INTRODUCTION
Haemolytic uraemic syndrome (HUS) and thrombotic thrombocytopaenic purpura (TTP) are rare disorders characterized by microangiopathic haemolytic anaemia, microthrombi, and multi-organ injury. HUS is one of the most common causes of acute renal failure in childhood worldwide [Reference Williams1]. Occasionally, cases may experience long-term renal impairment, and the determinants of long-term outcomes are unclear [Reference Siegler2, Reference Garg3]. HUS is a subset of thrombotic microangiopathy that has a number of aetiologies including several infectious agents, the most common of these being Escherichia coli O157 [Reference Tarr, Gordon and Chandler4]. Previous surveillance of childhood HUS in Scotland identified E. coli O157 in over 90% of cases [Reference Lynn5]. Clinical surveillance is particularly relevant in Scotland, where consistently higher rates of infection with E. coli O157 have been reported than in other parts of the United Kingdom or Europe (Fig.).
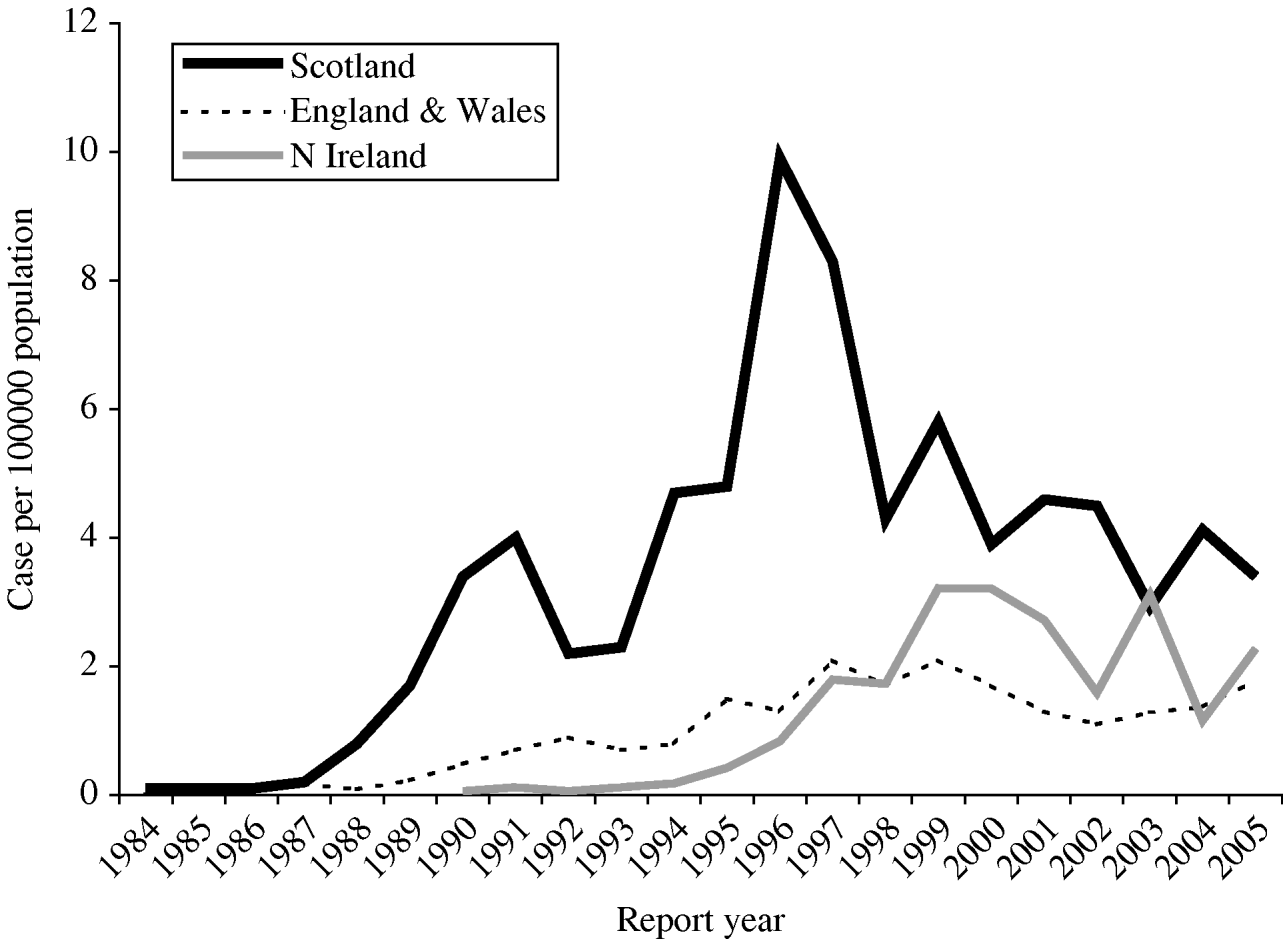
Fig. Incidence of E. coli O157 infection in Scotland compared with England and Northern Ireland (1984–2005). [Data out-with Scotland courtesy of Health Protection Agency: CDSC Northern Ireland and Laboratory of Enteric Pathogens/CDSC Colindale (E&W).]
