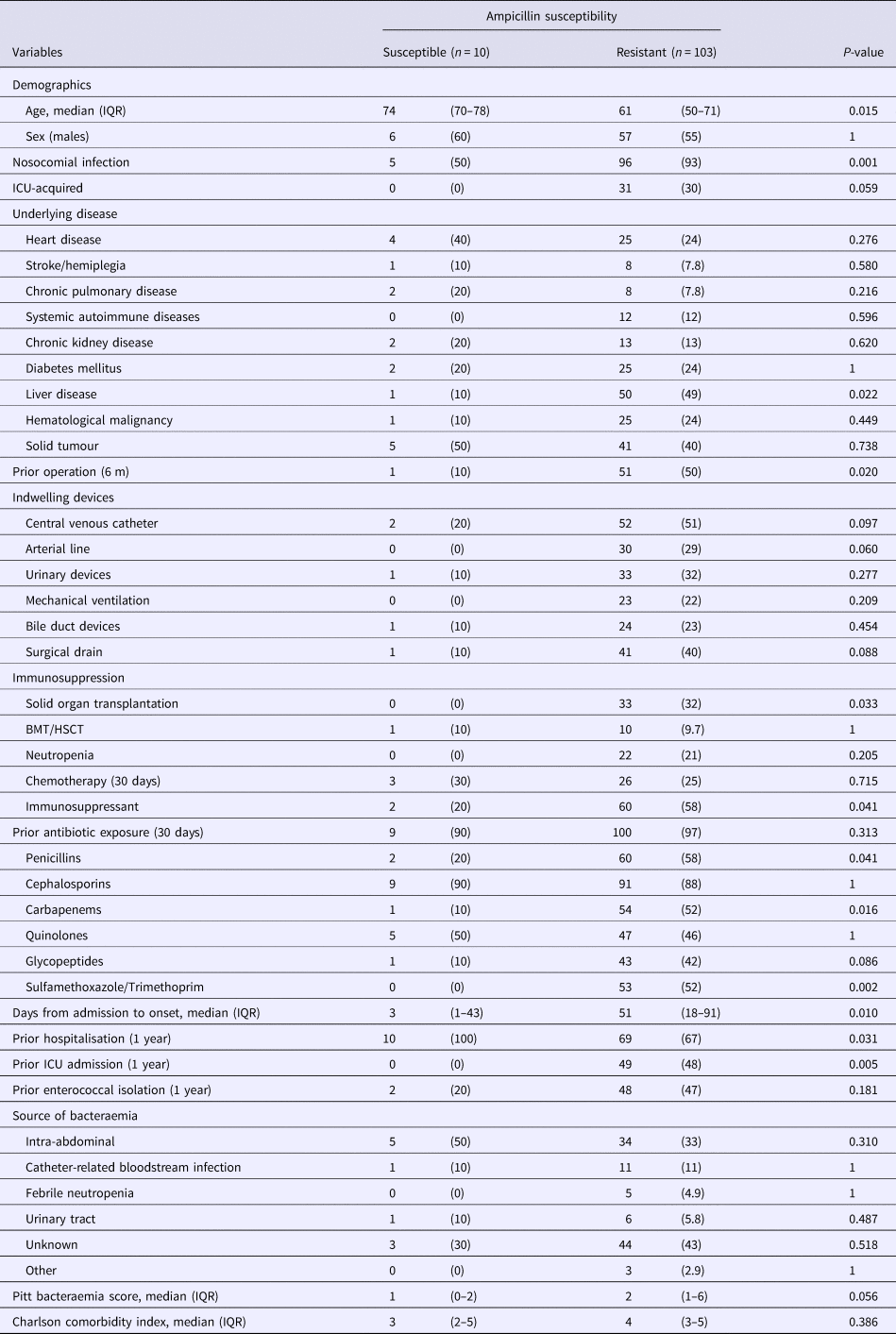
Data represent the number (%) of patients unless otherwise indicated.
IQR, interquartile range; ICU, intensive care unit; BMT, bone marrow transplantation; HSCT, hematopoietic stem cell transplantation.
