Although a genetic basis for schizophrenia is now virtually undisputed (Reference Cardno, Marshall and CoidCardno et al, 1999), cohort studies have provided evidence both for (Reference Hultman, Öhman and CnattingiusHultman et al, 1997; Reference Jones, Rantakallio and HartikainenJones et al, 1998; Reference Dalman, Allebeck and CullbergDalman et al, 1999) and against (Reference Done, Johnstone and FrithDone et al, 1991; Reference Buka, Tsuang and LipsittBuka et al, 1993) an association between obstetric adversity and later development of the disease. In a meta-analysis, Geddes & Lawrie (Reference Geddes and Lawrie1995) concluded that while there was some evidence of an association, there was also evidence for publication bias, as relatively few small negative studies had been published. Many studies within that meta-analysis had small numbers of index cases, relied on maternal recall of obstetric complications and failed to control for social class at the time of birth. We set out to address some of these methodological difficulties by using contemporaneous birth records of patients from a geographically defined case register and closely matched controls.
METHOD
Subjects
The Dublin Psychiatric Case Register is based on an integrated community service for a population of approximately 253 000. A specialist team compiles data for all in-patient and out-patient contacts with the psychiatric services. We initially selected 1779 Dublin-born patients who had been given an ICD-9 (World Health Organization, 1978) diagnosis of schizophrenia (ICD-9 295.0-295.9) between 1972 and 1992. Four hundred and thirty-one cases were included in the final analyses. The reasons for excluding cases are shown in Table 1.
Table 1 Case tracing results

| Cases lost to sample | Sample size (n) | Percentage of sample that are searchable singleton maternity hospital births | |
|---|---|---|---|
| Register | — | 1779 | — |
| Insufficient register data to allow tracing | 349 | 1430 | — |
| Estimate of sample not born in study hospitals (19.8%) | 283 | 1147 | — |
| Twin births | 40 | 1107 | — |
| Found cases | — | 541 | 49% |
| Missing social class data for matching | 110 | 431 | 39% |
Birth records
The maternity hospital labour ward diary detailed the following: parental names; age of mother; parity, number of pregnancies, length of gestation; paternal occupation; gender of child; date of birth; mode of delivery; presentation; whether twin or singleton; birth weight; premature rupture of membranes; nature of labour; hours in labour; child's and mother's health immediately after delivery; transfer to baby unit; immediate baptism. The diary, including a free-text record of the birth and delivery, was recorded verbatim. Similar data relating to home births, including a 9-day follow-up record of the baby's health, were also recorded.
Controls
The previous same-gender singleton live birth recorded in the labour ward diary, matched for maternal age, parity, social class and home/hospital birth, was selected as a control. The maternal age of the index case ±2 years was chosen as the age cut-off point for the maternal control. Parity status was classified as follows: prima gravida (first delivery), multi gravida (2-4 deliveries) or grand multi gravida (more than four deliveries). Social class, based on paternal occupation, was matched according to the classification system of O'Hare et al (Reference O'Hare, Whelan and Commins1991), which is also used by the Irish Central Statistics Office. One of the hospitals did not record paternal occupation, so where possible we obtained this information from the General Register Office birth register. Because we were unable to establish whether controls lived to the age at which the risk of developing schizophrenia occurs, we identified 22 deaths in the first year of life in the General Register Office death register and replaced them with appropriate controls. All birth records were rated blindly according to two obstetric complication scales, scale 1 (Reference Lewis, Owen, Murray, Schulz and TammingaLewis et al, 1989) and scale 2 (Reference Parnas, Schulsinger and TeasdaleParnas et al, 1982).
Analyses
Individual items from the obstetric complication scales were analysed separately using matched pairwise techniques. Odds ratios (ORs) and confidence intervals (CIs) were calculated for binary variables. Conditional logistic regression was used to compute for the development of the disorder after allowing for case-control matching. This procedure was repeated for each of the items in the separate scales.
RESULTS
The final study group comprised 431 cases (256 males, 175 females). This group had fewer married females and fewer in the higher social classes than the original selection. Cases were indistinguishable from controls in terms of maternal age, maternal parity and social class of origin (Table 2).