Whereas childhood HUS most often follows bloody diarrhoea caused by verocytotoxin-producing E. coli (VTEC), TTP frequently occurs in adults with a number of possible identifiable precipitants. It is generally accepted that the pathogenesis of TTP involves an acquired inhibitor, or congenital absence, of the von Willebrand factor metalloprotease [Reference Tsai and Lian6, Reference Furlan7]. This protease was recently identified as a member of the ADAMTS family, namely ADAMTS13 [Reference Levy8]. In contrast, patients with VTEC-associated HUS have normal ADAMTS13 activity [Reference Tsai9]. There has been much debate as to whether TTP and HUS are two distinct entities or the same disease along a clinical spectrum [Reference Remuzzi10–Reference Dlott12]. Although the cause of thrombotic microangiopathy might not be immediately apparent when patients present, the term HUS/TTP is inexplicit and does not encompass all forms of thrombotic microangiopathies.
Here, we report a population-based epidemiological surveillance study of thrombotic microangiopathies in all age groups, with particular emphasis on the links between these syndromes and precipitants of HUS and TTP, including infections, vascular procedures, and medications [Reference Sadler13]. Furthermore, we sought to identify outcomes and describe management strategies by clinicians. Without current surveillance of HUS and TTP, neither incidence nor outcomes are well established in adults or children.
METHODS
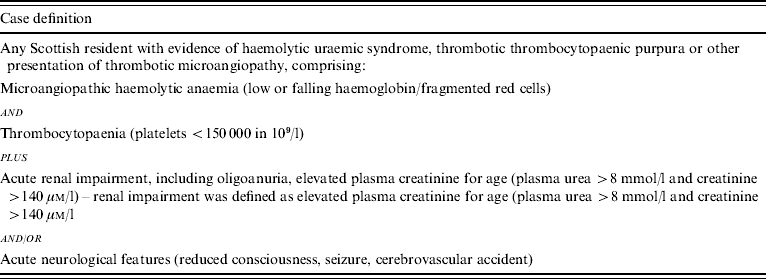
Cases were ascertained prospectively over 3 years of an active, continuing national surveillance programme in 2003. Consultants from six hospitals throughout Scotland were approached for participation in the study. Consultants in haematology, infectious diseases, microbiology, nephrology and paediatrics were sent fortnightly e-mails with the case definition as in Table 1, and asked to indicate whether they had a ‘case of thrombotic microangiopathy to report’ or ‘nil return’. Further information on possible cases was obtained by telephone including date of onset, gender, age, hospital and possible public health implications (e.g. infection with VTEC). A unique study number was created to maintain subject anonymity. Questionnaires, information sheets and consent forms were subsequently sent to the reporting clinician via post or e-mail. All completed forms and questionnaires were returned to Health Protection Scotland (HPS) and the data were entered into an Epi-Info (Version 6) database (CDC, Atlanta, GA, USA).
Table 1. Case definition for thrombotic microangiopathy
Statistical methods
The statistical significances of associations between categorical variables were investigated using χ2 or Fisher's exact tests. Normally distributed quantitative variables were compared using t tests with results displayed as mean (±s.e.m.). All analyses were performed using SPSS version 11 (SPSS Inc., Chicago, IL, USA) with a significance level of 5%.
