Significant outcomes
Psilocybin does not show an antidepressant-like effect in the Flinders Sensitive Line rat model of depression.
Other animal models of depression and behavioural tests might be more appropriate to study the therapeutic effects of psilocybin.
Limitations
These studies only used one animal model of depression and one behavioural test, and results may, therefore, not be valid for other models or tests.
Introduction
Depression is major public health concern. It affects hundreds of millions of people, is the leading cause of disability-adjusted life years (DALYs) globally (Ferrari et al., Reference Ferrari, Charlson, Norman, Patten, Freedman and Murray2013), and the economic costs pose a large burden on the society (Gustavsson et al., Reference Gustavsson, Svensson, Jacobi, Allgulander, Alonso and Beghi2011). Current treatment options, such as antidepressant medication and cognitive behavioural therapy, can be effective for some patients; however, around 20% do not respond to any intervention, the time to response is often several weeks, and many of those that do respond relapse (Rush et al., Reference Rush, Trivedi, Wisniewski, Nierenberg, Stewart and Warden2006). These strong limitations call for the development of new, more effective ways to treat depression. One way to do this is by re-evaluating and repurposing old drugs, as has been the case of ketamine (Canuso et al., Reference Canuso, Singh, Fedgchin, Alphs, Lane and Lim2018; Daly et al., Reference Daly, Singh, Fedgchin, Cooper, Lim and Shelton2018). Most recently, psilocybin, a psychedelic serotonin receptor agonist found in ‘magic mushrooms’, has shown a promising therapeutic potential for treatment-resistant depression (Carhart-Harris et al., Reference Carhart-Harris, Bolstridge, Rucker, Day, Erritzoe and Kaelen2016), end-of-life anxiety (Griffiths et al., Reference Griffiths, Johnson, Carducci, Umbricht, Richards and Richards2016; Ross et al., Reference Ross, Bossis, Guss, Agin-Liebes, Malone and Cohen2016), and addiction (Johnson et al., Reference Johnson, Garcia-Romeu, Cosimano and Griffiths2014; Bogenschutz et al., Reference Bogenschutz, Forcehimes, Pommy, Wilcox, Barbosa and Strassman2015). Psilocybin acutely induces powerful subjective effects, produces visual hallucinations, enhances positive mood, alters states of consciousness (Preller and Vollenweider, Reference Preller and Vollenweider2018), and has been used as a model of psychosis both in humans (Vollenweider et al., Reference Vollenweider, Vollenweider-Scherpenhuyzen, Babler, Vogel and Hell1998; Gouzoulis-Mayfrank et al., Reference Gouzoulis-Mayfrank, Heekeren, Thelen, Lindenblatt, Kovar and Sass1998; Vollenweider and Geyer, Reference Vollenweider and Geyer2001) and in animals (Halberstadt and Geyer, Reference Halberstadt and Geyer2013). In a recent pilot study, a carefully designed session of two oral administrations of a low and a moderate dose (10 and 25 mg) of psilocybin along with psychological support before, during, and after the administration showed a rapid-acting (1 week) and long-lasting (3 months) antidepressant effect in patients with treatment-resistant depression (Carhart-Harris et al., Reference Carhart-Harris, Bolstridge, Rucker, Day, Erritzoe and Kaelen2016). This is in contrast to the rapid antidepressant effect of ketamine, which typically only lasts up to a week (Fond et al., Reference Fond, Loundou, Rabu, Macgregor, Lancon and Brittner2014). This potentially represents an entirely new approach for the treatment of depression, in terms of pharmacological action, serotonin receptor 2A agonism, and the overall treatment paradigm – a single acute versus chronic treatment.
The underlying mechanisms of psilocybin’s antidepressant effects are, however, largely unknown, and a full comprehension may include both psychological and biological components. Understanding the neurobiological mechanisms may help optimise safety and efficacy of psilocybin treatment, reveal novel molecular targets to pursue in preclinical research, and, in general, improve our understanding of both depression and psychedelic drug effects in the brain. However, understanding the relation between neurobiological findings in animals and the antidepressant effect in humans requires valid, translational animal models. In the published literature, there are no reports of any antidepressant-like effects of psilocybin or psilocin in any animal model of depression.
