INTRODUCTION
Streptococcus pneumoniae is an important cause of community-acquired pneumonia, bacteraemia, and meningitis. Invasive pneumococcal disease (IPD) is defined as isolation of S. pneumoniae from a normally sterile body site (typically blood or cerebrospinal fluid), and carries with it significant morbidity and mortality, even in the modern antibiotic era. The mortality rate of pneumococcal bacteraemia in a large prospective international study was 16·9% in those with age >65 years and the presence of underlying disease or risk factors for immunosuppression significantly associated with increased mortality [Reference Yu1]. Pneumococcal meningitis also leads to poor outcomes, carrying a mortality rate of 21% [Reference Schuchat2]. Various states of immunodeficiency, including underlying malignancy, are recognized as risk factors for IPD [3, Reference Dworkin4]. Vaccination with the 23-valent pneumococcal polysaccharide vaccine (PPV23) is efficacious against IPD and recommended for patients in high-risk groups, including all patients with malignancy [Reference Moberley5–7]. Unfortunately, many patients with IPD who have an indication for vaccination are not vaccinated despite multiple encounters with the healthcare system [Reference Kyaw8].
Large-scale population studies have found increased rates of IPD in paediatric and adult patients with haematological and solid-organ malignancies [Reference Kyaw9–Reference Hjuler11], but condition-specific data is lacking. We conducted a large 5-year retrospective study of all adult patients aged ⩾18 years with IPD in the province of Alberta and obtained provincial prevalence data for various malignancies from the Alberta Cancer Board to determine the rates of IPD in patients with selected underlying malignancies.
METHODS
Demographics and definitions
The study was conducted in the province of Alberta from 2000 to 2004, which at the time of the study was divided into nine health regions. The population was 2 967 755 in 2000 and 3 179 036 in 2004 [12]. Cases of IPD were defined as the isolation of S. pneumoniae from any normally sterile body site, including blood, cerebrospinal fluid, pleural fluid, biopsy tissue, synovial fluid, pericardial fluid, and peritoneal fluid [13]. In Alberta, IPD is a notifiable disease reportable to the Provincial Health Office. S. pneumoniae isolates recovered from patients with IPD are submitted to the National Centre for Streptococcus (NCS) located in Edmonton, Alberta, for capsular serotyping and antimicrobial resistance profiling for trending analysis. Isolates were submitted to the NCS prospectively from acute diagnostic microbiology laboratories in Alberta during the study period.
To ensure as complete as possible the capture of all patients with IPD in Alberta during the study period, a number of databases were utilized. These included all patients identified by identification of S. pneumoniae isolates sent to the NCS, all patients reported to the provincial health office, and all patients captured in both the Calgary area S. pneumoniae Research Group database (Calgary, AB) and the Community Acquired Pneumonia Study database (Edmonton, AB). All four databases were combined to form the final dataset, and duplicate patients (identified by personal health number) were counted only once. An extensive retrospective chart review of all identified patients was then performed for all identified IPD cases occurring during the survey period. Current underlying malignancies were recorded as described in the chart. Haematological malignancies were defined as any leukaemia, any lymphoma, or multiple myeloma.
In the laboratory, upon receipt of S. pneumoniae isolates, bacteria were stored at −70°C until serotyping and susceptibility assays were performed. Only one isolate from each IPD case was included in the review unless the isolates were collected ⩾1 month apart or were of a different serotype if <1 month had elapsed between episodes of IPD.
Annual incidence rates of IPD for the general population were calculated between 2000 and 2004 using provincial population estimates from Alberta Health and Wellness [12]. Condition-specific incidence rates of IPD between 2000 and 2004 were calculated based on annual prevalence data of haematological and solid-organ malignancies obtained from the provincial cancer registry, maintained by the Alberta Cancer Board [Reference Wang14]. The registry records all new cancer cases throughout the province and also tracks all cancer-related deaths using information from Alberta Vital Statistics and Statistics Canada. Malignancies considered in our study included lung cancer (small-cell and non small-cell), multiple myeloma, chronic lymphocytic leukaemia (CLL), acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL), Hodgkin's lymphoma, and non-Hodgkin's lymphoma.
The study received approval from the institutional research review committees of all nine health regions in Alberta and also from the University of Alberta and the University of Calgary.
