In recent decades, a number of studies have examined the comorbidity of substance use and mental disorders. Reference Smart, Hayes, Sanson and Toumbourou1–Reference Siegfried4 One particular comorbidity that has been examined in some detail is the association between cigarette smoking and major depression, with a number of epidemiological studies showing that cigarette smoking is frequently comorbid with depression. Reference Rohde, Kahler, Lewinsohn and Brown5–Reference Patton, Hibbert, Rosier, Carlin, Caust and Bowes22 The apparent comorbidity between cigarette smoking and depression may be explained in at least two ways. First, it could be argued that both smoking and depression are caused by common underlying genetic and environmental factors. Evidence for this position has been provided by studies that have demonstrated shared genetic and environmental influences in the comorbidity of smoking and depression. Reference Rohde, Kahler, Lewinsohn and Brown5,Reference Haarasilta, Marttunen, Kaprio and Aro6,Reference Hu, Davies and Kandel8,Reference Mykletun, Overland, Aaro, Liabo and Stewart10,Reference Munafo, Hitsman, Rende, Metcalfe and Niaura15,Reference Fergusson, Goodwin and Horwood17,Reference Kendler, Neale, MacLean, Heath, Eaves and Kessler19,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21 However, a number of these studies have also found evidence for residual associations between cigarette smoking and depression after controlling for shared genetic and environmental influence, suggesting that there may be an association between the two that cannot be accounted for by common confounding factors. Reference Haarasilta, Marttunen, Kaprio and Aro6,Reference Hu, Davies and Kandel8,Reference Mykletun, Overland, Aaro, Liabo and Stewart10,Reference Munafo, Hitsman, Rende, Metcalfe and Niaura15,Reference Fergusson, Goodwin and Horwood17,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21
Second, it could also be argued that cigarette smoking and depression are related in a causal manner, such that either: depression increases the risks of smoking; or smoking increases the risks of depression. Indeed, several studies have argued that cigarette smoking may arise or increase as a result of self-medication of depressive symptoms. Reference Breslau, Kilbey and Andreski18,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21–Reference Lerman, Caporaso, Main, Audrain, Boyd and Bowman25 On the other hand, further studies have found evidence that smoking increases the risks of depression. Reference Steuber and Danner12,Reference Klungsoyr, Nygard, Sorensen and Sandanger14–Reference Pasco, Williams, Jacka, Ng, Henry and Nicholson16,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21
In a previous paper Reference Fergusson, Boden and Horwood26 we have developed a general approach to examining the comorbidity of disorders using a two-stage analytic strategy. In the first stage, fixed-effects regression models were used to control the associations between comorbid conditions, such as smoking and depression, for common, non-observed sources of confounding. These sources of confounding included all non-observed genes and environment, as well as time-dynamic confounding factors. In the second stage, structural equation modelling methods were used to model reciprocal pathways between the comorbid conditions. The present study employs a similar approach, described below.
Although it is often believed that epidemiological research can only control for the effects of observed confounders, this is not strictly correct and there are a number of analytic approaches that permit the control of non-observed confounders in non-experimental research. The best known of these is the discordant twin design in which monozygotic twins who are discordant for some exposure variable (e.g. smoking) are compared on an outcome measure (e.g. depression). Since the twin pairs share both common genes and common environment this comparison controls for these factors. Reference Kendler, Neale, MacLean, Heath, Eaves and Kessler19,Reference Kendler27
The principles underlying the discordant twin design can also be applied to longitudinal data on singletons via the fixed-effects regression model. Subject to the availability of longitudinal data, it proves possible to estimate the associations between a time-varying exposure variable (such as smoking) and a time-varying outcome measure (such as depression) net of any non-observed fixed factors that are associated with the outcome and that may be correlated with the exposure variable. In effect, this model makes it possible to eliminate one major source of confounding from fixed factors. Reference Hamerle, Ronning, Arminger, Clogg and Sobel28,Reference Hausman, Hall and Griliches29 However, the model does not address the issue of confounders that may vary over time and to control for such confounding, the fixed-effects model needs to be augmented by observed time-dynamic confounding factors.
Establishing that smoking and depression are related even following control for confounding is an important step in ascertaining a causal relationship between smoking and depression. However, such analysis does not resolve the issue of the direction of causality: even with well-collected longitudinal data, establishing which factor is antecedent and which factor is consequent proves difficult. Reference Breslau, Kilbey and Andreski18 Furthermore, there is a possibility that smoking and depression are reciprocally related to each other by a feedback loop in which smoking increases risks of depression, while at the same time the onset of depression leads to an increased consumption of tobacco. Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21 Structural equation models provide one means to address this issue by devising statistical models that permit reciprocal relationships between indices of smoking and depression and using these models to provide a guide to likely patterns of causation. Reference Fergusson, Goodwin and Horwood17,Reference Kendler, Gardner and Prescott30
In this paper we address these issues using data gathered over the course of a longitudinal study in which measures of depression and smoking were obtained at regular intervals. In the first stage of the analyses fixed-effects regression modelling was used to control the associations between smoking and depression for common confounding factors including non-observed common genes and environment. In the second stage, methods of structural equation modelling were used to explore the direction of causality.