There were substantial differences between patient characteristics with E. faecalis and E. faecium bacteraemia (Supplemental Table S2) and therefore prognostic factors for both species were investigated separately. Table 2 shows that by single-variable analysis, the factor significantly associated with 30-day mortality in patients with E. faecalis was bacteraemia from an unknown source. By multivariable analysis, BSI from an unknown source remained significantly associated with 30-day mortality in patients with E. faecalis bacteraemia (HR, 4.17; 95% CI 1.25–13.9, P = 0.020) as well as a higher PBS (HR, 1.27; 95% CI 1.09–1.48, P = 0.002) (per 1-point increase in score).
Table 2. Comparison of the baseline demographics and clinical characteristics of 30-day survivors and non-survivors with Enterococcus faecalis bacteraemia
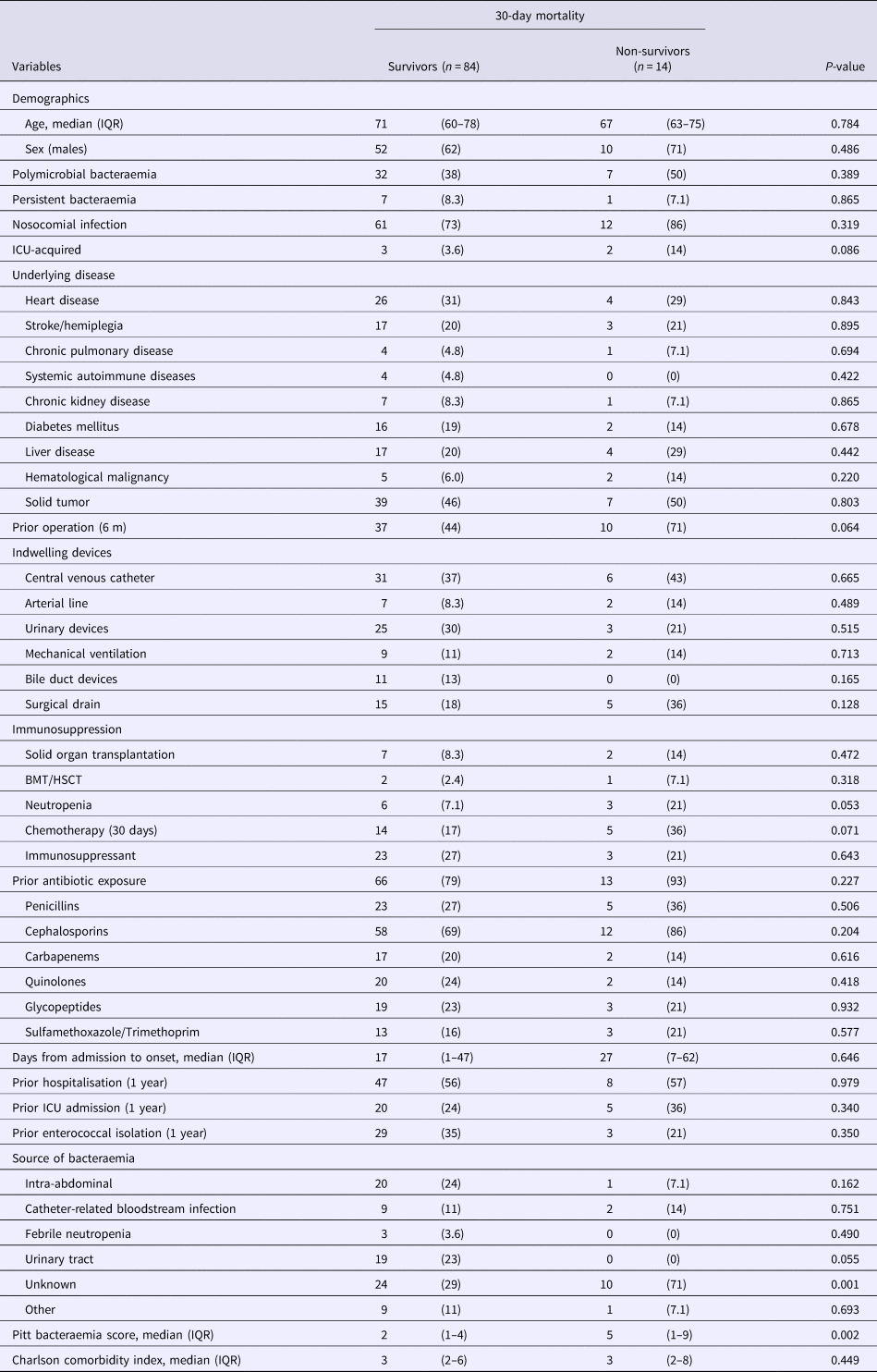
Data represent the number (%) of patients unless otherwise indicated.