The present study, therefore, aimed to show an antidepressant-like effect of psilocybin and psilocin in a rat model of depression. We used the Flinders Sensitive Line (FSL) rat model of depression that exhibits several phenotypical features resembling depression, particularly increased passive behaviour following stress, shown as increased immobility in the forced swim test (FST) compared with its control strain – the Flinders Resistant Line (FRL). We designed a series of experiments to address the optimal dose, treatment paradigm, and time between injection and tests. We hypothesised that treatment with psilocybin or psilocin would decrease immobility in the FST, corresponding to an antidepressant-like effect.
Methods
Ethical statement
The Danish National Committee for Ethics in Animal Experimentation had approved all animal procedures prior to initiation of the experiments (2012-15-2934-00254).
Study design
Four consecutive experiments (E1, E2, E3, E4) were conducted with FSL and FRL rats (Table 1). In each experiment, the antidepressant-like effect was evaluated as the decrease in immobility time between the FSL group treated with psilocin or psilocybin and the FSL group treated with saline. Individual animals were randomly allocated using a random number generator (www.random.org) to the treatment groups as summarised in Table 1. Rats were treated and tested in a pseudo-random order, with each model group (FRL vs. FSL) and treatment group (vehicle vs. psilocin/psilocybin) evenly distributed over the time interval. All behavioural tests were conducted between 8 a.m. and 2 p.m., depending on the experiment, with a maximal time slot of 2.5 h from the first to the last animal tested.
Table 1 Summary of the experiments E1-4. PSI = Psilocin. PSY = Psilocybin. FRL = Flinders Resistant Line. FSL = Flinders Sensitive Line. n = number of animals in cohort.

PSI, psilocin; PSY, psilocybin.
Animals
Male Flinders Line rats (FSL and FRL; see Table 1 for age and weight), from the colony maintained at Aarhus University, were housed in pairs (Cage 1291H Eurostandard Type III H, 425 × 266 × 185 mm; Techniplast, Buguggiate, Italy) at 22 ± 2°C on a 12-h light/dark cycle (lights on at 07:00 a.m.). The animals had ad libitum access to chow pellets and tap water. The welfare of the animals was assessed daily. The number of animals used for every experiment was determined based on priori sample size calculations performed with G*Power. We assumed an effect size of Cohen’s d = 1.6, based on the assumption that a putative antidepressant effect should reduce immobility in the FST by at least 20% to be considered significant (Slattery and Cryan, Reference Slattery and Cryan2012), and a standard deviation (SD) of 12.5%, based on previous FSL experiments. We assumed an alpha error probability of 0.05 and a power of 0.80. This gave a sample size of eight animals per group and resulted in a power of 0.84.
Drugs
Psilocin or psilocybin (both drugs from THC Pharm, Frankfurt, Germany) was dissolved in 0.9% sterile saline solution, acidified with hydrochloric acid, and subsequently adjusted to pH = 5–6. For vehicle controls, 0.9% sterile saline solution was used. Physiologically, psilocybin is a prodrug and is instantly hydrolysed in the blood by phosphatases to the active compound, psilocin. Psilocybin and psilocin can, therefore, be considered as equipotent at equimolar doses. Due to different molar masses, psilocin is approximately 1.4 times more potent per milligram than psilocybin.