Serotyping of S. pneumoniae isolates
Isolates received at the NCS were confirmed as S. pneumoniae based on morphology and optochin susceptibility [Reference Facklam, Washington, Balows, Hausler, Herrmann, Isenberg and Shadomy15]. Serotyping was performed at the NCS by Quellung reaction using commercial antisera prepared at the World Health Organization (WHO) Collaborating Center for Reference and Research on Pneumococci, located at the Statens Seruminstitut Copenhagen, Denmark [Reference Lund, Henrichsen, Bergan and Norris16]. Strains that failed to type were confirmed as S. pneumoniae using Accuprobe™ (Genprobe, USA).
Statistical analysis
Incidence rates and serotype prevalence were compared between various malignancies and the general adult population (all adults aged ⩾18 years) using Fisher's exact test. All statistical analyses were performed using SPSS version 16.0 (SPSS Inc., USA).
RESULTS
Incidence rates of IPD for patients with various malignancies vs. general population
A total of 1768 cases of IPD were identified in Alberta between 2000 and 2004 for which laboratory and clinical data were both complete. Of these cases 1273 occurred in patients aged ⩾18 years, for an incidence rate of 11·0 cases/100 000 population per year (95% CI 10·44–11·65). Of these, 152 (11·9%) cases occurred in adult patients with some form of underlying malignancy. Sixty-one (4·8%) cases involved patients with an underlying haematological malignancy, 82 (6·4%) cases involved patients with an underlying solid-organ malignancy (including cutaneous malignancies), and four (0·3%) occurred in patients who had haematological and solid-organ malignancies together. In the remaining five (0·4%) cases, the underlying malignancy was not classifiable based on the information in the database.
None of the four patients with haematological and solid-organ malignancies occurring together had lung cancer, hence these patients were grouped with their respective haematological malignancy in the final condition-specific analysis. Ten patients with haematological malignancy (six with lymphoma, four with leukaemia) were not able to be classified further based on information available from the database – these patients were not included in the condition-specific analysis. In total, 84 cases were included in the final condition-specific analysis, comprised of 29 patients with lung cancer and 55 patients with classifiable haematological malignancy.
The condition-specific incidence rates of IPD identified during the study period are given in Table 1 and compared to rates of IPD in the general adult population aged ⩾18 years. There was an increased risk of IPD in all six malignancies compared to the remainder of the general adult population aged ⩾18 years. With respect to the haematological malignancies, the risk of IPD steadily increased from patients with lymphoma (4·4 times greater with Hodgkin's, 5·8 times greater with non-Hodgkin's) to leukaemia (11·9 times greater with AML or ALL, 12·6 times greater with CLL), and was highest in those with multiple myeloma (62·8 times greater). There was a 13·4 times greater risk of IPD in those with lung cancer.
Table 1. Rates of invasive pneumococcal disease (IPD) in patients with underlying malignancy, 2000–2004
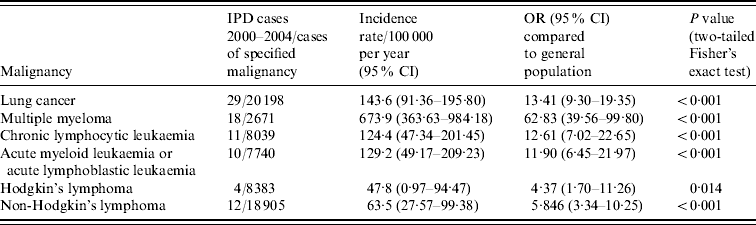
OR, Odds ratio; CI, confidence interval.
Pooled analysis of haematological malignancies including unclassifed cases
Given the number of unclassified haematological malignancies not included in the condition-specific analysis, we performed a pooled analysis which included these cases. Table 2 gives the results of a pooled analysis of all cases of lymphoma and leukaemia, including the 10 unclassified cases described above, as well as of all haematological malignancies together. The risk of IPD with any lymphoma was 7·5 times greater than the remainder of the general population aged ⩾18 years, which increased to 14·7 times greater with any leukaemia. A pooled analysis of all haematological malignancies including the 10 unclassifiable cases revealed an overall incidence rate of 142·1 cases/100 000 per year (95% CI 107·59–176·64), for a 13·6 times increased risk of IPD compared to the remainder of the adult population aged ⩾18 years (95% CI 10·63–17·50, P<0·001).
Table 2. Analysis of rates of invasive pneumococcal disease (IPD) in patients with haematological malignancies (including patients with unclassified haematological malignancies), 2000–2004
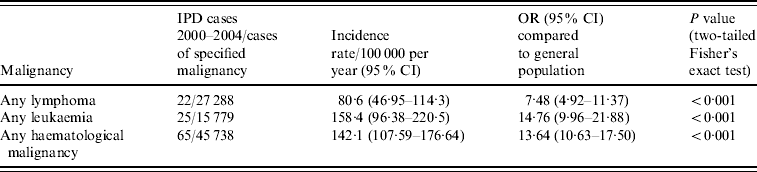
OR, Odds ratio; CI, confidence interval.
