Introduction
Although celebrated for its impressive avian diversity (Terborgh et al. Reference Terborgh, Robinson, Parker, Munn and Pierpont1990, Mittermeier et al. Reference Mittermeier, Mittermeier, Brooks, Pilgrim, Konstant, Fonseca and Kormos2002, Johnson et al. Reference Johnson, Stouffer and Vargas2011), Amazonia harbours habitats that are relatively poor in bird species such as white sand vegetation (WSV). This vegetation grows in nutrient impoverished sandy soils in patches of variable size generally surrounded by lowland terra firme forest in interfluvial regions (Anderson Reference Anderson1981, Ferreira Reference Ferreira2009). WSV is found in several countries across the Amazon basin (e.g. Peru, Guyana) with its distribution concentrated in north-western Amazon, south-eastern Colombia and southern Venezuela, especially along the Rio Negro basin (Anderson Reference Anderson1981, Pires and Prance Reference Pires, Prance, Prance and Lovejoy1985, Adeney Reference Adeney2009, Ferreira Reference Ferreira2009). Adeney (Reference Adeney2009) estimated that WSV occupies nearly 113,605 km2 or only 1.6% of the Amazon basin area.
The physiognomy of WSV varies from open grassy fields to high canopy forests and this variability is associated with soils with different textures, nutrient contents, frequency of fires and levels of flooding (Bongers Reference Bongers, Engelen and Klinge1985, Nascimento et al. Reference Nascimento, Bueno, Fritsch, Herbillon, Allard, Melfi, Astolfo, Boucher and Li2004, Vicentini Reference Vicentini, Borges, Iwanaga, Durigan and Pinheiro2004, Adeney Reference Adeney2009, Damasco et al. Reference Damasco, Vicentini, Castilho, Pimentel and Nascimento2012). Soils that support WSV are generally seasonally saturated by water, and recently this environment was recognised as a relevant type of wetland in the Amazon basin (Junk et al. Reference Junk, Piedade, Schöngart, Cohn-Haft, Adeney and Wittmann2011).
In the last decade, ornithologists described many new bird species from WSV, an unequivocal demonstration of its biotic peculiarity (e.g. Whitney and Alonso Reference Whitney and Alonso1998, Alonso and Whitney Reference Alonso and Whitney2001, Whitney and Alonso Reference Whitney and Alonso2005, Cohn-Haft and Bravo Reference Cohn-Haft, Bravo, del Hoyo, Elliott, Sargatal and Christie2013, Cohn-Haft et al. Reference Cohn-Haft, Santos, Fernandes, Ribas, del Hoyo, Elliott, Sargatal and Christie2013). Bird communities of WSV are characterised by low number of species, high individual dominance, elevated level of endemism and they represent an important contribution to regional turn-over of species (Oren Reference Oren1981, Alonso et al. Reference Alonso, Metz and Fine2013, Borges Reference Borges2013).
Although few studies characterised the avifauna of WSV in specific sites (Borges Reference Borges2004, Alonso et al. Reference Alonso, Metz and Fine2013, Borges Reference Borges2013, Borges et al. Reference Borges, Whittaker and Almeida2014), the recent expansion of ornithological inventories through the Amazon basin provides an opportunity to review the avifauna associated to this environment.
Our main objective here is to highlight the uniqueness of WSV avifauna through a careful literature compilation to identify the bird species that characterise WSV. In addition we provide the first comparison of WSV and Amazonian savanna avifaunas in order to emphasise the distinctiveness of the bird composition of the two main Amazonian non-forest vegetation types (Pires and Prance Reference Pires, Prance, Prance and Lovejoy1985).
This study is largely based on our field experience with WSV avifauna in different parts of Amazonia (e.g. Borges Reference Borges2004, Dantas et al. Reference Dantas, Faccio and Lima2011, Guilherme and Borges Reference Guilherme and Borges2011) and reflects a qualitative assessment of the bird-habitat association that we expect to be useful for further quantitative studies in community ecology and biogeography of this distinctive avifauna.
