Introduction
Feeding underwater is fundamental for all aquatic vertebrates. While the variations of feeding styles in extant and extinct vertebrates are sometimes difficult to study, especially for obligately aquatic time, the cycle of prey acquiring, processing, and swallowing is well understood in model taxa. This research points to fundamental constraints related to vertebrate development of the skull and jaws, as well as the physics of prey items in water (Lauder and Shaffer Reference Lauder, Shaffer, Hanken and Hall1993; Marshall and Pyenson Reference Marshall, Pyenson, Bels and Whishaw2019). Comparisons among distantly related lineages of secondarily aquatic vertebrates can still be informative; for example, marine reptiles and marine mammals have convergently evolved similar dentition and rostra, and even lineages within these two groups have evolved parallel feeding kinematics (McCurry et al. Reference McCurry, Walmsley, Fitzgerald and McHenry2017, Reference McCurry, Evans, Fitzgerald, McHenry, Bevitt and Pyenson2019; Marshall and Pyenson Reference Marshall, Pyenson, Bels and Whishaw2019; McCurry and Pyenson Reference McCurry and Pyenson2019).
The aforementioned examples focus on convergence using skeletal features, especially those related to crania, because they are well represented in the fossil record. Secondarily aquatic vertebrates include lineages of amniotes from the past 300 Myr of tetrapod evolution, and it is clear that evolutionary convergence spans anatomical scales from cellular to tissue to whole functional complexes (Lindgren et al. Reference Lindgren, Kaddumi and Polcyn2013; Kelley and Pyenson Reference Kelley and Pyenson2015; Delsett et al. Reference Delsett, Friis, Kölbl-Ebert and Hurum2022). The most-cited example of marine convergent evolution is the fish-shaped body of parvipelvian ichthyosaurs and odontocete cetaceans (Morris Reference Morris1998; McGhee Reference McGhee2011; Fig. 1), which is a comparison that illustrates how convergence can be detected at all scales, from bone microstructure to body shape and ecological role (Bernard et al. Reference Bernard, Lécuyer, Vincent, Amiot, Bardet, Buffetaut, Cuny, Fourel, Martineau, Mazin and Prieur2010; McGhee Reference McGhee2011; Lindgren et al. Reference Lindgren, Sjövall, Carney, Uvdal, Gren, Dyke, Schultz, Shawkey, Barnes and Polcyn2014, Reference Lindgren, Sjövall, Thiel, Zheng, Ito, Wakamatsu, Hauff, Kear, Engdahl, Alwmark, Eriksson, Jarenmark, Sachs, Ahlberg, Marone, Kuriyama, Gustafsson, Malmberg, Thomen, Rodríguez-Meizoso, Uvdal, Ojika and Schweitzer2018; Houssaye et al. Reference Houssaye, Martin Sander and Klein2016).

Figure 1. Fish-shaped marine tetrapods and their hyoid apparatuses. A–C, Delphinoids; D–F, ophthalmosaurid ichthyosaurs. A, Common dolphin (Delphinus delphis) photographed by Wayne Hoggards, NOAA. B, Skeleton of common bottlenose dolphin (Tursiops truncatus), with the hyoid apparatus colored, modified from Cozzi et al. (Reference Cozzi, Huggenberger and Oelschläger2017). C, Ventral view of T. truncatus skull with colored hyoid apparatus. D, Drawing of Early Cretaceous Keilhauia nui by Esther van Hulsen. E, Line drawing of Ophthalmosaurus icenicus modified from Moon and Kirton (Reference Moon and Kirton2016), with hyoid apparatus colored. F, Ophthalmosaurid skull in ventral view modified from McGowan and Motani (Reference McGowan and Motani2003). Note that the actual placement and architecture of the ophthalmosaurid hyoid apparatus is unknown (see text). Abbreviations: BH, basihyal; H, hyoid; HC, hyoid corpus; S, stylohyal; T, thyrohyal.