Demographic and clinical characteristics of patients with IPD and underlying malignancy
Selected demographic and clinical characteristics on the IPD cases in patients with underlying malignancy are provided in Table 3, organized by malignancy. Unclassified cases of lymphoma (six patients) and leukaemia (four patients) were also included. There were 26/29 (90%) patients with lung cancer and IPD that had a history of smoking, but in none of the other malignancies did rates of smoking exceed that of the general population aged ⩾18 years with IPD. Smoking history was not available in 19 cases. Eighty-five of the 94 (90%) patients had pneumococcal bacteraemia, eight had S. pneumoniae isolated from pleural fluid, and one had meningitis. Eighty-three of the 94 (88%) patients required admission to hospital, 10 of whom required admission to either an intensive care unit or coronary care unit setting, with the remaining 73 being admitted to a medical or surgical unit. Twenty-six of the 83 (31%) patients admitted to hospital died, with 20 (24%) of these cases being directly attributed to IPD or complications thereof. The remaining six patients who died were listed in the database as having died of other causes.
Table 3. Selected demographic and clinical characteristics of patients with invasive pneumococcal disease (IPD) and underlying malignancy
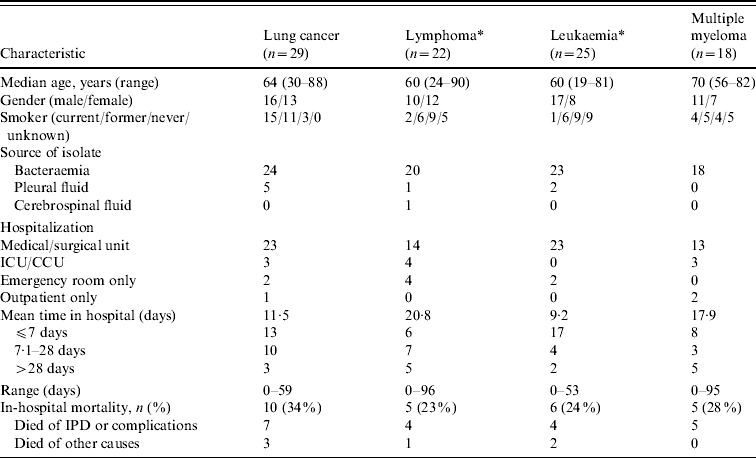
ICU/CCU, Intensive care unit/coronary care unit.
* Unclassified cases of lymphoma and leukaemia were included.
S. pneumoniae serotypes of isolates from cases of IPD with underlying malignancy
There was an increased prevalence of serotype 6A (11/84; 13% vs. 48/1273, 4%, OR 4·7, 95% CI 2·3–9·5, P<0·001) compared to the general adult population aged ⩾18 years, but no other serotypes predominated. Six cases were caused by serotype 9V and five cases by each of serotypes 3, 4, 6B, 19F, and 23F. Four cases were caused by each of serotypes 8, 22F, and 35B. Two cases were caused by each of serotypes 9N, 10A, 11A, 12F, 18C, 23A, 33F, and 38. A single case was caused by each of serotypes 1, 7F, 9L, 13, 14, 15A, 15B, 15C, 17F, 19A, 23B, and 35A. Serotype data was not available in two cases.
Twenty-nine (35%) cases were caused by serotypes in the 7-valent conjugate vaccine (PCV7, Prevnar®, Wyeth, USA), 31 (37%) cases were caused by serotypes in the 10-valent conjugate vaccine (PCV10, Synflorix®, GlaxoSmithKline, USA) and 48 (57%) cases were caused by serotypes in the 13-valent conjugate vaccine (PCV13, Prevnar 13). Fifty-seven (69%) cases were caused by serotypes in the 23-valent polysaccharide vaccine (PPV23, Pneumovax® 23, Merck, USA).
DISCUSSION
A 2005 study from the USA estimated the incidence of IPD in patients with haematological malignancy at 503·1 cases/100 000 per year (95% CI 272·6–334·6) and for solid-organ malignancy at 300·4 cases/100 000 per year (95% CI 422·2–622·3). There was a 38·3 times greater risk for patients with haematological malignancy and a 22·9 times greater risk for those with solid-organ malignancy compared to that of healthy adults [Reference Kyaw9]. Data from Scotland revealed increased rates of IPD in patients with haematological malignancy (733·7 cases/100 000 per year) and non-haematological malignancy (216·1 cases/100 000 per year) [Reference Kyaw10]. Data on the incidence of IPD in adults with specific underlying malignancies is limited, although a recent study from Germany found a 10 times greater risk of IPD in children with ALL [Reference Meisel17].