Methods
Study area
A general description of WSV is complicated by its highly variable vegetation structure and plant species composition (Anderson Reference Anderson1981, Ferreira Reference Ferreira2009). For ornithological purposes we classified the diversity of WSV physiognomies into the following general categories (Figure 1): i) open fields with sparse vegetation and grassy aspect; ii) scrub areas with exposed patches of sand; iii) areas densely covered by bushes without exposed sandy patches; iv) low forests with canopy reaching a maximum of 10 m; v) high forests with canopy reaching 25–30 m.

Figure 1. Structural heterogeneity of white-sand vegetation (WSV) in the Amazon basin: A) scrub with exposed sand patches in Uatumã region; B) sand fields with grassy aspect in the Aracá river region; C) Chamizales in Jenarro Herrera, Peru, note the low canopy forest and small diameter of the trees; D) dense and almost impenetrable scrub in Jaú National Park. Photographs by S. H. Borges (a, b, d) and A. Vicentini (c).
Here we consider the categories i–iii as white-sand campinas (WSC), categories iv–v as white-sand forests or campinaranas (WSF) and use white-sand vegetation (WSV) as a general term for all white sand habitats types. We emphasise, however, that the vegetation structure of WSV is highly heterogeneous and the categories described above are in fact distributed along an ecological continuum. Our categorisation is intended to be useful for a better understanding of local bird species distribution.
Amazonian savanna patches are physiognomically similar to WSC, but grow in different soils and have distinct floristic composition compared to WSC (Adeney Reference Adeney2009, IBGE 2012). Amazonian savannas occupy nearly 3–4% of the Amazon basin and are distributed mainly in Marajó Island, the Atlantic coast of Amapá, along the Trombetas River, in Roraima State and as smaller patches in southern Amazonia (Pires and Prance Reference Pires, Prance, Prance and Lovejoy1985, Miranda and Carneiro Filho Reference Miranda and Carneiro Filho1994).
Analysis
We consulted more than 30 published references and our unpublished data to build a checklist of bird species recorded in WSV (Appendix S1 in the online supplementary material). Each bird species included in this main list was categorised by its use of WSV as: i) sporadic species: visitors from other habitats that apparently do not use WSV consistently as foraging or breeding sites; ii) regular species: birds that regularly incorporate WSV in their territories as foraging and breeding sites. The regular use of WSV varied regionally for some species depending on the landscape context in which WSV patches are found (e.g. WSV near savannas or near flooded forests); iii) WSV near-restricted species: species registered almost exclusively in WSV, but that may also be found in habitats with vegetation structure similar to WSV such as black-water flooded forests or savannas; iv) WSV restricted species: birds so far recorded exclusively in WSV.
This qualitative categorisation is based on our field experience with WSV avifauna and the literature consulted and needs to be substantiated by quantitative studies. We anticipate that the status of regular and sporadic species may change or be adapted to local studies. In contrast, the categories ‘near-restricted’ and ‘restricted’ are likely to be more stable along the distribution of WSV.
We also determined through the literature the probable source habitats of the regular species and the alternative habitats used by near-restricted species. The distributions of near-restricted and restricted species were mapped into the areas of endemism of Amazonian birds (Silva et al. Reference Silva, Rylands and Fonseca2005, Borges and Silva Reference Borges and Silva2012) in order to investigate the biogeographic relationships of the WSV avifauna.
Previous studies compared the bird species composition of WSF (categories 4 and 5 above) with that found in flooded and terra firme forests in central and western Amazonia (Borges Reference Borges2004, Alonso et al. Reference Alonso, Metz and Fine2013, Borges Reference Borges2013). Here we take a further step in demonstrating the uniqueness of WSV avifauna, comparing bird assemblages of WSC (categories 1 to 3 above) and Amazonian savannas. We compared bird species lists from 12 open vegetation sites in Amazonia – six in Amazonian savannas and six in WSC (Table 1). These inventories have a wide geographic coverage and include open vegetation patches in different landscape contexts (Figure 2).