Parvipelvian ichthyosaurs from the Jurassic and Cretaceous had an overall similar body shape to living delphinoids, often assumed to indicate a similar lifestyle as fast-swimming predators. Still, feeding and diet in ichthyosaurs are not well known (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013). Fossilized stomach contents show that cephalopods were a major food source but also that ichthyosaurs were opportunistic feeders, similar to extant odontocete whales (Pollard Reference Pollard1968; Massare Reference Massare1987; Böttcher Reference Böttcher1989; Kear et al. Reference Kear, Boles and Smith2003; Massare et al. Reference Massare, Buchholtz, Kenney and Chomat2006; Fischer et al. Reference Fischer, Bardet, Benson, Arkhangelsky and Friedman2016). Studies of ichthyosaur tooth morphology and braincase elements suggest that there was niche differentiation among relatively similar-looking parvipelvians, including taxa that possibly fed on large prey (Dick and Maxwell Reference Dick and Maxwell2015; Fischer et al. Reference Fischer, Bardet, Benson, Arkhangelsky and Friedman2016; Cortés et al. Reference Cortés, Maxwell and Larsson2021). Studies that sampled more broadly within these groups and generated clade-wide comparisons would better test these broad-brush examples of convergence (Carroll Reference Carroll1997; Caldwell Reference Caldwell2002; Kelley and Pyenson Reference Kelley and Pyenson2015; Houssaye et al. Reference Houssaye, Martin Sander and Klein2016).
Many marine vertebrates, especially those with terrestrial ancestors, such as whales and ichthyosaurs, have evolved adaptations for raptorial feeding, suction feeding, or filter feeding, or they use a combination of methods (Werth Reference Werth and Schwenk2000). A crucial component for marine tetrapod feeding is the hyoid apparatus, which supports the tongue for transport of food and moves to generate suction. The hyoid apparatus consists of endoskeletal elements that have a splanchnocranial origin, and its architecture varies across vertebrate groups. The shape and size of the hyoid apparatus vary depending on feeding mode, and because feeding modes and hyoid morphology are relatively well known in extant cetaceans, this can be used to infer feeding modes in extinct taxa for which only the skeleton is known (Werth Reference Werth2007; Johnston and Berta Reference Johnston and Berta2011; Cooper et al. Reference Cooper, Hieronymus, Vinyard, Bajpai, Thewissen, Hembree, Platt and Smith2014; Peredo et al. Reference Peredo, Pyenson, Marshall and Uhen2018). Odontocetes have a wider and longer hyoid apparatus on average compared with other mammals (Reidenberg and Laitman Reference Reidenberg and Laitman1994), consisting of two pairs of (usually) ossified rods (stylohyals and thyrohyals) connected to the medial basihyal (Fig. 1). On the other hand, the internal microstructural architecture of the hyoid apparatus and its adaptations to feeding mode are relatively unknown, constituting an untapped source of information, as the inner structure of skeletal elements often reflects their biomechanical use (de Ricqlès Reference de Ricqlès1977).
When preserved and collected, the hyoid apparatus in ichthyosaurs usually consists of a single pair of ossified rods, of which the homology is uncertain (Owen Reference Owen1840; Sollas Reference Sollas1918; Kear Reference Kear2005; Ji et al. Reference Ji, Jiang, Motani, Hao, Sun and Cai2013; Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013), and its shape, size and configuration of elements, as well as their exact placement, are unknown (Marek et al. Reference Marek, Moon, Williams and Benton2015; Moon and Kirton Reference Moon and Kirton2016). In rare cases, a third element interpreted as the hyoid corpus (or basihyal) is identified (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013; Miedema and Maxwell Reference Miedema and Maxwell2022). The size and shape of hyoids have been used to infer feeding modes in Triassic to Middle Jurassic taxa. It was suggested that suction feeding was widespread among early ichthyosaurs such as Shastasaurus and Guanlingsaurus, because they possess long hyoids relative to their rostra (Sander et al. Reference Sander, Chen, Cheng and Wang2011). However, this interpretation has been contested, because the hyoids are too slender and short compared with those of known suction feeders among other vertebrates (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013).
Even less is known about the youngest ichthyosaur clade, the ophthalmosaurids, which include all but one species from the Late Jurassic until the extinction of the ichthyosaurs in the middle Cretaceous (Fischer et al. Reference Fischer, Appleby, Naish, Liston, Riding, Brindley and Godefroit2013). For this family, knowledge is lacking on hyoid anatomy and architecture and the relation between the hyoid apparatus, feeding mode, and diet. In this paper, we describe and discuss ophthalmosaurid hyoids, aiming to characterize their external morphology and internal microstructure and detect possible convergent evolution patterns for feeding in ichthyosaurs and odontocetes. We build on the work by Motani et al. (Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013) for comparisons with earlier ichthyosaurs, and describe the internal microstructure of hyoids in ichthyosaurs and, to our knowledge, in two odontocete species for the first time (except Kiprijanoff Reference Kiprijanoff1881). We hypothesize that the microstructure of the hyoid elements in both animals reflects their engagement in repeated dorsoventral movements. These similarities should be most notable for the stylohyals compared with thyrohyals in odontocete whales, as the former are the suspensory elements. Any difference between ichthyosaurs and odontocetes in microanatomy should co-occur with a difference in relative size or shape, as both inner and outer morphology reflect movements of the hyoid apparatus.