In our 5-year retrospective analysis, we identified 29 cases of IPD in patients with lung cancer in the province of Alberta between 2000 and 2004, a 13·4 times greater incidence of IPD compared to the remainder of the adult population aged ⩾18 years. If lung cancer is considered a surrogate for solid-organ malignancy, the incidence rate and odds ratio calculated in our study are lower than results previously estimated for solid-organ malignancies [Reference Kyaw9, Reference Kyaw10].
In our study, the highest rates of IPD were found in patients with multiple myeloma, who had a 62·8 times greater risk compared to the remainder of the general population aged ⩾18 years. Patients with multiple myeloma have been well-described as being at increased risk of bacterial infections with both Gram-positive and Gram-negative organisms, although the reasons for this have not been clearly elucidated [Reference Salonen and Nikoskelainen18]. Multiple myeloma leads to defects in complement activation and neutrophil function, as well as functional hypogammaglobulinaemia [Reference Jacobson and Zolla-Pazner19]. Decreased CD4 counts and decreased CD4/CD8 ratios have also been found in patients with multiple myeloma [Reference Pilarski20]. More modestly increased rates of IPD were found in patients with other haematological malignancies including lymphoma, CLL, and AML/ALL. The overall rate in patients with underlying haematological malignancy of 142·1 cases/100 000 per year is less than has been previously reported [Reference Kyaw9, Reference Kyaw10].
Serotype 6A, which was the only serotype to have an increased prevalence compared to the general adult population, is one of the six serotypes included in PCV13 but not in PCV7 or PCV10. Overall, 19/152 (12·5%) cases of IPD in patients with underlying malignancy were caused by one of the six serotypes included in PCV13 but not PCV7 or PCV10. The use of PCV13 directly in this high-risk population as well as in children may confer beneficial protective effects against IPD in patients with underlying malignancy via both direct and indirect (‘herd’) effects [Reference Musher21].
There are limitations to our study. We only captured patients with a positive isolate, and it is possible that cases of IPD were missed if cultures were not done or if they were negative (e.g. if drawn after the administration of antibiotics). Mortality rates reflect patients who died in hospital only. We relied on documentation of malignancy on the patient's chart, and it is possible that cases were missed because a patient's medical history was not documented. In some cases not enough detail was provided in the chart (i.e. only ‘lymphoma’ or ‘leukaemia’ were reported) to classify patients into the appropriate condition-specific groups for analysis. Hence, our study provides a minimal estimate of the risk of IPD and the actual risk may be higher. Pneumococcal vaccination status of patients could not be accounted for in our study. Age and smoking status may have confounded results, as may have other factors not accounted for in this study.
Our study reinforces that the risk of IPD is significantly increased in lung cancer and various haematological malignancies, and a systemic PPV23 vaccination strategy among those providers who care for these patients should be considered. Evaluation of an expanded valency conjugate vaccine in this high-risk population via prospective studies is warranted.
ACKNOWLEDGEMENTS
We acknowledge the staff of the Acute Diagnostic Microbiology Laboratories in Alberta who submitted isolates from cases of IPD to the National Centre for Streptococcus, Edmonton, Alberta. We acknowledge the contributions of Carol Mangan, Stephanie Hui, Linda Hastie, and the efforts of the Data Collection Team who include Anne Witschen, Lynne Korobanik, Freda Anderson, Loy Bacon, Shannon Pyra, Janine Schouten, Ambreen Mithani, and Natalie Chui. We also thank Janice Pitchko for designing and maintaining the study databases and Heather Mangan for her assistance with the database.
The procurement of the laboratory data for this work was supported by Alberta Health & Wellness (Edmonton, AB) and the National Microbiology Laboratory (Winnipeg, MB). This research was funded by the Provincial Laboratory for Public Health (Edmonton, AB) and a grant-in-aid from Wyeth Canada (Toronto, ON). The funders had no role in the study design or implementation, interpretation of data, analysis or manuscript preparation.
DECLARATION OF INTEREST
T.J.M., J.D.K., and G.J.T. have received compensation from Wyeth for consulting and speaking about pneumococcal vaccination, and financial support from Wyeth for performing epidemiological analyses of cases of invasive pneumococcal disease in the province of Alberta.