IQR, interquartile range; ICU, intensive care unit; BMT, bone marrow transplantation; HSCT, hematopoietic stem cell transplantation.
A comparison of the clinical characteristics of patients who died within or survived 30 days with E. faecium bacteraemia are presented in Supplemental Table S3. By single-variable analysis, a number of factors were significantly associated with earlier death in patients with E. faecium bacteraemia including an indwelling central venous line and arterial line, mechanical ventilation, bone marrow transplantation/haematopoietic stem cell transplantation, neutropenia, prior exposure to sulfamethoxazole/trimethoprim, bacteraemia from an unknown source and increased PBS. Conversely, intra-abdominal infection was the sole factor significantly associated with patient survival for more than 30 days. Moreover, a Kaplan–Meier plot of survival time showed that the isolation of ampicillin-resistant E. faecium strains was not significantly associated with 30-day mortality (P = 0.630) (Fig. 2). Finally, by multivariable analysis, the factors significantly associated with 30-day mortality in patients with E. faecium bacteraemia were bacteraemia from an unknown source (HR, 2.91; 95% CI 1.36–6.21, P = 0.006) and higher PBS (HR, 1.36; 95% CI 1.21–1.52, P < 0.001) (per 1-point increase in score).
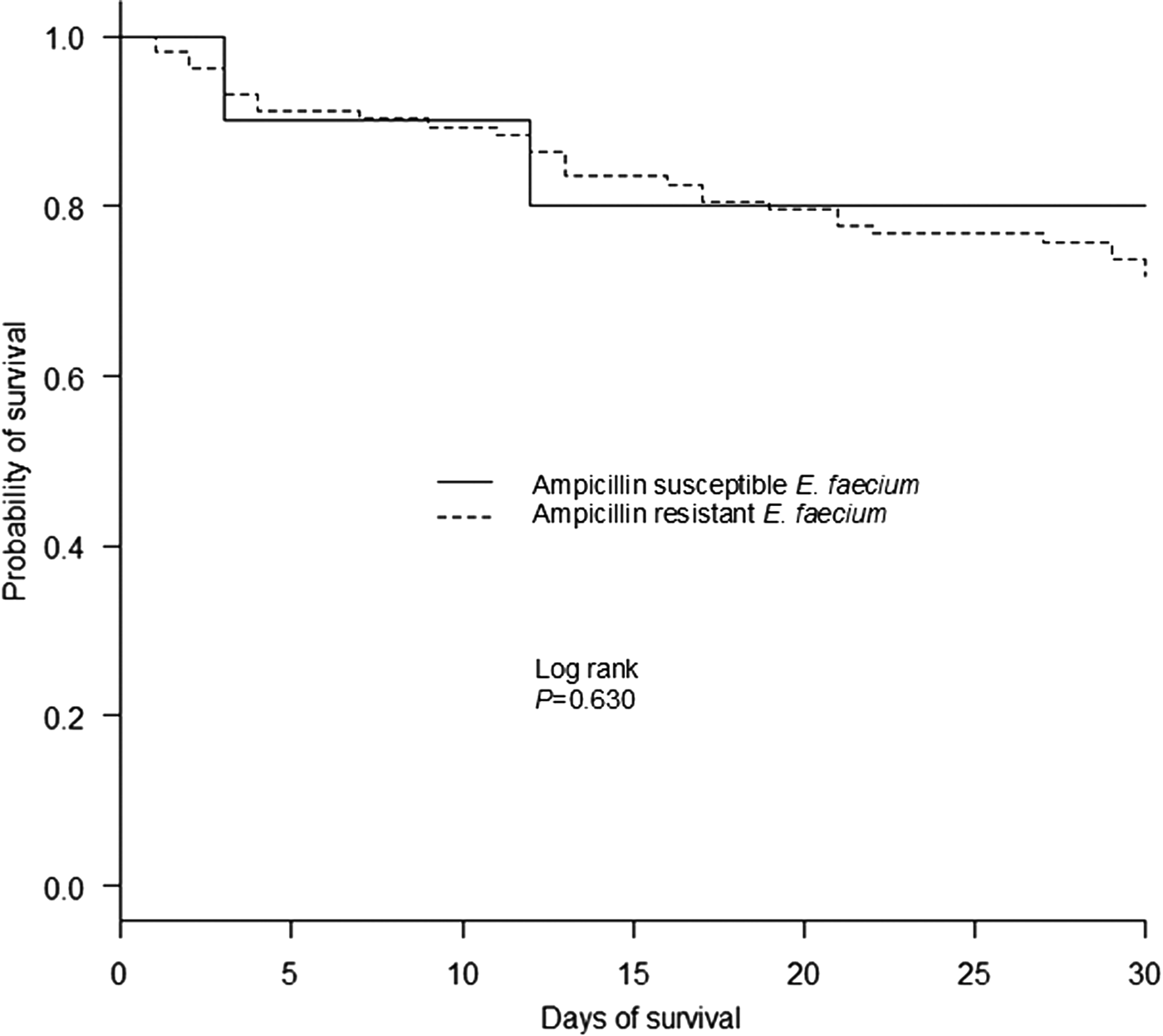
Fig. 2. Kaplan–Meier curve for all-cause 30-day mortality, according to the isolated E. faecium susceptibility to ampicillin.
Discussion
This study investigated the predictive and prognostic factors for ampicillin-resistant enterococcal bacteraemia. As all the E. faecalis isolates were susceptible to ampicillin, we chose to focus on factors predictive of ampicillin-resistant strains in E. faecium bacteraemia alone and the key finding was that nosocomial acquisition was the factor most predictive in these cases. By contrast, prognostic factors for both E. faecalis and E. faecium