Institutional Abbreviations
AM, Australian Museum, Sydney, Australia; BM, University Museum of Bergen, Bergen, Norway; CAMSM, Sedgwick Museum of Earth Sciences, Cambridge, U.K.; CMNH Carnegie Museum of Natural History, Pittsburgh, Pennsylvania, U.S.A.; CN, Natural History Museum of Denmark, Copenhagen, Denmark; IRSNB, Institut royal des Sciences naturelles de Belgique, Brussels, Belgium; MSVG, Museo di speleo-paleontologia e archeologia di San Vittore di Genga, Ancona, Marche, Italy; NHMO, zoological collections, Natural History Museum, Oslo, Norway; NHMUK, Natural History Museum, London, U.K.; PMO, paleontological collections, Natural History Museum, Oslo, Norway; SMSS, Städtisches Museum Schloss Salder, Salzgitter, Germany; SNHM, Staatliches Naturhistorisches Museum Braunschweig, Germany; UPM, Paleontological Museum of Undory, Ul'yanovsk, Russia.
Material and Methods
Size and Shape of Ophthalmosaurid Ichthyosaur Hyoids
Thirteen ophthalmosaurid specimens with one or both hyoid rods preserved were studied (Table 1), out of which 11 preserved at least one complete hyoid so that measurements could be used for calculations. For 8 out of the 13 specimens, anterior-posterior orientation was known. To compare the ophthalmosaurids with the published data on Triassic–Middle Jurassic non-ophthalmosaurids (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013), the same measurements were collected from publications, colleagues, and museum visits: anteroposterior length in a straight line (HL) and width taken half-way (HW). Both were log10 transformed for analysis. Hyobranchial robustness was calculated as in Motani et al. (Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013); HW:HL, similar to the “stylohyal robustness index” in Bloodworth and Marshall (Reference Bloodworth and Marshall2007) and in Johnston and Berta (Reference Johnston and Berta2011). Among the ophthalmosaurid specimens with hyoids (Table 1), none were preserved in articulation in such a way that mandibular width (MW of Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013) or width at the end of the toothrow (TW of Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013) could be measured. This limitation also meant that the metric Mandibular Pressure Concentration Index could not be calculated for the ophthalmosaurids.
Table 1. Ophthalmosauridae specimens used in this study. Abbreviations: HL, hyoid length; HW, hyoid width; ML, mandible length. See Institutional Abbreviations in text for museum acronyms.

Log-transformed measurements were subjected to reduced major axis (RMA) (Model II) regression in Past v. 4.09 (Hammer et al. Reference Hammer, Harper and Ryan2001), with calculation of parameters and standard errors following Warton et al. (Reference Warton, Wright, Falster and Westoby2006). Phylogenetic generalized least squares (PGLS; Grafen and Hamilton Reference Grafen and Hamilton1989; Pagel Reference Pagel1999) was calculated in Past v. 4.12 using a phylogeny consistent with the highly polytomous ichthyosaur consensus tree of Moon (Reference Moon2017). The tree for the taxa included in this study is shown in Figure 2. Polytomies were coded using zero branch lengths. Standard errors on regression parameters were calculated according to Smaers and Rohlf (Reference Smaers and Rohlf2016). The maximum-likelihood estimation of phylogenetic signal (lambda) was based on the regression residuals as recommended by Revell (Reference Revell2010) and was used for transforming the PGLS variance–covariance matrix.