Table 1. Sampling effort, methods applied and number of bird species in ornithological inventories in eleven sites of Amazonian savannas and white sand campinas (WSC).

1) cp: mist-net captures, sc: specimens collections, qc: qualitative census, com: compilation of previous studies, qt: quantitative census, 2) three numbers refer to total species, semi-dependent plus forest independent species and only independent forest species, respectively, 3) 1- Silva et al. Reference Silva, Oren, Roma and Henriques1997, 2- Santos et al. Reference Santos, Silveira and Silva2011, 3- Moskovits et al. (Reference Moskovits, Fitzpatrick and Willard1985), 4- Silva (Reference Silva, Miliken and Ratter1998), 5- Sanaiotti and Cintra Reference Sanaiotti and Cintra2001, 6- Aleixo and Poletto Reference Aleixo and Poletto2007, 7- Vasconcelos et al. Reference Vasconcelos, Dantas and Silva2011, 8- Borges Reference Borges2004, 9- Borges and Almeida Reference Borges and Almeida2011, 10- Borges Reference Borges2013, 11- SHB and CC, unpublished data, 12- Naka et al. Reference Naka, Cohn-Haft, Mallet-Rodrigues, Santos and Torres2006, 13- CC unpublished data, 14- RA unpublished data, 15- SHB, CC and RA, unpublished data, 16- Borges et al. Reference Borges, Whittaker and Almeida2014, 17- EG and AA, unpublished data. * not informed in the original publication, estimated as a minimum sampling effort; ** NP = National Park, SP = State Park, ES = Ecological Station, SDR = Sustainable Development Reserve.
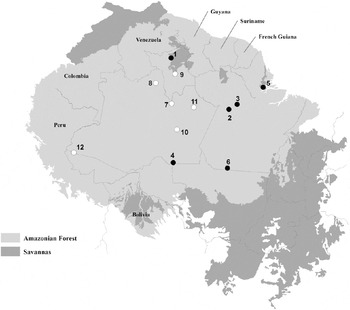
Figure 2. Locations of bird inventories in Amazonian savannas (black circles) and white-sand campinas (white circles) in the Amazon basin. Numbers corresponds to 1) Maracá Ecological Station; 2) Alter do Chão; 3) Monte Alegre; 4) Manicoré; 5) Macapá; 6) Serra do Cachimbo; 7) Jaú National Park; 8) Aracá river; 9) Viruá National Park; 10) Matupiri State Park; 11) Uatumã Sustainable Development Reserve; 12) Mâncio Lima/Guajará. Redrawn after Aleixo and Poletto (Reference Aleixo and Poletto2007).
Comparisons among heterogeneous species lists are complicated due to unequal sampling effort, different methods used, and differences in criteria applied to assign habitat association (Remsen Reference Remsen1994). For this reason we took some precautions to make the studies as comparable as possible. We selected only inventories that used similar field methods including qualitative censuses, specimen collections and mist-net captures (Table 1). To make the comparisons among sites more reliable we selected only bird species recorded in open and semi-open habitats based on habitat codes reported in the original publications (e.g. S for savanna in Silva et al. Reference Silva, Oren, Roma and Henriques1997).
After assembling a main checklist, each species was categorised as forest-dependent, forest semi-dependent, and non-forest following a distributional analysis of Cerrado birds (Silva Reference Silva1995). Using the classification of Silva (Reference Silva1995) it was possible to categorise 72.4% of the species compiled. We used the habitat categories provided by Stotz et al. (Reference Stotz, Fitzpatrick, Parker and Moskovits1996) to complement our categorisation. A small number of species (< 2% of species in the final list) were categorised by us, either because they did not appear in the lists of Silva (Reference Silva1995) and Stotz et al. (Reference Stotz, Fitzpatrick, Parker and Moskovits1996) or because categories suggested by the authors did not coincide with our field experience.