Method
Participants
The data were gathered during the course of the Christchurch Health and Development Study (CHDS). In this study a birth cohort of 1265 children (635 males, 630 females) born in the Christchurch (New Zealand) urban region in mid-1977 has been studied at birth, 4 months, 1 year and annually to age 16 years, and again at ages 18, 21 and 25 years. Reference Fergusson and Horwood31,Reference Fergusson, Horwood, Shannon and Lawton32 All study information was collected on the basis of signed consent from study participants.
Symptoms of major depression, ages 18, 21 and 25
At ages 18, 21 and 25 years, study participants were interviewed on a structured mental health interview designed to assess aspects of mental health and psychosocial adjustment. In these assessments, items from the Composite International Diagnostic Interview (CIDI) 33 pertaining to major depression were used to assess DSM–IV 34 symptom criteria for major depression, using the full range of questions pertaining to depressive symptoms, over the previous 12 months. For the purposes of the present analyses, the dichotomous responses (yes; no) to each individual symptom item in the symptom reports were summed at ages 18, 21, and 25 to create a measure of the total number of symptoms of depression reported during the year prior to each assessment.
In addition, for the purposes of examining the robustness of the data to alternative methods of measurement using structural equation modelling, participants were classified on three-level ordinal scale measures reflecting the severity of depression symptomatology in each year. This scale was: 0, had no depressive symptoms; 1, had depressive symptoms but did not meet criteria for major depression; 2, met criteria for major depression. At age 18, 74.5% of participants reported no symptoms; 7.2% reported some symptoms; and 18.3% met criteria for major depression; at age 21, 73.6% of participants reported no symptoms; 8.2% reported some symptoms; and 18.2% met criteria for major depression; and at age 25, 79.3% of participants reported no symptoms; 7.0% reported some symptoms; and 13.7% met criteria for major depression.
Cigarette smoking, ages 18, 21 and 25
At ages 18, 21 and 25, participants were questioned as to the frequency with which they had smoked cigarettes during the month prior to the assessment. Those who reported smoking were further questioned using custom-written survey items to assess DSM–IV symptom criteria for nicotine dependence (see online supplement). For the purposes of the present analyses, the responses to each individual symptom item in the symptom reports were summed for ages 18, 21 and 25 to create a measure of the total number of definite symptoms of nicotine dependence reported during the month prior to each of the three assessments. All non-smokers at each age period received scores of no symptoms on the measures of nicotine-dependence symptoms.
In addition, for the purposes of examining the robustness of the data to alternative methods of measurement using structural equation modelling, participants were classified on three-level ordinal scale measures reflecting the severity of nicotine-dependence symptomatology in each year. This scale was: 0, had no nicotine dependence symptoms; 1, had nicotine-dependence symptoms but did not meet criteria for nicotine dependence; 2, met criteria for nicotine dependence. At age 18, 83.0% of participants reported no symptoms; 3.1% reported some symptoms; and 13.9% met criteria for nicotine dependence; at age 21, 63.1% of participants reported no symptoms; 12.4% reported some symptoms; and 24.5% met criteria for nicotine dependence; and at age 25, 65.1% of participants reported no symptoms; 11.9% reported some symptoms; and 23.0% met criteria for nicotine dependence.
In addition, for the purposes of examining whether the findings of the study were robust to alternative methods of measuring nicotine intake, the smoking frequency data described above were used to create a six-point measure reflecting the number of cigarettes participants reported smoking per day. The categories were: non-smoker; <1 per day; 1–4 per day; 5–9 per day; 10–20 per day; and 21 plus per day.
Time-dynamic covariate factors
The following time-dynamic covariate factors were chosen from the database of the study on the basis of: their associations with symptoms of either nicotine dependence or depression in preliminary analyses; and previous research examining smoking and depression among the present cohort. Reference Fergusson, Goodwin and Horwood17,Reference Fergusson, Lynskey and Horwood35 These covariate factors included the following, each assessed during the periods 17–18, 20–21 and 24–25 years.