Table 2 Matching criteria: a comparison of cases and controls (n=431)

| Criterion | Cases | Controls | T or z | P |
|---|---|---|---|---|
| Maternal age (years) | 30.7 (s.d.=5.9) | 30.6 (s.d.=5.8) | T=0.88 | 0.381 |
| Number of pregnancies | 3.98 (s.d.=2.9) | 3.91 (s.d.=2.9) | T=-0.78 | 0.441 |
| Social class | 4.98 (s.d.=1.2) | 4.98 (s.d.=1.2) | z=-0.02 | 0.982 |
Scales
Cases did not differ from controls on scale 1 in terms of either definite (OR 1.05, 95% CI 0.72-1.54, P=0.85) or equivocal (OR 0.92, 95% CI 0.61-1.40, P=0.76) complications. Because we were evaluating contemporaneous labour ward records rather than depending on maternal recall, we also combined the definite and equivocal complications categories and found no difference between patients and controls. Similarly, scale 2 did not distinguish between cases and controls in terms of frequency, severity or total complications score.
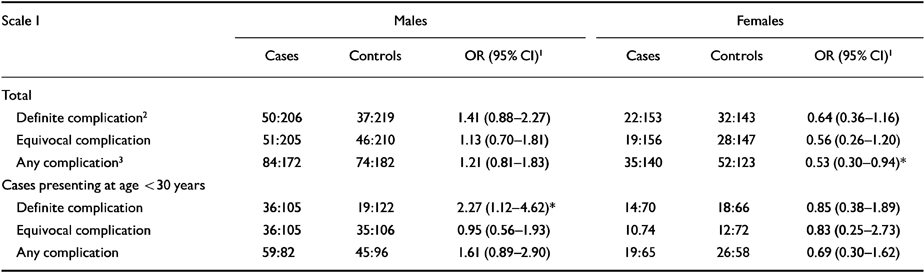
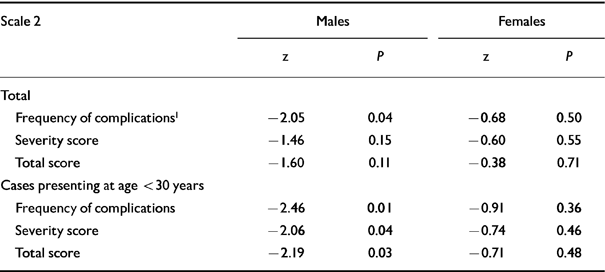
In the light of previous findings (Reference O'Callaghan, Gibson and ColohanO'Callaghan et al, 1992; Reference Kirov, Jones and HarveyKirov et al, 1996; Reference Verdoux, Geddes and TakeiVerdoux et al, 1997; Reference Smith, Kopala and LapointeSmith et al, 1998) we split the study groups by age at first diagnosis and gender and found that only males diagnosed with schizophrenia before the age of 30 (Table 3) had a greater number of definite complications than controls on scale 1. Similarly, using scale 2, male patients had a higher frequency of and more severe complications than their matched controls (Table 4).
Table 3 Obstetric complication scale 1: summary scores (Lewis & Murray, 1989).