We excluded from this comparative analysis: i) aquatic species, since some sites do not have habitat types associated with water; ii) migrant species, since some studies do not cover different seasons; iii) aerial species, which are difficult to associate with a specific habitat; iv) strictly forest species, because most of the sampling efforts in the compared inventories were applied to low-canopy forests, scrublands or open habitats.
Incidence matrices of non-forest and forest semi-dependent bird species were analysed through cluster analysis with the Jaccard index as a measure of similarity among sites, and group average as linkage strategy. Differences in species composition between Amazonian savannas and WSC were tested through an analysis of similarity (ANOSIM) (Clarke and Warwick Reference Clarke and Warwick2006).
We also performed an indicator species analysis (Dufrêne and Legendre Reference Dufrêne and Legendre1997) to identify bird species associated with savannas or WSC. For this analysis we used only species recorded in at least three sites and the significance of association were tested through permutation tests. Cluster analysis and ANOSIM were performed in Primer 6.0 (Clarke and Gorley Reference Clarke and Gorley2006) and indicator species analysis was performed in PC-ORD 5.0 (McCune and Mefford Reference McCune and Mefford1999).
Results
White-sand vegetation avifauna
The literature compilation and our data resulted in a check-list of 544 bird species recorded in WSV with most (58%) being sporadically found in this habitat (Appendix S1). We categorised 195 species as regular users of WSV and 35 as restricted or near-restricted to WSV. We considered species of the last two categories as bird indicators of WSV (Table 2).
Table 2. Bird indicator species of white-sand vegetation (WSV) in the Amazon basin identified through literature compilation. Codes and numbers in front of species name are: Alternative habitats: sv (savanna), bw (black-water flooded forests), tf (terra firme forest); Areas of endemism: 1) Guiana, 2) Imeri, 3) Jaú, 4) Napo, 5) Inambari, 6) Rondônia, 7) Tapajós, 8) Xingu, 9) Belém; IUCN (2013)Red List categories: LC - Least Concern; NT - Near Threatened; NE - recently described taxa that are not formally evaluated by IUCN; VU - Vulnerable. Taxonomy follows Comitê Brasileiro de Registros Ornitológicos (2014) and Remsen et al. (Reference Remsen, Cadena, Jaramillo, Nores, Pacheco, Pérez-Emán, Robbins, Stiles, Stotz and Zimmer2014) for species not recorded on Brazilian territory.

Black-water flooded forests, terra firme forests and savannas were the most relevant habitat sources for regular species recorded in WSV. Black-water flooded forest was also the main alternative habitat for birds categorised as near-restricted species, being used by nearly half of the species assigned to this category (Table 2).
The nine currently recognised areas of endemism (AoE) in Amazonia house at least one WSV bird indicator (Table 2). This includes species widely distributed (e.g. Xenopipo atronitens and Polytmus theresiae) and species recorded in only one or two areas of endemism (e.g. Cyanocorax helprini, Herpslochmus gentryi, Polioptila clementsi). The Napo area of endemism housed the largest number of WSV indicator species with five restricted species (Table 2). There are also examples of congeneric species with allopatric distribution in different areas of endemism (e.g. Cyanocorax hafferi - AoE Inambari; C. helprini – AoE Imeri).
Amazonian open vegetation: species diversity and composition
At least 235 bird species were recorded in Amazonian savannas and WSC, with 111 non-forest and 124 semi-dependent forest birds (Appendix S2 in the online supplementary material). Considering all species, there were more bird species in savannas than in WSC sites (mean ± standard error: 83.16 ± 2.79 vs. 54 ± 10.61 species, Mann Whitney U test, P = 0.05). This difference becomes larger when only non-forest birds were considered (mean ± standard error: 52.83 ± 3.91 vs. 16.16 ± 3.61, Mann Whitney U test, P = 0.002).