Stressful life events were assessed by responses to items from the Feeling Bad Scale Reference Lewis, Seigel and Lewis36 and custom-written survey items to provide an index of the number of stressful life events during each year. Anxiety disorder at ages 18, 21 and 25 was measured via items from the (CIDI) 33 to assess DSM–IV symptom criteria for a range of anxiety disorders. Cannabis use was assessed at each year by questioning the frequency with which participants had used cannabis during each year since the previous assessment. Other illicit drug use was assessed at each year by questioning participants whether they had used illicit drugs other than cannabis during each year since the previous assessment. Affiliation with ‘deviant’ peers was assessed via questions pertaining to friends’ use of alcohol, tobacco or other illicit drugs, or involvement in criminal activity, problems with aggressive behaviour or being in trouble with the law, in the year prior to each assessment. Unemployment was assessed by asking participants about their experience of unemployment in each year and classified into four levels reflecting the duration of unemployment in the year. Partner substance use and criminal offending was assessed on the basis of participant reports of the extent to which their partner: used tobacco, alcohol or illicit drugs or had problems resulting from alcohol or illicit drugs; and engaged in criminal offending, had problems with aggressive behaviour or were in trouble with the law, in the year prior to each assessment.
Statistical methods
Associations between smoking and depression.
In the first stage of the analysis the association between symptoms of nicotine dependence and depression in each year was assessed using a Poisson regression model in which the rates of depressive symptoms were modelled as a log-linear function of nicotine dependence symptoms. In each case the significance of the association was assessed using the Wald chi-squared statistic for the effect of nicotine-dependence symptoms from the fitted model. In addition, the pooled association between nicotine-dependence symptoms and depressive symptoms was estimated via generalised estimating equation methods Reference Liang and Zeger37,Reference Zeger and Liang38 to fit a population-averaged regression model in which the rate of depressive symptoms at each of the three time periods was modelled as a log-linear function of nicotine-dependence symptoms during each time period. This model was of the form:
where log(Y it ) was the log rate of depressive symptoms reported by the ith subject in a given interval t, and X it represented nicotine-dependence symptoms during the interval t. In this model observations from the same individual over time were permitted to be correlated with an unstructured correlation matrix. In addition, the associations were modelled using depressive symptoms as the exposure variable, and nicotine-dependence symptoms as the outcome variable. All models were fitted using Stata 8.0 for Windows. From the fitted models, estimates of the incidence rate ratio (IRR) and 95% confidence intervals were calculated.
In order to examine the robustness of the data to alternative measures of modelling nicotine intake, further models of the form described above were fitted in which the measure of nicotine dependence was substituted by a measure of daily cigarette-smoking frequency.
Fixed-effects model for covariate adjustment
To adjust the associations between smoking and depression for unobserved fixed and observed time-dynamic confounding factors, a conditional fixed-effects Poisson regression model was fitted to the joint data over the three measurement periods. This model was of the form:
In this model the α i are individual specific terms that are assumed to reflect the effects of all fixed sources of variation in the outcome Y it , and Z ikt are the set of observed time-dynamic covariates. The fixed effects α i are assumed to be constant over time and to be correlated with other predictors in the model. The major advantage of the fixed-effects model is that it can adjust for all sources of fixed covariate effects, including non-observed fixed confounders. Reference Hamerle, Ronning, Arminger, Clogg and Sobel28
In addition to the model described above, the fixed-effects models were also fitted using depressive symptoms as the exposure variable, and nicotine-dependence symptoms as the outcome variable. Also, in order to examine the robustness of the data to alternative measures of modelling nicotine intake, further models of the form described above were fitted in which the measure of nicotine dependence was substituted by a measure of daily cigarette smoking frequency.
Structural equation modelling
To explore issues of causal direction, a series of structural equation models was fitted to the symptom count measures of depression and nicotine dependence observed for the three intervals 17–18, 20–21 and 24–25 years. These models incorporated both fixed effects influencing the measures of depression and smoking over time and the potential to examine both unidirectional and reciprocal effects between depression and smoking within time intervals.
Figure 1 depicts a model with reciprocal causal effects between depression and smoking. This model assumed that:
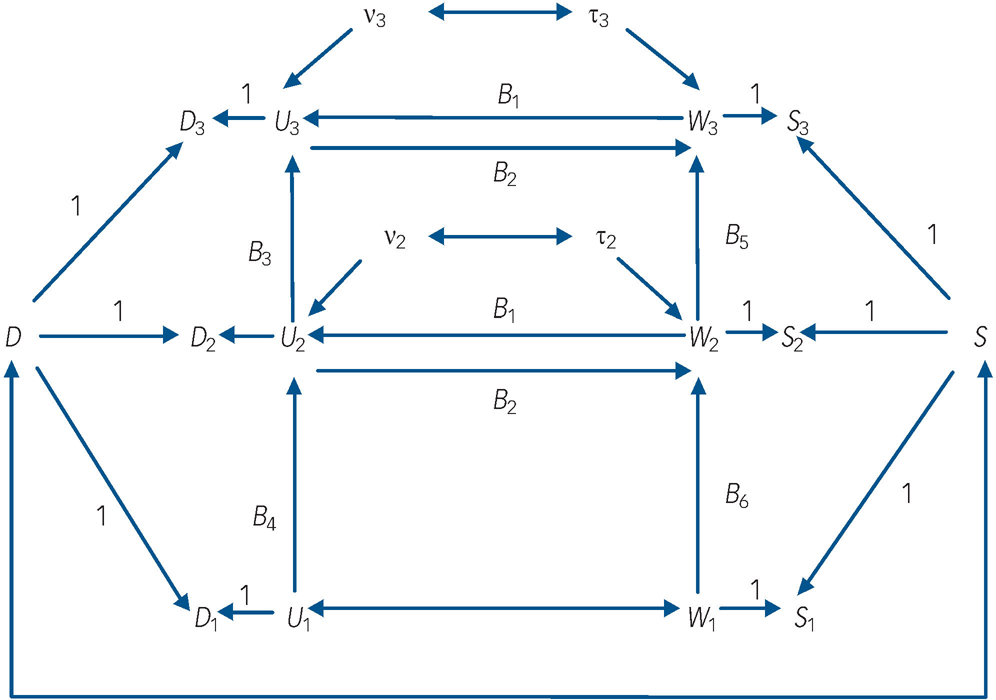
Fig. 1 Autoregressive model of depression and nicotine-dependence symptomatology incorporating fixed effects and reciprocal paths between time-dynamic components of depression and nicotine-dependence symptoms.