| Scale 1 | Males | Females | ||||
|---|---|---|---|---|---|---|
| Cases | Controls | OR (95% CI)1 | Cases | Controls | OR (95% CI)1 | |
| Total | ||||||
| Definite complication2 | 50:206 | 37:219 | 1.41 (0.88-2.27) | 22:153 | 32:143 | 0.64 (0.36-1.16) |
| Equivocal complication | 51:205 | 46:210 | 1.13 (0.70-1.81) | 19:156 | 28:147 | 0.56 (0.26-1.20) |
| Any complication3 | 84:172 | 74:182 | 1.21 (0.81-1.83) | 35:140 | 52:123 | 0.53 (0.30-0.94)* |
| Cases presenting at age <30 years | ||||||
| Definite complication | 36:105 | 19:122 | 2.27 (1.12-4.62)* | 14:70 | 18:66 | 0.85 (0.38-1.89) |
| Equivocal complication | 36:105 | 35:106 | 0.95 (0.56-1.93) | 10.74 | 12:72 | 0.83 (0.25-2.73) |
| Any complication | 59:82 | 45:96 | 1.61 (0.89-2.90) | 19:65 | 26:58 | 0.69 (0.30-1.62) |
Table 4 Obstetric complication scale 2: summary scores (Reference Parnas, Schulsinger and TeasdaleParnas et al, 1982)
| Scale 2 | Males | Females | ||
|---|---|---|---|---|
| z | P | z | P | |
| Total | ||||
| Frequency of complications1 | -2.05 | 0.04 | -0.68 | 0.50 |
| Severity score | -1.46 | 0.15 | -0.60 | 0.55 |
| Total score | -1.60 | 0.11 | -0.38 | 0.71 |
| Cases presenting at age <30 years | ||||
| Frequency of complications | -2.46 | 0.01 | -0.91 | 0.36 |
| Severity score | -2.06 | 0.04 | -0.74 | 0.46 |
| Total score | -2.19 | 0.03 | -0.71 | 0.48 |
Individual complications
The specific complications of Caesarean section (OR 4.00, 95% CI 1.08-22.1, P=0.04) and narrow pelvis (OR 7.00, 95% CI 0.90-320, P=0.07) distinguished patients from controls. The individual complications, classified by gender and age of presentation, for each scale are shown in Tables 5 and 6. We found that Caesarean section (both emergency and not otherwise specified (NOS)) distinguished between cases and controls (OR 7.82 × 1014, 95% CI 0-0, P=0.004) and was specific to males. Those born by Caesarean section presented to the psychiatric services at a significantly younger age (mean 24.01 years, s.d.=6.3; T=3.76, P=0.003) than those born by normal delivery (mean 31.4 years, s.d.=10.6). Conditional logistic regression analysis of the males confirmed that only Caesarean section was significant (log likelihood removal = 354.9, d.f.=1, P≤0.001) in differentiating between cases and controls. For females, only low birth weight (log likelihood removal = 242.6, d.f.=1, P=0.002) distinguished between the two groups.
Table 5 Individual complications from scale 1 (Reference Lewis, Owen, Murray, Schulz and TammingaLewis et al, 1989): individual complication odds ratios between cases and controls. Sample divided initially by gender and then by age of presentation to psychiatric services

| Complication | Total sample | Case-control pairs presenting at age <30 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||||
| Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI) | |
| Definite complications | ||||||||||||