Treatments
Saline, psilocin, or psilocybin was injected intraperitoneally at a volume of 1 ml, in the animals’ home cage. The following doses were used: psilocin 0.50 and 2.0 mg/kg, psilocybin 2.0, 3.0, and 10 mg/kg, as summarised in Table 1. All injections were conducted between 08.00 a.m. and 11.00 a.m. During dose administrations, the investigators were blinded to the strain of the animal, but not to the treatment. The route of administration was chosen to ensure reliable absorption and high bioavailability. Doses were chosen based on the published literature with psilocybin in rats (Davis and Walters, Reference Davis and Walters1977; Geyer et al., Reference Geyer, Light, Rose, Petersen, Horwitt and Adams1979; Rambousek et al., Reference Rambousek, Palenicek, Vales and Stuchlik2014; Tyls et al., Reference Tyls, Palenicek, Kaderabek, Lipski, Kubesova and Horacek2016) and therapeutic human doses (Carhart-Harris et al., Reference Carhart-Harris, Bolstridge, Rucker, Day, Erritzoe and Kaelen2016; Griffiths et al., Reference Griffiths, Johnson, Carducci, Umbricht, Richards and Richards2016; Ross et al., Reference Ross, Bossis, Guss, Agin-Liebes, Malone and Cohen2016) converted using an allometric scaling factor of 6.2 from humans to rats (Nair and Jacob, Reference Nair and Jacob2016) (psilocybin 25 mg/70 kg × 6.2 = 2.2 mg/kg). Based on rat pharmacokinetics (Saito et al., Reference Saito, Toyo’oka, Fukushima, Kato, Shirota and Goda2004), 3 h was chosen as the earliest time point for behavioural testing to ensure that any behavioural effects would be due to persistent changes and not to the acute effects of the drug.
Open field test
The open field test (OFT) was used to assess locomotor activity. The test was conducted in a sound-proof and dimly-lit room. Animals were placed in the middle of a novel arena consisting of a square black plastic box (100 × 100 × 30 cm) and left for 5 min. Their activity was recorded using an infrared camera, and the total distance travelled within 5 min was quantified using Ethovision XT 12 video tracking software, Noldus. Between each session, the arena was cleaned with 70% ethanol.
Forced swim test
Depressive-like behaviour was assessed using immobility in the FST as a measure of behavioral despair. A modified FST was applied with no pre-swim session, as previously described (du Jardin et al., Reference du Jardin, Liebenberg, Müller, Elfving, Sanchez and Wegener2016), and elaborated in the discussion. The apparatus used for the FST consisted of Plexiglas cylinders 60 cm tall, 20 cm in diameter, filled with 40 cm water at a temperature of 24 ± 2°C. Immediately after the OFT, rats were subjected to the FST for 5 min, and their activity was video-recorded for later scoring. Each video was scored for immobility, swimming, and struggling behaviour, determined every 5 s, by a single, trained observer. The observer was blinded to strain and treatment group. Each animal was scored at least twice. If the discrepancy in immobility time between the two scorings exceeded 20 s, the animal was scored a third time. The averages of the two scorings nearest to each other in immobility time were calculated and reported in seconds.
Statistical analysis
The unit of analysis was a single animal. ROUT test was used for the identification of outliers at a significance level of Q = 0.01. Two-way analysis of variance (ANOVA) was conducted to assess the main effects and interaction of strain and treatment in the OFT and FST and was followed by post hoc Tukey’s multiple comparisons tests. Calculations were carried out using GraphPad Prism 7 software. All data are presented as mean ± SEM, and significance was determined at p < 0.05.
Results
Open field test
The OFT was used to assess the effects of psilocin and psilocybin on locomotor activity in four separate experiments (Fig. 1). In all the experiments, FSL rats showed a higher locomotor activity than FRL rats (two-way ANOVA; E1: F (1,42) = 22.17, p < 0.0001; E2: F (1,30) = 112.9, p < 0.0001; E3: F (1,31) = 49.04, p = 0.0001; E4: F (1,32) = 26.41, p < 0.0001). Full results of statistical test can be found in the supplementary material, Supp. Table 1. Treatment with psilocin or psilocybin had no statistically significant effect on locomotor activity in the OFT in any of the experiments (two-way ANOVA; E1: F (2,42) = 1.617, p = 0.2106; E2: F (1,30) = 0.1895, p = 0.6664; E3: F (1,31) = 1.284, p = 0.265; E4: F (1,32) = 1.663, p = 0.2064). This suggests that psilocin and psilocybin do not have any stimulant or depressant effects on locomotor activity at these time points, which otherwise could have confounded FST results.