Dt , depression symptoms at time t; St , nicotine-dependence symptoms at time t; D, fixed-effects component of Dt ; S, fixed-effects component of St ; Ut , time-dynamic component of Dt ; Wt , time-dynamic component of St ; ν, disturbance term for Ut , τ, disturbance term for Wt .
-
(a) the observed symptom measures of depression (denoted D t , t = 1, 2, 3) at ages 17–18 (t = 1), 20–21 (t = 2) and 24–25 (t = 3) were influenced by fixed sources of variation (D) that were constant over time and by time-dynamic sources of variation (U t );
-
(b) the observed symptom measures of nicotine dependence (denoted S t , t = 1, 2, 3) were also influenced by fixed sources of variation (S) that were constant over time and time-dynamic sources of variation (W t );
-
(c) the fixed factors D and S were permitted to be correlated;
-
(d) the time-dynamic components of depressive symptoms (U t ) and nicotine-dependence symptoms (W t ) were linked by autoregressive processes in which past depressive symptoms predicted future depressive symptoms, and past nicotine-dependence symptoms predicted future nicotine-dependence symptoms respectively;
-
(e) the time-dynamic components of depression and nicotine-dependence symptoms were reciprocally related at t = 2, 3 so that current U t influenced current W t and vice versa. These reciprocal effects were assumed to be constant over time;
-
(f) the time-dynamic components U 1 and W 1 were assumed to be correlated rather than reciprocally related in order to assist with model identifiability.
In this model the fixed effects (D, S) are latent variables that summarise the net effect of all non-observed fixed factors that exert a constant effect on the measures of depressive symptoms and nicotine-dependence symptoms respectively over time. These factors include all childhood, family and personal characteristics that have a fixed effect on outcomes over time, and thus may include both genetic and environmental influences. The time-dynamic components of the model (U t , W t ) represent the effect of all other sources of variance in depressive symptoms and nicotine-dependence symptoms respectively that are not solely due to fixed factors. The equations defining this model were as follows:
model equations:
 \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \[\ \begin{array}{rl}&D_{t}=D+U_{t}(t=1,2,3);S_{t}=S+W_{t}{\ }(t=1,2,3)\\&U_{3}=B_{1}W_{3}+B_{3}U_{2}+v_{3};W_{3}=B_{2}U_{3}+B_{5}W_{2}+{\tau}_{3}\\&U_{2}=B_{1}W_{2}+B_{4}U_{1}+v_{2};W_{2}=B_{2}U_{2}+B_{6}W_{1}+{\tau}_{2}\end{array}\ \] \end{document}
\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \[\ \begin{array}{rl}&D_{t}=D+U_{t}(t=1,2,3);S_{t}=S+W_{t}{\ }(t=1,2,3)\\&U_{3}=B_{1}W_{3}+B_{3}U_{2}+v_{3};W_{3}=B_{2}U_{3}+B_{5}W_{2}+{\tau}_{3}\\&U_{2}=B_{1}W_{2}+B_{4}U_{1}+v_{2};W_{2}=B_{2}U_{2}+B_{6}W_{1}+{\tau}_{2}\end{array}\ \] \end{document}
model assumptions:
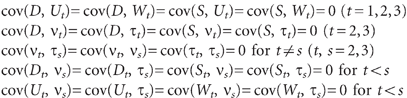 \batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \[\ \begin{array}{rl}&\mathrm{cov}(D,U_{t})=\mathrm{cov}(D,W_{t})=\mathrm{cov}(S,U_{t})=\mathrm{cov}(S,W_{t})=0{\ }(t=1,2,3)\\&\mathrm{cov}(D,v_{t})=\mathrm{cov}(D,{\tau}_{t})=\mathrm{cov}(S,v_{t})=\mathrm{cov}(S,{\tau}_{t})=0{\ }(t=2,3)\\&\mathrm{cov}(v_{t},{\tau}_{s})=\mathrm{cov}(v_{t},v_{s})=\mathrm{cov}({\tau}_{t},{\tau}_{s})=0{\ }\mathrm{for}{\ }t{\neq}s{\ }(t,s=2,3)\\&\mathrm{cov}(D_{t},v_{s})=\mathrm{cov}(D_{t},{\tau}_{s})=\mathrm{cov}(S_{t},v_{s})=\mathrm{cov}(S_{t},{\tau}_{s})=0{\ }\mathrm{for}{\ }t{<}s\\&\mathrm{cov}(U_{t},v_{s})=\mathrm{cov}(U_{t},{\tau}_{s})=\mathrm{cov}(W_{t},v_{s})=\mathrm{cov}(W_{t},{\tau}_{s})=0{\ }\mathrm{for}{\ }t{<}s\end{array}\ \] \end{document}