The avifaunas of WSC and Amazonian savannas had distinct species composition (Figure 3). According the R values in the ANOSIM tests, the level of species dissimilarity between environments depending on the set of species used; semi-dependent plus non-forest birds (R = 0.78, P = 0.02, 462 permutations) or only non-forest birds (R = 0.62, P = 0.02, 462 permutations). Both habitats shared approximately 37% (88/235) of the bird species. One hundred and nine (46%) and 38 (16%) species were exclusively recorded in savannas or WSC, respectively.
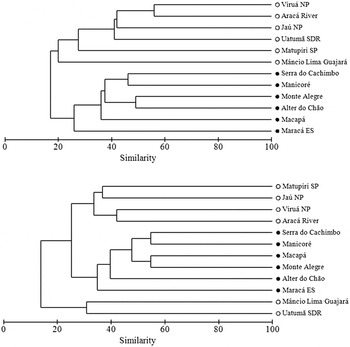
Figure 3. Grouping of sites based on bird distribution (presence/absence) in Amazonian savannas (black circles) and white-sand campinas (white circles). Upper panel shows grouping based on semi-dependent forest species plus non-forest species, lower panel shows grouping using only non-forest bird species.
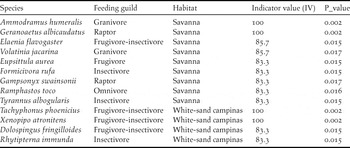
Indicator species analysis shows that 13 bird species were significantly associated with savannas (nine species) or WSC (four species) (Table 3). Some of these indicator species occur exclusively in sites of its respective preferred habitat (Indicator Value 100) and include birds with diverse feeding habits (Table 3).
Table 3. Bird species significantly associated with Amazonian savannas or white-sand campinas identified through indicator species analysis.
Discussion
Characteristics of WSV avifauna
The small number of species is the most evident attribute of the WSV bird assemblage. Quantitative comparisons showed that WSF houses a smaller number of species than terra firme and seasonally flooded forests in Peru and central Amazonia (Alonso et al. Reference Alonso, Metz and Fine2013, Borges Reference Borges2013). Although based on few sites and without standard sampling effort, the analyses presented here suggest that WSF also has a smaller number of species even when compared with physiognomically similar Amazonian savannas.
Number of species is only one possible measurement of community diversity (Magurran Reference Magurran2004) and considering WSV as a biologically impoverished habitat based only on this characteristic is unjustified. In fact, the Amazonian avifauna is characterised by different groups of species that use the major vegetation types and WSV has an important contribution to this species turnover as shown in central and western Amazonia (Alonso et al. Reference Alonso, Metz and Fine2013, Borges Reference Borges2013).
Bird species diversity is also affected by structural variability within WSV. In Jaú National Park, WSF harbours twice as many species compared with WSC (Borges Reference Borges2004, Reference Borges2013). In this case, species diversity is likely to be affected by the variation in vegetation structure observed along ecological gradients driven by flooding, soil texture and nutrient contents (Nascimento et al. Reference Nascimento, Bueno, Fritsch, Herbillon, Allard, Melfi, Astolfo, Boucher and Li2004, Damasco et al. Reference Damasco, Vicentini, Castilho, Pimentel and Nascimento2012), emphasising the relevance of soil properties for bird distribution.
The number of bird species in WSV sites increased with the area occupied by this vegetation (Oren Reference Oren1981). In Aracá region, for example, WSV covers nearly 300,000 hectares and houses more than 50 non-forest species compared with 25 non-forest bird species recorded in isolated patches of WSV with only 2,000 ha in Jaú National Park (Borges Reference Borges2013, Borges et al. Reference Borges, Whittaker and Almeida2014). A reasonable explanation for this area effect is that large patches of WSV generally present higher habitat heterogeneity. For example, open and grassy fields apparently are found only in WSV patches larger than 1,000 ha (Borges et al. unpubl. data) adding extra habitats that are selected by some nighthawks (Caprimulgidae) and other bird species apparently absent in small patches of WSV (SHB pers. obs). The species-area-habitat relationship in WSV avifauna, however, deserves a quantitative evaluation that will be explored in a separate publication.