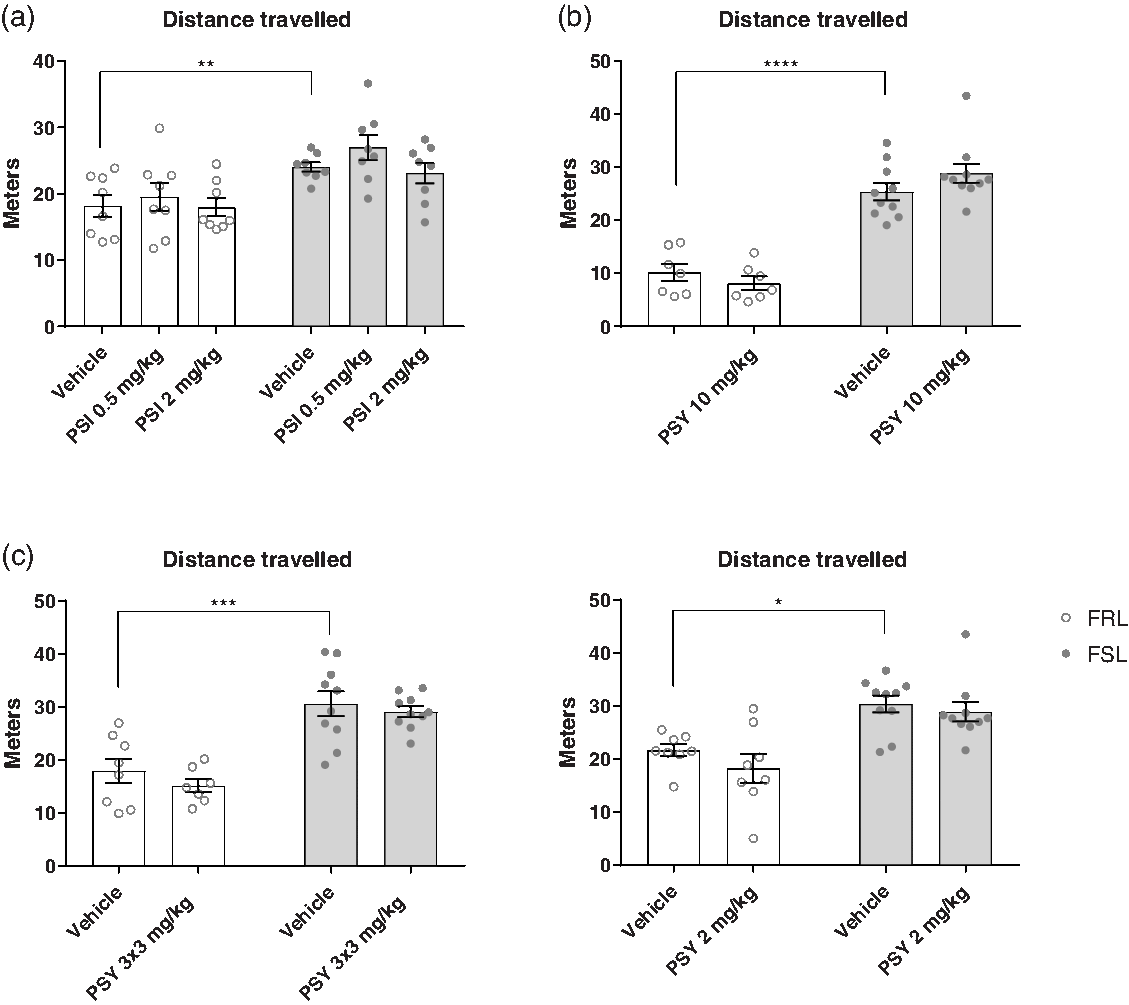
Fig. 1. Open field test (OFT). Effect of strain (FRL and FSL) and treatment with PSI (a) or PSY (b-d) on distance travelled in the OFT. a. E1. Effect of strain and treatment on distance travelled. FRL-vehicle (n = 8), FRL-PSI 0.50 mg/kg (n = 8), FRL-PSI 2 mg/kg (n = 8), FSL-vehicle (n = 8), FSL-PSI 0.50 mg/kg (n = 8), FSL-PSI 2 mg/kg (n = 8). b. E2. Effect of strain and treatment on distance travelled. FRL-vehicle (n = 7), FRL-PSY 10 mg/kg (n = 7), FSL-vehicle (n = 8), FSL-PSY 10 mg/kg (n = 8). c. E3. Effect of strain and treatment on distance travelled. FRL-vehicle (n = 8), FRL-PSY 3 x 3 mg/kg (n = 7, one animal died unexpectedly with no known cause of death), FSL-vehicle (n = 8), FSL-PSY 3 x 3 mg/kg (n = 8). d. E4. Effect of strain and treatment on distance travelled. FRL-vehicle (n = 8), FRL-PSY 2 mg/kg (n = 8), FSL-vehicle (n = 10), FSL-PSY 2 mg/kg (n = 9, 1 animal was excluded as a statistical outlier). Columns represent means and error bars represent ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Forced swim test
The FST was used to assess antidepressant-like effects of either psilocin or psilocybin in four separate experiments (Fig. 2). Treatment with psilocin or psilocybin had no statistically significant effect on immobility time in any of the experiments (two-way ANOVA; E1: F (2,42) = 0.196, p = 0.8228; E2: F (1,26) = 0.00462, p = 0.9463; E3: F (1,27) = 1.087, p = 0.3065; E4: F (1,32) = 0.4132, p = 0.5249). In all the experiments, FSL rats showed higher immobility time compared with FRL rats (two-way