\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \[\ \begin{array}{rl}&\mathrm{cov}(D,U_{t})=\mathrm{cov}(D,W_{t})=\mathrm{cov}(S,U_{t})=\mathrm{cov}(S,W_{t})=0{\ }(t=1,2,3)\\&\mathrm{cov}(D,v_{t})=\mathrm{cov}(D,{\tau}_{t})=\mathrm{cov}(S,v_{t})=\mathrm{cov}(S,{\tau}_{t})=0{\ }(t=2,3)\\&\mathrm{cov}(v_{t},{\tau}_{s})=\mathrm{cov}(v_{t},v_{s})=\mathrm{cov}({\tau}_{t},{\tau}_{s})=0{\ }\mathrm{for}{\ }t{\neq}s{\ }(t,s=2,3)\\&\mathrm{cov}(D_{t},v_{s})=\mathrm{cov}(D_{t},{\tau}_{s})=\mathrm{cov}(S_{t},v_{s})=\mathrm{cov}(S_{t},{\tau}_{s})=0{\ }\mathrm{for}{\ }t{<}s\\&\mathrm{cov}(U_{t},v_{s})=\mathrm{cov}(U_{t},{\tau}_{s})=\mathrm{cov}(W_{t},v_{s})=\mathrm{cov}(W_{t},{\tau}_{s})=0{\ }\mathrm{for}{\ }t{<}s\end{array}\ \] \end{document}
In these equations the terms ν t and τ t (t = 2,3) represent disturbance terms reflecting unexplained sources of variation in the time-dynamic components of depressive symptoms (U t ) and nicotine-dependence symptoms (W t ) respectively. The coefficients B 1, B 2 represent the reciprocal effects of nicotine-dependence symptoms on depressive symptoms and vice versa respectively within each time period. The coefficients B 3, B 4 and B 5, B 6 represent the across-time stabilities in the time-dynamic components of depressive symptoms and nicotine-dependence symptoms respectively.
The reciprocal cause model depicted in Fig. 1 was fitted to the observed measures of depressive symptoms and nicotine-dependence symptoms at ages 17–18, 20–21, 24–25 years. The fit of this model was then compared with the fit of two other models that assumed unidirectional causal effects between depression and nicotine-dependence symptoms. These models were: a model that assumed a unidirectional effect from nicotine-dependence symptoms to depressive symptoms (i.e. B 1 J 0; B 2 = 0); a model that assumed a unidirectional effect from depressive symptoms to nicotine-dependence symptoms (i.e. B 1 = 0; B 2 J 0).
Since the observed measures were markedly non-normally distributed the models were fitted to the covariance matrix of the observed data using the method of weighted least squares. All models were fitted using LISREL 8 for Windows. (Scientific Software International; www.ssicentral.com). Model goodness of fit was assessed on the basis of a number of indices including the following three: the model chi-squared goodness of fit statistic; the root mean squared error of approximation (RMSEA). Values of RMSEA less than 0.05 are assumed to be indicative of a well-fitting model; and the comparative fit index (CFI). This index varies between 0 and 1 with values close to 1 indicating a well-fitting model.
To examine the sensitivity of the results to model estimation methods a series of alternative models was fitted to the data. These included: a model that used the ordered categorical measures of nicotine dependence and depression described above; and a model that substituted a measure of cigarette-smoking frequency in place of the measure of nicotine dependence.
Sample size and sample bias
The present analyses are based on the sample of 1055 participants for whom data on cigarette smoking and depression symptomatology were available on at least one occasion from 18, 21 and 25 years. However, since not all participants were assessed at all ages the observed sample numbers varied between age 18 (n = 1025), age 21 (n = 1011) and age 25 (n = 1003). These samples represented between 79 and 81% of the initial cohort of 1265 participants.