RESULTS
Between 1 January 2003 and 31 December 2005, 110 reports of thrombotic microangiopathy were provided to HPS, of which 73 were designated by the reporting clinicians as HUS and 37 as TTP. These figures correspond to respective average annual incidences of 0·47 and 0·24 cases per 100 000. Clinicians also reported three patients with thrombocytopaenia but there was no renal impairment or neurological sequelae involved and upon recommendation by the relevant clinician, it was decided not to include these as cases of thrombotic microangiopathy. Forty-seven cases were male, and there was a significant association between gender and diagnosis of thrombotic microangiopathy (χ2, P=0·021). More males than expected had HUS and more females than expected had TTP. There was a significant age-related difference in type of thrombotic microangiopathy with HUS patients having a mean age of 18 (±2·6) years compared with TTP patients having a mean age of 48 (±3·3) years (χ2, P<0·001). Geographical origin as determined by NHS Board of residence was investigated as a predisposing factor to which type of thrombotic microangiopathy developed in the patient cohort. Rural and urban areas have been defined elsewhere based on standardized incidence rates (SIR) for zoonotic infection including E. coli O157 [Reference Stewart14]. Rural residents were more likely to be diagnosed with HUS, while urban residents were more likely to be diagnosed with TTP (P=0·018).
Predisposing factors for development of thrombotic microangiopathies
Table 2 demonstrates that, in this survey, illnesses designated as HUS are primarily associated with E. coli O157 infection and this association is highly significant (60/73, P<0·001). Other causes of HUS included VTEC of non-O157 serotype (serotypes O177, O145 and O-unidentifiable, one patient each). Diarrhoea-positive HUS was also seen in five other patients and while no pathogen was identified, the symptoms strongly suggested VTEC infection (bloody diarrhoea and abdominal cramps with no fever reported). One case of atypical HUS was reported where the predisposing factors considered to be clinically significant were infection with parvovirus B19 and recent immunization with the MMR vaccine. However, this patient was subsequently diagnosed as having severe combined immune deficiency.
Table 2. Predisposing factors in development of haemolytic uraemic syndrome (HUS) or thrombotic thrombocytopaenic purpura (TTP) – χ2 analyses performed on predisposing factors and development of thrombotic microangiopathy
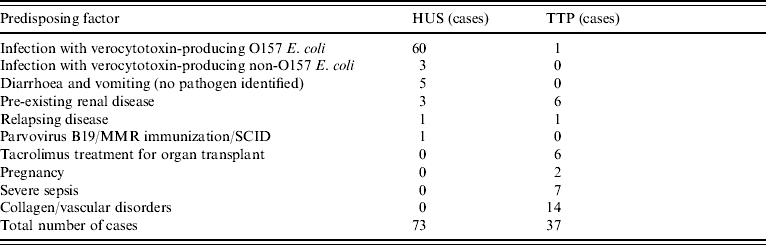
Identified predisposing factors for a diagnosis of TTP were more diverse. Unlike HUS, prior E. coli O157 infection was not significantly associated with TTP, this being identified in only one case. Moreover, this patient was on long-term aspirin treatment for pre-existing vascular disease. Overall, development of TTP was significantly associated with collagen/vascular conditions (14/37 cases, P<0·001). There was also significant association with severe sepsis and the development of TTP (7/37 cases, P<0·005).
Clinical parameters
A range of clinical parameters available at time of admission, are detailed in Table 3. Patients diagnosed as having HUS had significantly shorter hospital stays [14 (±1·5) days] than those with TTP [21 (±3) days] (P<0·02). Length of oliguria (defined as urine output <0·5 ml/kg per hour for 24 consecutive hours) was significantly associated with post-discharge or chronic renal impairment (P=0·020) and this was true for both HUS and TTP.
Table 3. Univariate analysis of clinical markers for haemolytic uraemic syndrome (HUS) and thrombotic thrombocytopaenic purpura (TTP)
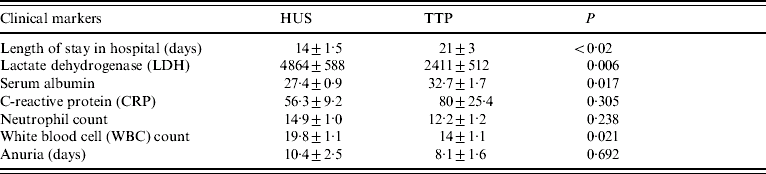
Values are mean±s.e.m.; significance relates to difference between thrombotic microangiopathy.