bacteraemia were found to be unknown acquisition source and increased PBS and additionally for E. faecium cases, presentation with neutropaenia. Previous studies have highlighted exposure to penicillins and carbapenems as predictive factors for ampicillin-resistant enterococcal bacteraemia [Reference Hamada13] and notable prognostic factors were clinical and co-morbid severity, nosocomial acquisition, isolation of E. faecium and several other underlying conditions [Reference Pérez-García14–Reference Fortún18]. However, in almost all of these studies, all enterococcus species were generally pooled into a single group and evaluated together [Reference Hamada13–Reference Billington15, Reference McBride, Upton and Roberts17, Reference Fortún18]. Owing to differences in their epidemiology and ampicillin susceptibility, we investigated predictive factors of ampicillin-resistant strains in E. faecium alone and prognostic factors for both E. faecalis and E. faecium bacteraemia separately. The high proportion of E. faecium (48.1%) in our case series is noteworthy in contrast to some previous studies, which reported bacteraemia frequencies for this species ranging from 14.1% to 37.0% [Reference Hamada13–Reference Fortún18]. Moreover, our observed rate of ampicillin-resistant strains (47.2%) in bacteraemia was markedly higher than earlier reports of 9.8% to 40.6% [Reference Hamada13, Reference Billington15–Reference McBride, Upton and Roberts17]. A possible explanation for this higher frequency may be due to a relatively high rate of ampicillin-resistant strains in Japan with frequencies ranging from 32.7% to 40.6% [Reference Hamada13, Reference Suzuki26]. Another reason for our high rate may be the specialist tertiary nature of a university hospital which provides advanced medical care for patients referred from community hospitals where almost all patients would have received earlier treatment, such as antibiotic therapy.