To adjust for possible sample selection bias resulting from sample attrition, a two-stage process was used. In the first instance, a sample selection model was constructed by using data gathered at birth to predict inclusion in the analysis sample. This showed that there were statistically significant (P<0.05) tendencies for the obtained sample to underrepresent children from more socially disadvantaged backgrounds (low parental education, low socioeconomic status, single-parent family). On the basis of the fitted selection models, the sample was then post-stratified into a series of groups and the probability of study participation estimated for each group.
For the Poisson regression models the data were then re-analysed with the observations for each individual weighted by the inverse of the probability of study participation, and using standard error estimates that were robust to the weighting procedures used. For the structural equation models analyses were conducted that both assumed that missing observations within the analysis sample were missing at random and weighted the observed data by the inverse of the probability of study participation. The analyses were conducted using weighted least squares procedures that were robust to the data-weighting and missing-data assumptions. In all cases the analyses produced essentially identical conclusions to the findings reported here, suggesting that the effects of missing data and selection bias on the results were likely to be minimal.
Results
Associations between nicotine-dependence symptoms and symptoms of depression, ages 17–25
Table 1 shows the associations between the number of symptoms of nicotine dependence and rates of depressive symptoms at ages 17–18, 20–21, and 24–25 years (the distribution of the three-category scales for nicotine dependence and smoking in Table 1 are shown in online Table DS1). In addition, Table 1 also reports on the pooled mean number of depressive symptoms over the three measurement periods, and provides an estimate of the IRRs and 95% confidence intervals, derived from Poisson regression models (see Methods) for the pooled (population-averaged) association between the symptoms of nicotine dependence and symptoms of depression at ages 17–18, 20–21, and 24–25. The Table shows that, at each age, increasing levels of nicotine-dependence symptoms were significantly (P<0.0001) associated with increasing rates of depressive symptoms, with these trends were reflected in the measures of nicotine dependence and depressive symptoms pooled over the three observation periods (likelihood ratio LR χ2(1) = 387.25, P<0.0001).
Table 1 Associations between nicotine-dependence symptoms and depressive symptoms, ages 17–18, 20–21 and 24–25a

| Number of nicotine dependence symptoms | |||||
|---|---|---|---|---|---|
| Depressive symptoms measure | 0 | 1-2 | 3-4 | 5-6 | P b |
| Depressive symptoms, mean (s.d.) | |||||
| Age 17-18 | 1.57 (3.00) | 1.88 (3.30) | 2.96 (3.78) | 5.38 (4.09) | <0.0001 |
| Age 20-21 | 1.44 (2.88) | 1.85 (3.29) | 2.57 (3.44) | 2.94 (3.87) | <0.0001 |
| Age 24-24 | 1.32 (2.83) | 0.89 (2.52) | 1.55 (3.21) | 2.46 (3.64) | <0.0001 |
| Depressive symptoms, pooled mean (s.d.) | 1.45 (2.91) | 1.51 (3.06) | 2.31 (3.49) | 3.15 (3.95) | <0.0001 |
| Pooled association, incidence rate ratio (95% CI) | 1 | 1.29 (1.26-1.32) | 1.66 (1.58-1.75) | 2.13 (1.98-2.31) | |
Overall, those reporting at least five symptoms of nicotine dependence had rates of depressive symptoms that were 2.13 times (95% CI 1.98–2.31) those of individuals who reported no symptoms of nicotine dependence.
In addition, Poisson regression models in which symptoms of nicotine dependence were regressed on depressive symptoms produced comparable results (LR χ2(1) = 108.21, P<0.0001). Also, analyses in which a measure of daily cigarette intake was used in place of the measure of nicotine-dependence symptoms produced comparable results (depressive symptoms regressed on cigarette intake: LR χ2(1) = 223.86, P<0.0001; cigarette intake regressed on depressive symptoms: LR χ2(1) = 25.47, P<0.0001).
Adjustment for covariate factors
The associations between nicotine-dependence symptoms and depressive symptoms were adjusted for non-observed genetic and environmental factors using fixed-effects regression methods. Fixed-effects models were fitted to the data for nicotine-dependence symptoms and depressive symptoms at ages 17–18, 20–21, and 24–25, and extended to include a series of observed time-dynamic covariate factors measured during the period 17–25 years (see Methods). Adjustment for fixed and time-dynamic covariate factors reduced the magnitude of the pooled association between nicotine-dependence symptoms and depressive symptoms (LR χ2(1) = 22.54, P<0.0001), but the association remained statistically significant.
In addition, fixed-effects models with time-dynamic covariate factors, in which symptoms of nicotine dependence were regressed on depressive symptoms, again showed a statistically significant association between nicotine-dependence symptoms and depressive symptoms (LR χ2(1) = 4.34, P<0.05). Again, analyses in which a measure of daily cigarette intake was used in place of the measure of nicotine-dependence symptoms produced comparable results (depressive symptoms regressed on cigarette intake: LR χ2(1) = 21.89, P<0.0001; cigarette intake regressed on depressive symptoms: LR χ2(1) = 868, P<0.05).