ANOVA; E1: F (1,42) = 93.5, p < 0.0001; E2: F (1,26) = 68.87, p < 0.0001; E3: F (1,27) = 38.33, p < 0.0001; E4: F (1,32) = 29.64, p < 0.0001). This suggests that psilocin and psilocybin do not exert an antidepressant-like effect in the FSL rats.
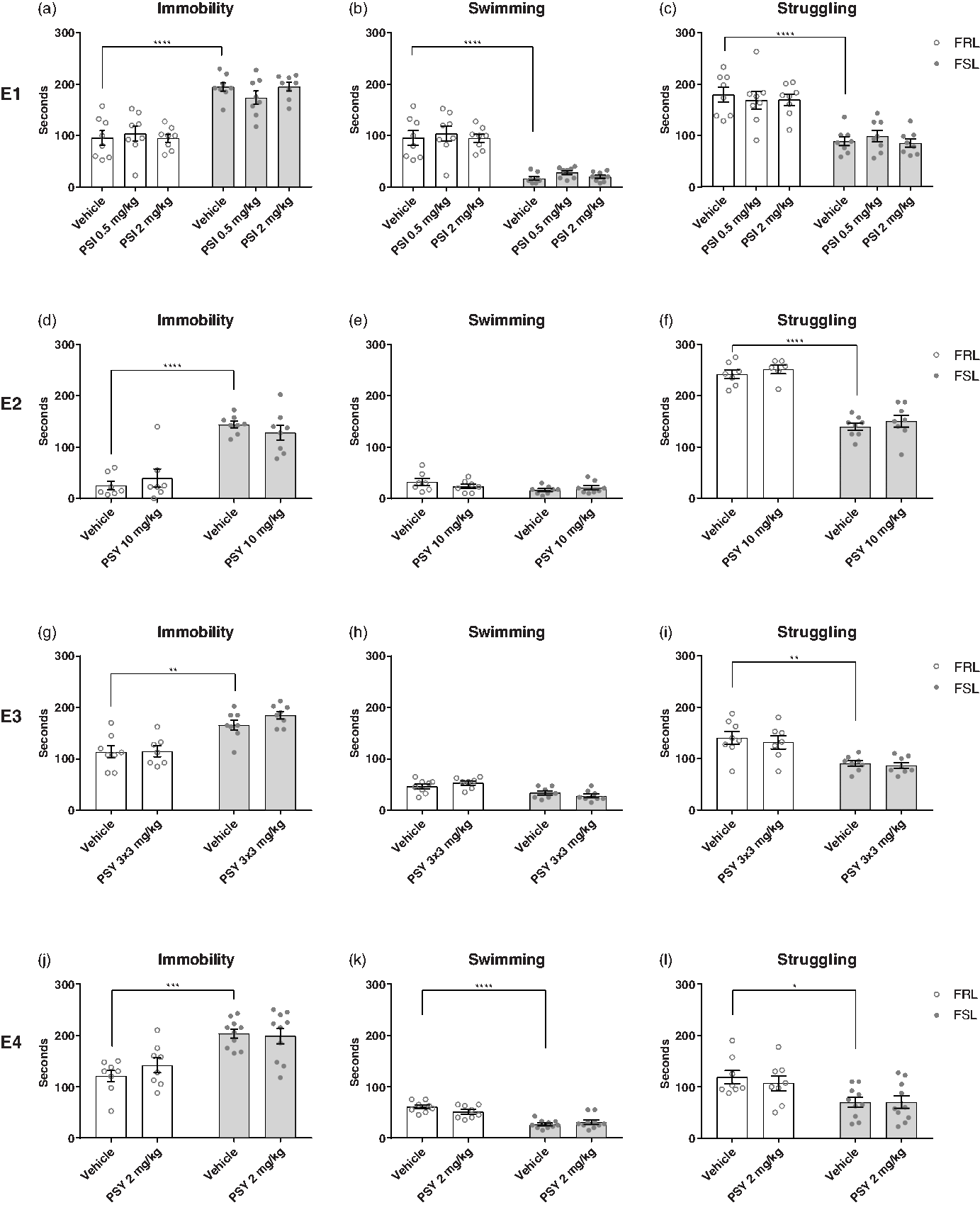
Fig. 2. Forced swim test (FST). Effect of strain (FRL and FSL) and treatment with PSI (E1) and PSY (E2-4) on immobility, swimming and struggling behaviour in the FST. E1. (a-c) effect of strain and treatment on immobility (a), swimming (b), and struggling (c) behaviour. FRL-vehicle (n = 8), FRL-PSI 0.50 mg/kg (n = 8), FRL-PSI 2 mg/kg (n = 8), FSL-vehicle (n = 8), FSL-PSI 0.50 mg/kg (n = 8), FSL-PSI 2 mg/kg (n = 8). E2. (d-f) effect of strain and treatment on immobility (d), swimming (e), and struggling (f) behaviour. FRL-vehicle (n = 7), FRL-PSY 10 mg/kg (n = 6-7, 1 animal excluded as a statistical outlier in the struggling-outcome), FSL-vehicle (n = 8), FSL-PSY 10 mg/kg (n = 8). E3. (g-i) effect of strain and treatment on immobility (g), swimming (h), and struggling (i) behaviour. FRL-vehicle (n = 8), FRL-PSY 3 x 3 mg/kg (n = 7, one animal died unexpectedly with no known cause of death), FSL-vehicle (n = 8), FSL-PSY 3 x 3 mg/kg (n = 8). E4. (j-l) effect of strain and treatment on immobility (j), swimming (k), and struggling (l) behaviour. FRL-vehicle (n = 8), FRL-PSY 2 mg/kg (n = 8), FSL-vehicle (n = 10), FSL-PSY 2 mg/kg (n = 10). Columns represent means and error bars represent ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Discussion