The avifauna of WSV receives an important contribution from other habitats, mainly the black-water flooded forests (Oren Reference Oren1981, Borges and Carvalhaes Reference Borges and Carvalhaes2000, Borges Reference Borges2004). Black-water flooded forests and WSV are floristically and structurally related habitats (Anderson Reference Anderson1981, Kubitzki Reference Kubitzki1990). We hypothesise that strips of flooded forest along black-water rivers function as corridors increasing connectivity of apparently isolated populations of WSV birds. Populations of Xenopipo atronitens (a WSV specialist), for example, separated by the channel of the Negro river (a black-water river) shared several haplotypes, whereas birds on opposite sides of the Solimões-Amazonas (white-water rivers) are more genetically distinct (Capurucho et al. Reference Capurucho, Cornelius, Borges, Cohn-Haft, Aleixo, Metzger and Ribas2013). Although these observations are suggestive, the effects of landscape connectivity and isolation of WSV patches on its bird community remain to be studied in more detail.
Another important aspect of the WSV bird community is its relationship with past events of expansion and retraction of Amazonian open vegetation. Capurucho et al. (Reference Capurucho, Cornelius, Borges, Cohn-Haft, Aleixo, Metzger and Ribas2013) proposed that Xenopipo atronitens populations have expanded since the Last Glacial Maximum (LGM). Phylogeographic studies of other WSC specialists (Polytmus theresiae, Elaenia ruficeps and Tachyphonus phoenicius) suggest that results found for X. atronitens can be applied to the other elements of WSV avifauna (Matos Reference Matos2014, Duarte pers. comm.). Taken together, phylogeographic studies of WSV birds suggest that events of climate change during the Pleistocene have likely affected the availability of open habitats in Amazonia, which in turn has caused changes in bird species ranges (Haffer Reference Haffer and Vieira2001, Capurucho et al. Reference Capurucho, Cornelius, Borges, Cohn-Haft, Aleixo, Metzger and Ribas2013, Matos Reference Matos2014).
Avifauna of Amazonian open vegetation
The avifauna of the two main open vegetation types of Amazonia (WSC and savannas) is clearly distinct in species composition, a pattern shared with their respective plant communities (Adeney Reference Adeney2009). The differences in plant and bird communities of Amazonian savannas and WSC are likely related to characteristics of their edaphic substrates. Based on a map of Brazilian soil classification system (IBGE 2012), savanna sites used in this study fall within five major soil classes (neosols, latosols, plinthosols and argisols). In contrast, all WSC sites grow over spodosols except for the WSC of Matupiri SP (planosol) and Uatumã SDR (latosol) sites.
The observed pattern suggests that the relationship of soil characteristics and bird species distribution already documented for birds associated to Amazonian forests (Pomara et al. Reference Pomara, Ruokolainen, Tuomisto and Young2012, Borges Reference Borges2013) could also be extended to non-forest bird communities. Further quantitative field studies will certainly elucidate details of the relationships among soils, vegetation and bird distribution in Amazonian open vegetation types.
The contribution of other biomes to the bird assemblages of Amazonian savannas and WSC is another difference between these vegetation types. Most of the open vegetation birds recorded exclusively at Amazonian savanna sites studied here are also found in other biomes, mainly Cerrado and Atlantic forest (van Perlo Reference van Perlo2009, Ridgely and Tudor Reference Ridgely and Tudor2009). In contrast, the majority of WSC indicator bird species have their distributions entirely within Amazonia, showing that the typical avifauna of Amazonian open vegetation is represented by WSC birds.