Results from structural equation models
The findings above are consistent with the view that nicotine-dependence symptoms and depressive symptoms may be linked by a cause and effect model. However, the analysis does not establish that this association is one in which increasing frequency of nicotine-dependence symptoms leads to increased frequency of depressive symptoms or vice versa. To address this issue, a series of structural equation models was fitted to the data to test alternative assumptions about the direction of association between nicotine-dependence symptoms and depressive symptoms (see Methods). Specifically, three models were fitted:
-
(a) Model 1: a model assuming a reciprocal association between depressive symptoms and nicotine-dependence symptoms within time;
-
(b) Model 2: a model assuming a unidirectional casual effect from nicotine-dependence symptoms to depressive symptoms;
-
(c) Model 3: a model assuming a unidirectional causal effect from depressive symptoms to nicotine-dependence symptoms.
In fitting these models the correlations shown in Fig. 1 between the disturbance terms ν t and τ t were fixed to zero as preliminary analyses showed that these coefficients were non-significant for all models and at all times.
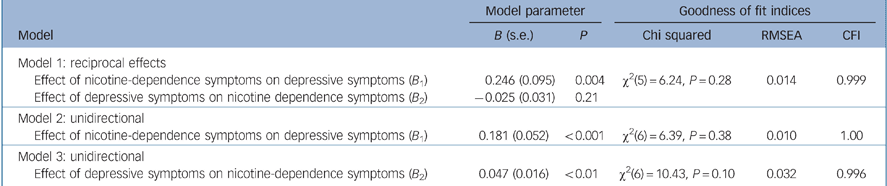
Table 2 shows estimates of the effects of nicotine-dependence symptoms and depressive symptoms on each other and associated goodness of fit statistics from the three models. The analyses suggested the following conclusions. First, Models 1 and 2 were well fitting and led to the same general conclusion: nicotine-dependence symptoms were significantly related to depressive symptoms (P<0.005) but depressive symptoms were not significantly related to nicotine-dependence symptoms (P = 0.21). The difference in chi-squared statistics for the two models was not statistically significant (Δχ2(1) = 0.15, P = 0.70) suggesting that the path from depressive symptoms to nicotine-dependence symptoms could be constrained to zero without affecting model fit. Consistent with these results Model 3 was significantly less well fitting than Models 1 and 2. In particular, the change in model chi-squared from Models 1 to 3 was significant (Δχ2(1) = 4.19, P = 0.04), suggesting that the pathway from nicotine-dependence symptoms to depressive symptoms could not be constrained to zero without affecting model fit.
Table 2 Summary of fitted model coefficients for the causal associations between depressive symptoms and nicotine-dependence symptoms and model goodness of fit indices
| Model parameter | Goodness of fit indices | ||||
|---|---|---|---|---|---|
| Model | B (s.e.) | P | Chi squared | RMSEA | CFI |
| Model 1: reciprocal effects | |||||
| Effect of nicotine-dependence symptoms on depressive symptoms (B 1) | 0.246 (0.095) | 0.004 | χ2(5) = 6.24, P = 0.28 | 0.014 | 0.999 |
| Effect of depressive symptoms on nicotine dependence symptoms (B 2) | -0.025 (0.031) | 0.21 | |||
| Model 2: unidirectional | |||||
| Effect of nicotine-dependence symptoms on depressive symptoms (B 1) | 0.181 (0.052) | < 0.001 | χ2(6) = 6.39, P = 0.38 | 0.010 | 1.00 |
| Model 3: unidirectional | |||||
| Effect of depressive symptoms on nicotine-dependence symptoms (B 2) | 0.047 (0.016) | < 0.01 | χ2(6) = 10.43, P = 0.10 | 0.032 | 0.996 |
As noted in Methods these estimates were obtained from models utilising continuous-count measures of DSM–IV symptom criteria for nicotine dependence or depression in each year (online Table DS1). To examine the sensitivity of the results to model estimation methods a series of alternative models was fitted to the data. These models included: a model that used ordered categorical measures of nicotine dependence and depression; and a model that substituted a measure of daily cigarette intake for the measure of nicotine dependence symptoms. Each of these models produced results consistent with the conclusions drawn above.
Discussion
In this analysis we have used data gathered over the course of a longitudinal study to examine the comorbidities of major depression and nicotine dependence. The analysis used advanced statistical modelling methods to control for non-observed sources of confounding and to explore causal pathways. The analysis led to the following conclusions.