The main finding of this study is that psilocin and psilocybin do not exert an antidepressant-like effect in the FSL rats, when evaluated using the FST and OFT. We found no effect of psilocin or psilocybin on immobility, struggling, or swimming behaviour in the FST, nor any effect on distance travelled in the OFT. This lack of effect was contrary to our hypothesis that psilocin and psilocybin would exhibit antidepressant-like effects, reflecting the antidepressant effects observed in human studies.
According to the published literature, the antidepressant potential of psilocin and psilocybin has not previously been investigated in an animal model of depression. Some studies have reported related effects of serotonergic psychedelics, such as normalisation of learning behaviour by lysergic acid diethylamide in the olfactory bulbectomy model of depression (Buchborn et al., Reference Buchborn, Schroder, Hollt and Grecksch2014), antidepressant-like effects of N,N-dimethyltryptamine (DMT) (Cameron et al., Reference Cameron, Benson, Dunlap and Olson2018), and Ayahuasca (Amazonian brew that contains DMT) (Pic-Taylor et al., Reference Pic-Taylor, da Motta, de Morais, Junior, Santos Ade and Campos2015) in the rodent FST. Other studies have investigated the therapeutic potential of psilocin or psilocybin in animal models of other psychiatric disorders such as anxiety (Horsley et al., Reference Horsley, Palenicek, Kolin and Vales2018), OCD (Sard et al., Reference Sard, Kumaran, Morency, Roth, Toth and He2005), and PTSD (Catlow et al., Reference Catlow, Song, Paredes, Kirstein and Sanchez-Ramos2013).