Treatment and outcomes
Patients with thrombotic microangiopathy were assessed for treatment regimens employed and for subsequent outcomes (Table 4). Treatment was not standardized throughout Scotland but there was a significant association between HUS and use of peritoneal dialysis (P=0·011). Other treatments for HUS included plasma exchange, haemodialysis, haemofiltration and steroid administration. Treatment for TTP consisted of plasma exchange and/or steroid administration and there was a strong association with these particular methods of treatment and diagnosis of TTP (both P<0·001).
Table 4. Treatment regimens and outcomes for haemolytic uraemic syndrome (HUS) and thrombotic thrombocytopaenic purpura (TTP)
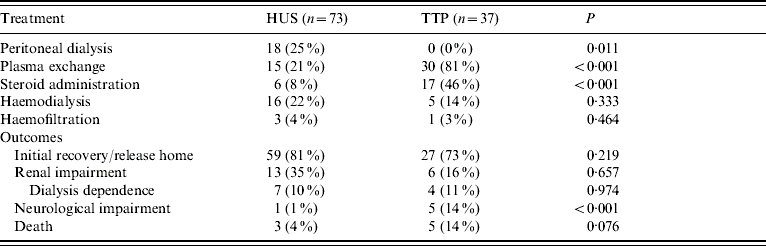
Significance relates to difference between thrombotic microangiopathy.
Of 110 patients diagnosed with thrombotic microangiopathy, 86 were discharged directly to home without dialysis. Eight patients died, while 19 patients had some form of renal impairment on discharge, with 11 of those 19 requiring long-term, post-discharge dialysis. There was no association between diagnosis of thrombotic microangiopathy and outcome. Patients were followed up for up to 3 years (K. G. J. Pollock, unpublished data).
DISCUSSION
Recent studies have contributed greatly to our current understanding of the molecular mechanisms leading to HUS and TTP [Reference Garg3, Reference Sadler13, Reference Ake15]. However, prompt diagnosis and relevant therapy still remain challenging because of the clinical similarities in these disorders. The study sought to extend current knowledge by monitoring all age groups with thrombotic microangiopathies, as well as the extent of clinical presentations of these disorders. This study provides a range of data including epidemiology, demography, clinical parameters, treatment regimens and outcomes, and demonstrates that clinical surveillance of thrombotic microangiopathies is an efficient and complementary approach to VTEC surveillance (which includes E. coli O157). While children are more likely to develop VTEC-associated HUS, cases do occur in the adult population and the similarity of presentation in adults and children with VTEC-associated HUS was high, with most (90%) HUS patients presenting with classic VTEC clinical features of bloody diarrhoea and abdominal pain. Clinical surveillance confirms that VTEC remains the most common cause of microangiopathy in Scotland and reinforces the need to identify and treat cases of VTEC sooner than we are managing to achieve at present.
In contrast to the predisposing factors associated with HUS (predominantly E. coli O157), patients with TTP had diverse predisposing factors and/or infections (particularly those with severe sepsis). The symptoms preceding HUS or TTP were also quite different and this suggests that while TTP and HUS share some features and on rare occasion can be difficult to distinguish, they are, in fact, two quite different entities with different pathophysiologies. This observation is in accordance with other studies [Reference Hosler, Cusumano and Hutchins11, Reference Marques, Mayfield and Blackall16]. Also, the majority of clinical markers and treatments employed for the two syndromes are quite distinct. For these reasons, we believe that the term HUS/TTP is imprecise and should be abandoned because current evidence indicates that TTP and HUS differ in pathogenesis and they do not encompass all forms of thrombotic microangiopathies. When the underlying cause of disease is uncertain, the appellation thrombotic microangiopathy is sufficient, appropriate and preferable.