| Rhesus incompatibility | 1:255 | 0:256 | 2.9 × 1014 (0-0) | 2:173 | 2:173 | 1.0 (0.1-16.0) | 1:140 | 0:140 | — | 1:83 | 2:82 | 5.1 × 10-16 (0-0) |
| Pre-eclampsia severe | 3:253 | 2:254 | 0.8 (0.2-3.4) | 0:175 | 1:174 | 1.3 × 10-15 (0-0) | 1:140 | 1:140 | 1.0 (0.1-16.0) | 0.84 | 0:84 | — |
| Antepartum haemorrhage | 3:253 | 2:254 | 0.5 (0.1-2.7) | 0:175 | 4:171 | 1.3 × 10-15 (0-0)* | 3:138 | 3:138 | 1.0 (0.1-7.1) | 0:84 | 2:82 | 1.3 × 10-15 (0-0) |
| Labour >36 h | 2:254 | 3:253 | 0.5 (0.1-4.0) | 2:173 | 3:172 | 0.5 (0.1-5.5) | 1:140 | 1:140 | 1.0 (0.1-16.0) | 0:84 | 1:83 | 1.3 × 10-15 (0-0) |
| Labour <3 h | 12:244 | 9:247 | 1.4 (0.6-3.4) | 8:167 | 10:165 | 0.8 (0.3-2.1) | 9:132 | 4:137 | 6.0 (0.7-49.8)* | 6:78 | 4:80 | 1.3 (0.30-5.96) |
| Cord prolapse | 1:255 | 2:254 | 0.5 (0.1-5.5) | 2:173 | 0:175 | 7.8 × 1014 (0-0) | 0:141 | 1:140 | 1.3 × 10-15 (0-0) | 2:82 | 0:84 | 7.8 × 1014 (0-0) |
| Gestational age <37 weeks | 11:245 | 5:251 | 2.2 (0.8-6.3) | 2:173 | 5:170 | 0.4 (0.1-2.1) | 9:132 | 1:140 | 8.0 (1.0-64.0)* | 1:83 | 3:81 | 0.3 (0.1-3.2) |
| Gestational age >42 weeks | 3:253 | 1:255 | 3.0 (0.3-28.8) | 2:173 | 3:172 | 0.7 (0.1-4.0) | 3:138 | 1:140 | 3.0 (0.3-28.8) | 1:83 | 3:81 | 0.3 (0.1-3.2) |
| Caesarean section (emergency) | 5:251 | 0:256 | 7.8 × 1014 (0-0) | 2:173 | 2:173 | 1.0 (0.2-7.1) | 5:136 | 0:141 | 7.8 × 1014 (0-0)* | 1:83 | 2:82 | 1.0 (0.1-16.0) |
| Breech/abnormal presentation | 11:245 | 6:250 | 1.8 (0.7-5.0) | 5:170 | 9:166 | 0.6 (0.2-1.7) | 7:134 | 2:139 | 1.5 (0.3-9.0) | 2:82 | 6:78 | 0.5 (0.1-2.7) |
| High/difficult forceps | 9:247 | 9:247 | 1.0 (0.4-2.7) | 3:172 | 0:175 | 7.8 × 1014 (0-0)* | 6:135 | 6:135 | 1.0 (0.3-4.0) | 2:82 | 0:84 | 7.8 × 1014 (0-0) |
| Birthweight <2000 g | 3:253 | 1:255 | 3.0 (0.3-28.8) | 0:175 | 2:173 | 1.3 × 10-15 (0-0) | 1:140 | 0:141 | — | 0:84 | 1:83 | 4.7 × 10-16 (0-0) |
| Equivocal complications | ||||||||||||
| Pre-eclampsia NOS | 8:248 | 6:250 | 1.4 (0.4-4.4) | 2:173 | 1:174 | 2.0 (0.2-22.1) | 7:134 | 6:135 | 2.0 (0.5-8.0) | 2:82 | 1:83 | 2.0 (0.2-22.1) |
| Labour > 24 h/difficult/precipitate | 10:246 | 11:245 | 0.9 (0.4-2.1) | 3:172 | 4:171 | 0.8 (0.2-3.4) | 7:134 | 7:134 | 0.7 (0.2-2.3) | 1:83 | 1:83 | 0.6 (0.1-16.0) |
| Cord knotted around neck | 1:255 | 1:255 | 1.0 (0.1-16.0) | 0:175 | 1:174 | 1.3 × 10-15 (0-0) | 1:140 | 0:141 | 7.8 × 1014 (0-0) | 0:84 | 0:84 | — |
| Premature/postmature | 2:254 | 2:254 | 1.0 (0.1-7.1) | 0:175 | 2:173 | 1.3 × 10-15 (0-0) | 1:140 | 1:140 | 1.00 (0.1-16.0) | 0:84 | 0:84 | — |
| Caesarean section NOS | 5:251 | 0:256 | 7.8 × 1014 (0-0) | 0:175 | 1:174 | 3.5 × 10-15 (0-0) | 3:138 | 0:141 | 7.8 × 1014 (0-0)* | 0:84 | 0:84 | — |
| Forceps/instrumental delivery | 15:241 | 16:240 | 1.2 (0.6-2.4) | 9:166 | 10:165 | 1.2 (0.4-3.5) | 12:129 | 14:127 | 1.0 (0.4-2.5) | 3:81 | 6:78 | 0.3 (0.1-3.2) |
| Birth weight <2500 g/small | 8:248 | 7:249 | 0.7 (0.3-1.8) | 2:173 | 10:165 | 1.2 (0.0-0.8)* | 3:138 | 3:138 | 0.4 (0.1-2.1) | 2:82 | 6:78 | 0.3 (0.1-1.7) |