Our results suggest that the FST is an inappropriate test, or the FSL rat an inappropriate model, for replicating the antidepressant effect of psilocybin. The FST was originally developed as an antidepressant ‘drug screen’ and has, as argued by Stanford (Stanford, Reference Stanford2017), sometimes been misinterpreted as a model of depression. In our study, the FST was not used as a model, but only as a depressive-like behavioral outcome measure, while the FSL strain was the model. Whether immobility in the FST can be interpreted as depressive-like has also been questioned, and the immobility has been suggested to reflect adaptive behaviour and not despair (Molendijk and de Kloet, Reference Molendijk and de Kloet2015). Furthermore, interpreting any single behaviour as depressive-like may be too anthropomorphic or simplistic (Slattery and Cryan, Reference Slattery and Cryan2014; Commons et al., Reference Commons, Cholanians, Babb and Ehlinger2017). Alongside these critiques, our negative results emphasise the need for re-evaluating and improving the conceptual framework for animal models of depression. The FSL rat has a high degree of face, construct, and predictive validities (Overstreet and Wegener, Reference Overstreet and Wegener2013). Importantly, the FSL rats have been shown to respond to traditional antidepressants such as tricyclic antidepressants, selective serotonin reuptake inhibitors, ketamine, and a large variety of experimental drugs (Overstreet and Wegener, Reference Overstreet and Wegener2013; du Jardin et al., Reference du Jardin, Liebenberg, Müller, Elfving, Sanchez and Wegener2016;) using the no pre-swim FST applied in the present study. In this setup, the pre-swim session, which is usually applied to induce acute stress (Detke et al., Reference Detke, Johnson and Lucki1997), is omitted, since the FSL rats show inherent depressive-like behaviour, without the pre-swim session (Overstreet and Wegener, Reference Overstreet and Wegener2013). The FSL rats showed a markedly lower expression of 5-HT2A receptor mRNA in the frontal cortex (35%) and hippocampus (37%), compared with FRL rats (100%) (du Jardin et al., Reference du Jardin, Liebenberg, Müller, Elfving, Sanchez and Wegener2016), and this may render this strain inappropriate for studying psilocybin and psilocin, since the activation of the 5-HT2A receptor has been shown to be necessary for their subjective effects in humans (Vollenweider et al., Reference Vollenweider, Vollenweider-Scherpenhuyzen, Babler, Vogel and Hell1998; Quednow et al., Reference Quednow, Kometer, Geyer and Vollenweider2012) and behavioral effects in rodents (Gonzalez-Maeso et al., Reference Gonzalez-Maeso, Weisstaub, Zhou, Chan, Ivic and Ang2007).
A wider interpretation of our results is that the antidepressant effect of psilocybin shown in humans is not directly translatable to animal models of depression at any circumstances. We are, of course, hesitant to make such a claim at this stage; however, several aspects, both biological and psychological, complicate back-translation from humans to animals. Biologically, the human 5-HT2A receptor showed a 15-fold higher affinity for psilocin than the rat 5-HT2A receptor (Gallaher et al., Reference Gallaher, Chen and Shih1993), due to a single amino acid substitution, Ser-242, in humans, monkeys, and pigs, and Ala-242 in rats and mice (Johnson et al., Reference Johnson, Loncharich, Baez and Nelson1994; Johnson et al., Reference Johnson, Baez, Kursar and Nelson1995). All other things being equal, this should give a tremendously different receptor interaction profile of psilocin, and consequently psilocin would always have a different effect in humans than it would in rodents. An issue of a different nature is the role of psychotherapy in human psilocybin studies. In all recent clinical studies with psilocybin, the administration of drugs was never the sole intervention, but was always accompanied by psychotherapy or psychological preparation, support during the trip, oftentimes pleasant music, and integration of experience after the treatment session. These factors could be necessary for achieving an antidepressant response, and are difficult, if not impossible, to translate into animal experiments. Also, higher cognitive abilities might be necessary for achieving a positive effect with psychedelics. In humans, psychedelic trips are often highly profound and meaningful, but we currently do not know whether this is the case in rats. The fact that ketamine did show a significant antidepressant-like effect in the FSL rat (Liebenberg et al., Reference Liebenberg, Joca and Wegener2015), while psilocybin did not, indicates different mechanisms of action; however, the appropriate level of analysis for this dissimilarity remains unestablished. Altogether, back-translating the antidepressant effect of psilocybin is a complicated matter.