A notable finding of the study was the increase in the rate of ampicillin-resistant E. faecium in healthcare-associated community-onset bacteraemia. According to previous studies, prior hospitalisation and the use of β-lactams and fluoroquinolones were associated with colonisation of patients by ampicillin-resistant enterococci on admission [Reference de Regt27, Reference Weinstein28]. We did not find prior hospitalisation to be significantly associated with ampicillin-resistant bacteraemia and neither was there a correlation between the amounts of prescribed β-lactams and fluoroquinolones and rates of ampicillin-resistant strains, which might suggest an absence of antimicrobial selection pressure.
The multivariable analysis suggested that nosocomial acquisition was the only predictive factor for ampicillin-resistant E. faecium bacteraemia. This contrasts with a report from Spain that the previous administration of β-lactams and urinary catheterisation were predictive factors of such cases [Reference Fortún18]. This difference might have been due to the proportions of nosocomial infection and ampicillin-resistant E. faecium which were markedly higher here (nosocomial infection: 67.3% vs. 89.3%, ampicillin-resistant E. faecium: 59.2% vs.91.2%) compared with the Spanish study. Predictive factors for ampicillin-resistant strains in enterococcal bacteraemias caused by all enterococcal species has been investigated previously in Japan [Reference Hamada13] which reported that exposure to penicillins and carbapenems and bacteraemia related to mucositis with febrile neutropenia were risk factors for ampicillin-resistant strains [Reference Hamada13]. Several studies have reported an association between the incidence of ampicillin-resistant enterococci and the prior administration of penicillins, cephalosporins, β-lactams, imipenem and fluoroquinolones [Reference Hamada13, Reference Fortún18, Reference Boyce29–Reference Gudiol32].
The prognostic factors for 30-day mortality in patients with both E. faecalis and E. faecium bacteraemia were increased PBS and bacteraemia from an unknown source. The severity of both bacteraemia and comorbidities were previously reported as prognostic factors for enterococcal bacteraemia [Reference Billington15, Reference Pinholt16, Reference Fortún18]. A population-based cohort study in Denmark which sought risk factors of 30-day mortality for E. faecalis and E. faecium bacteraemia separately identified an unknown focus of infection as one of the prognostic factors associated with E. faecalis [Reference Pinholt16]. Although the reasons for such an association are unclear, inadequate clinical investigation or an unrecognised primary focus of infection such as endocarditis may lead to antimicrobial treatment and surgical management for an insufficient duration [Reference Bouza10, Reference Dahl11].
We were unable to estimate the attributable mortality for enterococcal bacteraemia due to the absence of records of direct cause of death on medical charts and autopsies were rarely performed. We also did not include a matched control group without enterococcal bacteraemia. However, the survival time analysis indicated that the isolation of ampicillin-resistant strains from the patients’ blood was not associated with a poor prognosis and may imply that ampicillin susceptibility of the infecting strain is not critical to patient outcomes; nevertheless, more complex patient conditions such as focus of bacteraemia, comorbidity, neutropenia and severity of infection, most notably affected the prognosis.
The primary limitations of the study were associated with the retrospective design. Some data were missing or inaccurate due to incomplete documentation in the medical records and we were unable to determine the source of certain bacteraemias due to insufficient investigations; this, therefore, increased the number of cases with an unknown source. In addition, even with electronic medical charts, it was difficult to identify the exact time when the index cultures were collected and antibiotics were given. We obtained patient referral medical information if the patients had previously received medical care in different hospitals in order to collect as much information as possible on variables. However, some data from other hospitals at which the patients may have been treated in the previous year were sometimes incomplete. We did not record the duration of the antibiotic therapy and glycopeptide use and may have underestimated the incidence of enterococcal bacteraemia due to false-negative blood cultures; additionally, blood cultures might not have been collected in some cases in which bacteraemia was suspected.
In conclusion, the prognostic factors for E. faecalis bacteraemia were the severity of BSI and infection from an unknown source; these factors also applied to E. faecium bacteraemia. The Nosocomial acquisition was the sole predictive factor for ampicillin-resistant E. faecium bacteraemia. We suggest that when severe E. faecium bacteraemia is suspected in a setting with a high-degree of ampicillin resistance then glycopeptides should be administered as empirical treatment.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0950268818002479
Conflict of interest
None.