| Incubator/resuscitation/blue | 14:242 | 13:243 | 1.1 (0.5-2.6) | 5:170 | 3:172 | 1.7 (0.4-7.0) | 10:131 | 10:131 | 0.9 (0.3-2.6) | 2:82 | 3:81 | 1.0 (0.1-7.1) |
| Gross anomaly | 2:254 | 1:255 | 2.0 (0.2, 22.1) | 0:175 | 1:174 | 3.5 × 10-15 (0-0) | 1:140 | 1:140 | 1.0 (0.1-16.0) | 0:84 | 0:84 | — |
Table 6 Individual complications from scale 2 (Reference Parnas, Schulsinger and TeasdaleParnas et al, 1982): individual complication odds ratios between cases and controls. Sample divided initially by gender and then by age at presentation to psychiatric services

| Complication | Total sample | Case-control pairs presenting at age < 30 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||||||
| Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI) | Cases | Controls | OR (95% CI) | |
| Forceps | 23:233 | 25:231 | 0.9 (0.5-1.7) | 12:163 | 10:165 | 1.6 (0.5-4.9) | 17:124 | 19:122 | 0.8 (0.4-1.7) | 5:79 | 6:78 | 1.0 (0.1-7.1) |
| Caesarean section | 10:246 | 0:256 | 7.8 × 1014 (0-0)* | 2:173 | 3:172 | 0.7 (0.1-4.0) | 8:133 | 0:141 | 7.8 × 1014 (0-0)* | 1:83 | 2:82 | 1.0 (0.1-16.0) |
| Placental defects | 4:252 | 4:252 | 1.0 (0.2-3.4) | 1:174 | 3:172 | 0.3 (0.1-3.2) | 3:138 | 4:137 | 1.0 (0.1-7.1) | 1:83 | 1:83 | 1.0 (0.1-16.0) |
| Previous foetal loss | 4:252 | 5:251 | 0.8 (0.2-3.4) | 5:170 | 4:171 | 1.3 (0.3-6.0) | 3:138 | 5:136 | 0.3 (0.1-2.2) | 4:80 | 4:80 | 1.0 (0.2-5.0) |
| Bleeding after delivery | 5:251 | 4:252 | 1.3 (0.4-4.7) | 2:173 | 0:175 | 7.8 × 1014 (0-0) | 2:139 | 1:140 | 2.0 (0.2-22.1) | 1:83 | 0:84 | 7.8 × 1014 (0-0) |
| Narrow pelvis | 6:250 | 1:255 | 6.0 (0.7-49.8)* | 1:174 | 0:175 | 7.8 × 1014 (0-0) | 6:135 | 0:141 | 7.8 × 1014 (0-0)* | 0:84 | 0:84 | — |
| Mother's illness during pregnancy | 13:243 | 6:250 | 2.4 (0.9-6.8) | 6:169 | 7:168 | 0.9 (0.3-2.6) | 9:132 | 5:136 | 4.0 (0.9-18.8)* | 5:79 | 5:79 | 1.0 (0.3-3.5) |
| Labour time > 24 h | 5:251 | 6:250 | 0.8 (0.3-2.7) | 2:173 | 4:171 | 0.5 (0.1-2.7) | 3:138 | 2:139 | 1.5 (0.3-9.0) | 0:84 | 1:83 | 1.3 × 10-15 (0-0) |
| Mother's serious illness | 2:254 | 1:255 | 2.0 (0.2-22.1) | 2:173 | 2:173 | 1.0 (0.2-7.1) | 2:139 | 1:140 | 1.0 (0.1-16.0) | 0:84 | 0:84 | — |
| Bad foetal position | 11:244 | 6:250 | 1.8 (0.7-5.0) | 5:170 | 9:166 | 0.6 (0.2-1.7) | 7:134 | 2:139 | 1.5 (0.3-9.0) | 2:82 | 6:78 | 0.5 (0.1-2.7) |
| Contractions of pelvis during delivery | 0:256 | 0:256 | — | 1:174 | 0:175 | 7.8 × 1014 (0-0) | 0:141 | 0:141 | — | 1:83 | 0:84 | — |
| Primary uterine inertia | 1:255 | 2:254 | 0.5 (0.1-5.5) | 0:175 | 0:175 | — | 1:140 | 2:139 | 1.0 (0.1-16.0) | 0:84 | 0:84 | — |
| Prematurity with weight > 2500 g | 8:248 | 4:252 | 2.0 (0.6-6.7) | 1:174 | 2:173 | 0.5 (0.1-5.5) | 6:135 | 0:141 | 7.8 × 1014 (0-0)* | 0:84 | 0:84 | — |
| Labour time > 36 h | 2:254 | 2:254 | 1.0 (0.2-7.1) | 1:174 | 1:174 | 1.0 (0.1-16.0) | 1:140 | 1:140 | 1.0 (0.1-16.0) | 0:84 | 0:84 | — |
| Secondary uterine inertia | 9:247 | 6:250 | 1.5 (0.5-4.2) | 1:174 | 0:175 | 2.9 × 1014 (0-0) | 6:135 | 5:136 | 1.0 (0.3-3.5) | 1:83 | 0:84 | 7.8 × 1014 (0-0) |