The present studies have some limitations. The translationally relevant dose in rats is yet to be established, and we only covered a range from 0.5 to 10 mg/kg psilocybin. We chose to include a high (10 mg/kg) dose, which is higher than those commonly reported in the published literature [for review see (Halberstadt and Geyer, Reference Halberstadt and Geyer2018)], because of the lower 5-HT2A affinity in rats compared with humans (Gallaher et al., Reference Gallaher, Chen and Shih1993). Furthermore, microdialysis studies showed that dopamine levels in the nucleus accumbens (NAc) are strongly altered, selectively, by 10 mg/kg psilocin and not by 1 or 5 mg/kg (Sakashita et al., Reference Sakashita, Abe, Katagiri, Kambe, Saitoh and Utsunomiya2015). This could be relevant to its antidepressant effect, as the human doses that produce a therapeutic effect (0.30–0.35 mg/kg) have also been found to increase dopamine levels in ventral striatum (NAc and olfactory tuberculum) in human PET studies (Vollenweider et al., Reference Vollenweider, Vontobel, Hell and Leenders1999). Our studies are also limited by having only measured a single behavioral outcome in the FSL rats – immobility in the FST. This is potentially problematic in two ways. Firstly, it is possible that behavioral or neurobiological abnormalities in the FSL rats, which were unmeasured, were rescued by psilocybin/psilocin; and secondly, depression is a complex illness with a multifaceted symptomatology, which may in principle not be validly reducible to a single behavioral outcome measure.
We consider our results to carry a low risk of bias. Animals were individually randomised to experimental groups, allocation was concealed, the outcome assessor was blinded, incomplete outcome data has been addressed, and we reported all the obtained results. However, we have not provided group-specific baseline characteristics or adjusted for confounders, and investigators were only blinded to the strain of the animals during the injections and not the treatment group.
In conclusion, we found no antidepressant-like effect of psilocin or psilocybin in the FSL rats using the FST, despite the significant antidepressant effect observed in humans. Human studies suggest that the antidepressant effect of psilocybin could be of a different nature than classical antidepressants, and we therefore propose testing psilocybin in a range of other behavioural tests that measure other symptoms of depression.
Supplementary material
To view supplementary material for this article, please visit https://doi.org/10.1017/neu.2019.15
Author ORCIDs
Oskar Jefsen, 0000-0002-5831-5158
Acknowledgements
We express a special gratitude to the animal caretakers Tessa Rasmussen, Stine Dhiin, and Mie Nødgaard Hansen for having maintained experimental animals.
Authors’ contributions
OJ designed and executed experiments 2–4, analysed and interpreted all data. KH designed and executed experiments 1–2. SLC designed and executed experiment 1. BE, DJN, and GW reviewed and interpreted the data. HKM designed the experiments, reviewed and interpreted the data. All authors contributed on writing the manuscript.
Financial support
The study was supported financially by the Independent Research Fund Denmark (DFF-7025-00041), the Lundbeck Foundation (R-197-2016-1730), Torben and Alice Frimodts Fond, Fonden til Laegevidenskabens Fremme, and Dagmar Marshalls Fond.
Conflict of Interest
Gregers Wegener is Editor-in-Chief of Acta Neuropsychiatrica, but was not involved and actively withdrew during the review and decision process of this manuscript. Gregers Wegener reported having received lecture/consultancy fees from H. Lundbeck A/S, Servier SA, AstraZeneca AB, Eli Lilly A/S, Sun Pharma Pty Ltd., Pfizer, Inc., Shire A/S, HB Pharma A/S, Arla Foods Amba., and Mundipharma International, Ltd. David John Nutt is a scientific advisor for COMPASS Pathways that is currently conducting a multicentre clinical trial of psilocybin in Europe. All other authors report no potential conflicts of interest.
Animal welfare
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use of laboratory animals.