Conservation
Populations of WSV bird specialists are distributed in patches with different sizes and levels of isolation. This patchy distribution is likely to affect their population dynamics and probability of survival in the long term. Some bird species have low population density and their local maintenance depends on colonisation events from nearby patches. If population dynamics of WSV are consistent with island biogeography models (Whittaker and Fernández-Palacios Reference Whittaker and Fernández-Palacios2007), populations in small and isolated patches of this habitat should experience events of local extinction at higher rates than populations in larger and more connected patches. Therefore, maintaining connectivity among isolated patches should be a priority for conservation of WSV.
White sand vegetation is a fragile ecosystem with low capacity to regenerate after disturbance (Anderson Reference Anderson1981, Uhl et al. Reference Uhl, Jordan, Clark, Clark and Herrera1982). Field observations suggest that birds specialized in WSV disappear after the site is disturbed (SHB pers. obs.). The main human-driven disturbance of WSV is sand extraction for construction, threatening WSV patches close to large cities such as Belém, Manaus and Iquitos (Alonso et al. Reference Alonso, Metz and Fine2013, Ferreira Reference Ferreira, Chaves, Cunha, Rosário and Parolin2013a,Reference Ferreira, Santos, Bastos and Cunhab, SHB pers. obs.). This mining practice results in complete suppression of vegetation with drastic effects on WSV biota.
At least seven WSV indicator birds (20% of this avifauna) are classified in threatened categories (Table 2), with one (Polioptila clementsi) considered as ‘Critically Endangered’ (IUCN 2013). The fragility of the WSV ecosystem, its patchy distribution and small population sizes of some specialised birds makes WSV one of the most threatened vegetation types in the Amazon basin.
The current system of officially protected areas in the Amazon includes patches of WSV and could be instrumental in protecting this avifauna (Adeney Reference Adeney2009). Even so there are good opportunities to increase representation of this environment in officially protected areas in regions such as the Aracá river (Adeney Reference Adeney2009, Borges et al. Reference Borges, Whittaker and Almeida2014). Moreover, a detailed and updated gap analysis is necessary to quantity the representation of WSV found within the boundaries of the current protected areas in the Amazon region. This is especially urgent in areas where deforestation rates are increasing such as the southern portion of the Amazonas state (Adeney Reference Adeney2009). In this sense, there is an urgent need to map, investigate and protect small patches of WSV, especially in regions close to resource-demanding cities.
This study joins others in emphasising the relevance of WSV to interpret the evolution of Amazonian biological diversity (Brown and Benson Reference Brown and Benson1977, Oren Reference Oren1981, Fine et al. Reference Fine, Daly, Villa, Mesones and Cameron2005, Capurucho et al. Reference Capurucho, Cornelius, Borges, Cohn-Haft, Aleixo, Metzger and Ribas2013, Alonso et al. Reference Alonso, Metz and Fine2013). WSV is not a poor habitat in any sense. This vegetation type harbours threatened and unique biodiversity with great potential to help us understand the current structure as well as the environmental history of the most biodiverse biome of the planet.
Acknowledgements
Financial support for field work in WSV sites was provided by Fundação de Amparo a Pesquisa do Estado do Amazonas (FAPEAM) and Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP) through the FAPESP / FAPEAM joint funding programme (FAPESP 09/53365-0), Gordon and Betty Moore Foundation, and Fundação Vitória Amazônica (FVA). Federal (ICMBIO) and State (CEUC) environmental agencies provided permits for field work in protected areas. EG and CCR are grateful to CNPq for its support via project no. 474592/2010-3 (2010-2012) and fellowship no. 458311/2013-8, respectively. Jeff Stratford and three anonymous reviewers made important suggestions and corrections that significantly improved the manuscript. We dedicate this paper to Dr David C. Oren, pioneer in the studies of birds of Amazonian WSV, whose contributions are an important source of intellectual inspiration. This is contribution #001 of the Amazonia Insular Research Group.