| Bleeding during delivery | 1:255 | 2:254 | 3.8 × 10-15 (0-0) | 0:175 | 4:171 | 1.3 × 10-15 (0-0)* | 1:140 | 1:140 | 1.4 × 10-15 (0-0) | 0:84 | 3:81 | 4.7 × 10-16 (0-0) |
| Labour > 48 h | 0:256 | 1:255 | 3.5 × 10-15 (0-0) | 1:174 | 2:173 | 0.5 (0.1-5.5) | 0:141 | 0:141 | — | 0:84 | 1:83 | 1.3 × 10-15 (0-0) |
| Asphyxiation | 9:247 | 6:250 | 1.6 (0.5-4.9) | 2:173 | 0:175 | 7.8 × 1014 (0-0) | 7:134 | 4:137 | 1.7 (0.4-7.0) | 0:84 | 0:84 | — |
| Umbilical complications | 2:254 | 3:253 | 0.7 (0.1-4.0) | 2:173 | 1:174 | 2.0 (0.2-22.1) | 1:140 | 1:140 | 1.0 (0.1-16.0) | 2:82 | 0:84 | 7.8 × 1014 (0-0) |
| Eclampsia | 2:254 | 1:255 | 2.0 (0.2-22.1) | 0:175 | 0:175 | — | 0:141 | 0:141 | — | 0:84 | 0:84 | — |
| Prematurity with weight < 2500 g | 4:252 | 2:254 | 2.0 (0.4-10.9) | 1:174 | 3:172 | 0.3 (0.1-3.2) | 3:138 | 1:140 | 2.0 (0.22-22.1) | 1:83 | 2:82 | 0.5 (0.1-5.5) |
DISCUSSION
Limitations
The patients received a clinical discharge diagnosis by a psychiatrist according to ICD-8 (World Health Organization, 1974) or ICD-9 rather than a research diagnosis. Less than 50% of the targeted sample were found, more male than female birth records were identified and the age at first diagnosis was younger for located cases. These results could be accounted for by our inability to trace the birth records of married females. Some home births were excluded because their records contained insufficient detail. Social class is related to obstetric outcome (Reference Wilcox, Smith and JohnsonWilcox et al, 1995), so we excluded 110 cases that could not be matched adequately for social class of origin. Finally, the labour ward diaries contained only limited information on early pregnancy and the postnatal period.
Scales and individual labour and delivery complications
Despite applying two frequently used obstetric complication scales we failed to find differences between cases and controls in terms of overall complication scores. There is an apparent difference between these overall results and other large studies in this field (Reference Kendell, Juszczak and ColeKendell et al, 1996; Reference Hultman, Öhman and CnattingiusHultman et al, 1997). One potential explanation for the contradictory results may lie in the patient selection procedures. Many case-control and cohort studies (Reference Done, Johnstone and FrithDone et al, 1991; Reference McCreadie, Hall and BerryMcCreadie et al, 1992; Reference Verdoux and BourgeoisVerdoux & Bourgeois, 1993; Reference Kendell, Juszczak and ColeKendell et al, 1996) have relied on information from patients born in recent years when either their mothers were alive or birth records, particularly computerised information, were available. Such patients are more likely to have had a young age at onset. While this study indicates that young male patients do have an excess of obstetric complications we failed to find any general effect of obstetric adversity among persons developing the disorder.