First, in agreement with previous research, Reference Rohde, Kahler, Lewinsohn and Brown5–Reference Patton, Hibbert, Rosier, Carlin, Caust and Bowes22 there was evidence of significant comorbidity between nicotine-dependence symptoms and depressive symptoms at ages 18, 21 and 25. Second, adjustment for non-observed fixed sources of confounding and observed time-dynamic covariate factors showed that the association between depression and smoking could not be entirely explained by these sources of confounding. In addition, further analyses in which nicotine-dependence symptoms were regressed on depressive symptoms produced similar estimates. Also, analyses in which the measure of nicotine dependence was replaced by a measure of daily cigarette intake produced comparable results, suggesting that the findings were robust to alternative methods of measurement. In general, these findings are consistent with previous research that has concluded that the comorbidity between cigarette smoking and depression cannot be explained by common sources of confounding, including common genes and common environment. Reference Haarasilta, Marttunen, Kaprio and Aro6,Reference Hu, Davies and Kandel8,Reference Mykletun, Overland, Aaro, Liabo and Stewart10,Reference Munafo, Hitsman, Rende, Metcalfe and Niaura15,Reference Fergusson, Goodwin and Horwood17,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21 These findings are also consistent with the view that there is likely to be a direct cause and effect association between smoking and depression.
To explore possible pathways between cigarette smoking and depression, methods of structural equation modelling were used to fit a reciprocal causation model. This analysis suggested that the best-fitting model was one in which there was a unidirectional association from symptoms of nicotine dependence to depressive symptoms, but no reverse effect from depressive symptoms to nicotine dependence. Again, analyses using either an alternative measure of nicotine dependence and depression, or a measure of daily cigarette intake in place of nicotine dependence, produced similar results, suggesting that the models were robust to alternative methods of measurement. Collectively this evidence is consistent with the conclusion that there is a cause and effect relationship between smoking and depression in which cigarette smoking increases the risk of symptoms of depression. Reference Steuber and Danner12,Reference Klungsoyr, Nygard, Sorensen and Sandanger14–Reference Pasco, Williams, Jacka, Ng, Henry and Nicholson16,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21 The underlying mechanisms that give rise to such an association are unclear; however, it has been proposed that this linkage may arise from the effects of nicotine on neurotransmitter activity in the brain, causing changes to neurotransmitter activity and leading to increased risk of depression. Reference Haustein, Haffner and Woodcock39,Reference Picciotto, Caldarone, King and Zachariou40
It should also be noted that the findings of the present study are not in agreement with a number of studies that have suggested a causal (self-medication) pathway from depression to smoking. Reference Breslau, Kilbey and Andreski18,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21–Reference Crone and Reijneveld23,Reference Lerman, Caporaso, Main, Audrain, Boyd and Bowman25 There are several reasons why the findings may differ from those of other studies. First, some studies have not tested assumptions regarding causal pathways using structural models. Reference Breslau, Kilbey and Andreski18,Reference Breslau, Peterson, Schultz, Chilcoat and Andreski21 Second, other studies have used retrospective recall, Reference Patton, Hibbert, Rosier, Carlin, Caust and Bowes22 whereas the present study was prospective in nature. Third, at least one study modelled the associations between smoking and internalising disorders, rather than depression per se. Reference Crone and Reijneveld23 Finally, a further study found evidence for a causal link from depression to smoking only among a subset of participants with a particular genetic variant. Reference Lerman, Caporaso, Main, Audrain, Boyd and Bowman25
The findings of the present study are also not in agreement with a number of studies that have failed to find a persistent association between cigarette smoking and major depression. Reference Rohde, Kahler, Lewinsohn and Brown5,Reference Kendler, Neale, MacLean, Heath, Eaves and Kessler19,Reference Roy, Parker, Mitchell and Wilhelm41 Again, there may be a number of reasons why the findings of the present study differ from previous findings. For example, one study employed a design in which a subsample of participants was selected for further study at subsequent time points, Reference Rohde, Kahler, Lewinsohn and Brown5 whereas another study compared a selected sample of currently depressed smokers with currently depressed non-smokers. Reference Roy, Parker, Mitchell and Wilhelm41 In addition, one study employed retrospective recall for measures of depression and smoking. Reference Kendler, Neale, MacLean, Heath, Eaves and Kessler19
Limitations
Finally, it is important to recognise that the conclusions drawn in this analysis rely on some underlying assumptions that are necessary to identify the models we have presented. The most pervasive of these assumptions is that the pattern of comorbidity being studied is represented by a stable causal process that was operative throughout the course of this study. This is clearly a strong assumption, but it is essential for both the fixed-effects and reciprocal-causes models. Further research may be required to examine whether the assumptions regarding the stability of patterns of comorbidity between smoking and depression are correct. Furthermore, it is likely that the models that we have used to represent these data are only approximations to a more complex set of conditions. For these reasons the findings of this study should be viewed as suggestive rather than definitive.
Funding
This research was funded by grants from the Health Research Council of New Zealand, the National Child Health Research Foundation, the Canterbury Medical Research Foundation and the New Zealand Lottery Grants Board.






eLetters
No eLetters have been published for this article.