HUS patients were routinely treated with peritoneal dialysis or haemodialysis, although in some instances, treatment involved only careful monitoring and parenteral volume expansion. A recent study encouraged the hospitalization of children infected with E. coli O157 and administration of intravenous isotonic fluids, as soon as possible in illness, even before microbiological culture results were known [Reference Ake15]. This volume expansion was associated with relative renal protection, as shown by the association between parenteral volume expansion before the development of HUS and attenuated renal injury during HUS. Interestingly, children who present early in illness during VTEC infections have higher rates of developing HUS [Reference Wong17] possibly because they have more severe or fulminant vascular injury. However, infected children who seek medical attention early in illness present a paradoxical opportunity: while they are more likely to develop HUS, they also stand to benefit from an opportunity to receive prompt hospital treatment well before renal damage ensues. The potential value of recognizing, and volume expanding such children early in illness should not be overlooked.
Patients with TTP were routinely treated with plasma exchange and/or steroids, as is common practice in other centres [Reference Marques, Mayfield and Blackall16, Reference Wong17]. The mortality rate from TTP has decreased significantly since plasma exchange was introduced [Reference Vesely18, Reference Bell19], although in our cohort 14% of TTP cases did not respond to plasma exchange and subsequently died. Measurement of ADAMTS13 activity may be useful for early diagnosis, with severe ADAMTS13 deficiency appearing to be specific for TTP [Reference Tsai and Lian6]. In particular, the discrimination of TTP from HUS, especially in adults, can be an urgent issue for treatment because most diarrhoea-positive HUS cases are associated with VTEC. Recent advances in assay methods should facilitate laboratory testing of ADAMTS13 for patients with thrombotic microangiopathy. Ideally, all patients in the study diagnosed with TTP would have had plasma tested for ADAMTS13 levels to confirm a diagnosis of TTP but this was not feasible at all Scottish centres. However, a clinical diagnosis of TTP has been made in other studies without the benefit of such assays [Reference Zakarija20].
While the case definition for thrombotic microangiopathy is sensitive and probably identifies most thrombotic microangiopathies in Scotland, one of the main limitations of the study is the probable under-reporting of cancer-associated TTP. Only one case has been reported in 3 years and this probably represents a fraction of the actual numbers for several reasons. Clinicians who specialize in this area are less likely to report thrombotic microangiopathy, where malignancy is terminal or highly advanced and would also be less likely to seek consent for participation in the study. We did not target such specialists and in this respect, we may have biased the clinician sample. One of the main confounding factors for the diagnosis of TTP is age; specifically, patients with thrombotic microangiopathy who are older are more likely to be on anticoagulant therapy for vascular conditions or have other chronic problems and this partly explains the association between age and being diagnosed with TTP caused by endothelial damage incurred over the life experience.
Future surveillance and follow-up questionnaires in this cohort could identify cases that recover initially but subsequently develop occult nephropathy or neurological problems. Follow-up studies have produced conflicting results with regard to renal outcome after an episode of thrombotic microangiopathy [Reference Brandt21, Reference Van Dyck, Proesmans and Depraetere22]. Brandt and colleagues prospectively studied a cohort of individuals following an outbreak of VTEC-associated HUS in Washington State and found that cholelithiasis and other gastrointestinal sequelae were associated with HUS [Reference Brandt21]. Furthermore, renal sequelae were identified in 35% of the cohort but the follow-up period was limited to only 3 years and the short follow-up period may underestimate the extent and severity of eventual renal sequelae. In a study looking at recovery after diarrhoea-associated HUS, 31% of children had an increased urinary albumin excretion, 18% had a reduced glomerular filtration rate and 10% had both, in association with a higher systolic blood pressure, indicating a significant nephropathy in this group [Reference Fitzpatrick, Shah and Trompeter23]. However, in the Scottish experience, the outcome for children with VTEC-associated HUS is much better than that quoted above and this corroborates the outcome experience of international colleagues [Reference Siegler2, Reference Garg3, Reference Garg24]. Subsets of patients with HUS should be particularly scrutinized for chronic renal sequelae rates, particularly those in whom anuria occurs.
ACKNOWLEDGEMENTS
Professor Phillip Tarr, Washington University of St Louis, MO, USA is thanked for helpful discussion and editorial comments. The Scottish HUS steering group is also thanked for helpful discussion.
DECLARATION OF INTEREST
None.