Persons who later developed schizophrenia were more likely to have been born by Caesarean section. This finding complements those of the recent meta-analysis by Verdoux et al (Reference Verdoux, Geddes and Takei1997) and a National Register study in Denmark (Reference Bennedsen, Mortensen and OlesenBennedsen et al, 1998), although in the present study the effect was confined to males. We confirm, using a case register study, the meta-analysis result (Reference Verdoux, Geddes and TakeiVerdoux et al, 1997) that Caesarean section is related to a younger age at first presentation with schizophrenia.
Although this association is interesting, the complication itself is non-specific. Caesarean section is the result of the obstetrician's judgement to intervene in response to a variety of potential risks. Most of the patients in this study were born when the rate of Caesarean section was less than 3% and was rarely an elective procedure. The commonest recorded reasons for section were major antepartum haemorrhage, deep transverse arrest or failure of labour to progress where foetal distress was apparent. Cephalopelvic disproportion was noted in 25% of cases born by Caesarean section, and a clinical note recording ‘narrow pelvis’ was found more frequently among cases than among controls.
One of the commonest reasons for a narrow maternal pelvis is poor nutrition during adolescence. Mothers born and raised in Third World countries who migrated to the USA are on average shorter and have narrower pelvic dimensions than mothers born in the USA. Those immigrant women who eat a high-protein diet and receive adequate prenatal care give birth to relatively large infants, which results in cephalopelvic disproportion and severe dystocia (Reference Abitbol, Taylor-Randall and BartonAbitbol et al, 1997). In addition to the direct effects of malnutrition (Reference Susser and LinSusser & Lin, 1992), cephalopelvic disproportion merits consideration as a nutritionally related risk factor, particularly for groups previously described as having increased risk of schizophrenia (Reference WarnerWarner, 1995; Reference Harrison, Glazebrook and BrewinHarrison et al, 1997).
The reported palatal (Reference O'Callaghan, Larkin and KinsellaO'Callaghan et al, 1991; Reference Cantor-Graae, McNeil and TorreyCantor-Graae et al, 1994; Reference Lane, Kinsella and MurphyLane et al, 1997) and craniofacial abnormalities among patients with schizophrenia may not entirely result from genetic factors, as we considered previously but may be related to a moulding process in a narrow pelvis (Reference de la Fuentede la Fuente, 1991). However, a narrow pelvis in itself does not necessarily result in damage to the foetus, since when identified it commonly results in operative delivery.
Among patients, a gestational age of less than 37 weeks occurred more frequently among males whose first diagnosis was before the age of 30. While this result has been reported previously (Reference Jones, Rantakallio and HartikainenJones et al, 1998), such cases, as with Caesarean section and cephalopelvic disproportion, accounted for a modest proportion of our study group. Indeed, despite several individual complications being associated with the later development of schizophrenia, the proportion of cases affected was extremely small, indicating that obstetric adversity is not associated with the majority of cases of schizophrenia, especially when the age at onset is over 30 years.
Although this paper describes a large case-control study which used contemporaneous records, the fact that many individual labour and delivery complications occur at a frequency of less than 10% in the general population suggests that the cross-referencing of population-based obstetric databases with psychiatric case registers is necessary in order to address definitively the contribution of individual complications to the risk of schizophrenia.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
▪ Labour and delivery complications are relevant to some, but certainly not all, patients with schizophrenia.
-
▪ Delivery by Caesarean section was more common among patients than controls and was associated with a younger age at first diagnosis among males.
-
▪ Male cases were more frequently associated with complications.
LIMITATIONS
-
▪ Only 49% of the estimated number of traceable records were found.
-
▪ The study was confined to labour and delivery complications. Only limited data for the pregnancies and perinatal period were identified.
-
▪ Despite the sample size, we believe that the power of the present study was limited.
ACKNOWLEDGEMENTS
We thank the staff and clinicians of the Dublin Psychiatric Case Register and the masters and filing staff of the four hospitals included in this study. We are grateful for permission from the Minister of Health to access the General Registry Office (GRO) records and to the staff of the GRO for assisting us. Permission was kindly granted by the Masters of the three major Dublin maternity hospitals (the National Maternity Hospital and Rotunda and Coombe Hospitals) to access labour ward diaries, which have been retained at each hospital since 1896. Additionally, we were granted access to the maternity unit of St James's Hospital which, although no longer functioning as an obstetric unit, has retained some records.









eLetters
No eLetters have been published for this article.