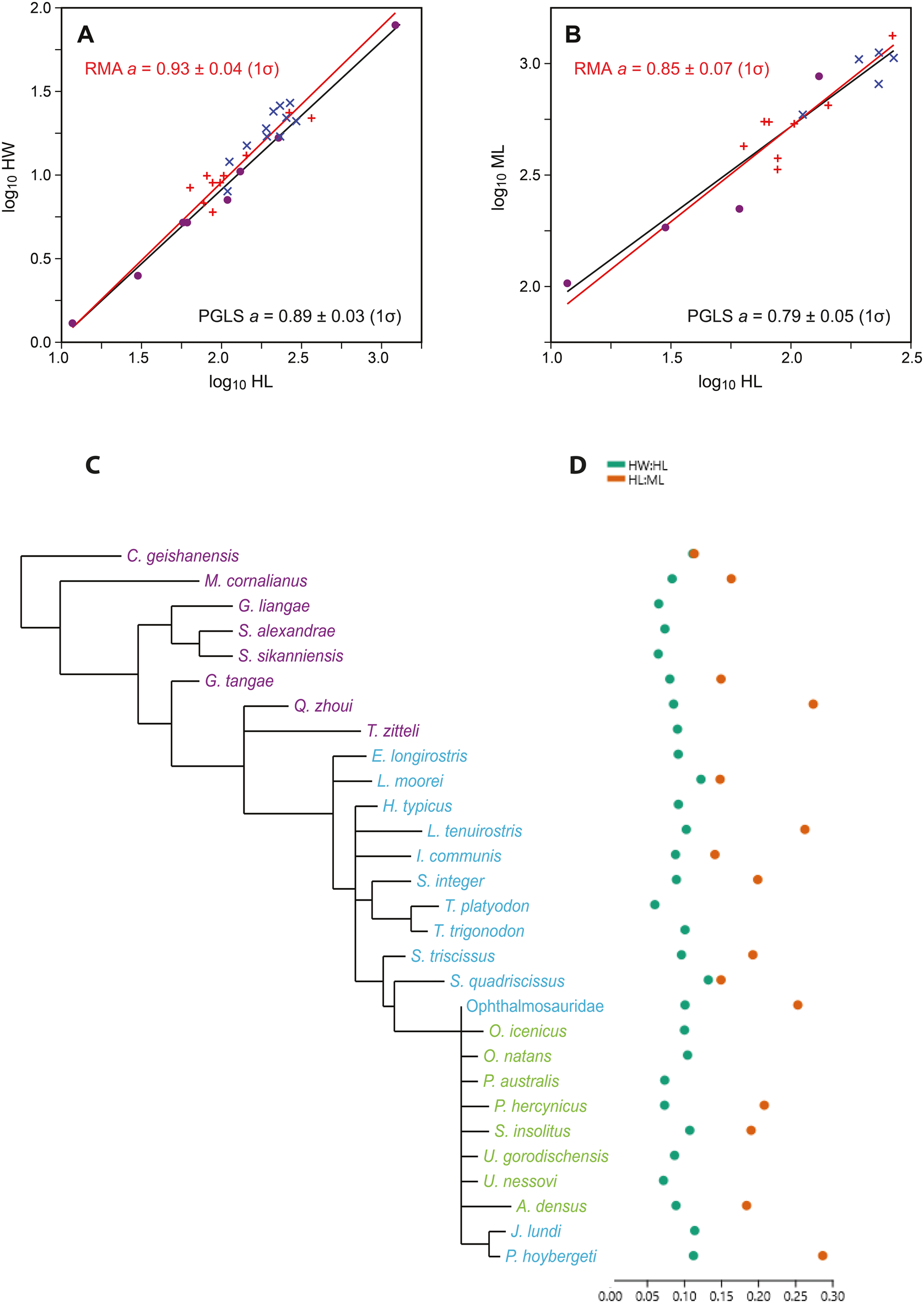
Figure 2. A, Log-transformed ichthyosaur hyoid lengths (HL) vs. widths (HW), with reduced major axis (RMA; red) and phylogenetic generalized least squares (PGLS; black) regression lines. Dots indicate Triassic taxa; red crosses indicate Early to Middle Jurassic; blue crosses indicate Late Jurassic–Cretaceous Ophthalmosauridae. B, HL vs. mandibular lengths (ML). C, Phylogeny of the ichthyosaur taxa included in the analysis (based on Moon Reference Moon2017). D, The two indexes for ichthyosaur hyoids (HW:HL and HL:ML), referring to taxa in C.
To compare hyoid robustness in ichthyosaurs and toothed whales, we assembled a dataset with measurements taken from osteological natural history museum collections in Bergen, Oslo, and Copenhagen (66 specimens) and measurements previously used in Johnston and Berta (Reference Johnston and Berta2011; 102 specimens). For comparison to the ichthyosaur hyoid elements, the stylohyals were used, because they represent the suspensory portion of the hyoid apparatus.
To evaluate the relative length of the hyoid, its length was compared with mandibular length (HL:ML). We were able to measure this trait for 12 non-ophthalmosaurid specimens based on the data from Motani et al. (Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013), and 5 ophthalmosaurid specimens and 66 odontocete specimens measured for this study.
For both indexes (HW:HL, and relative length of hyoid HL:ML), we compared both ichthyosaurs and odontocetes (Fig. 3). Mandible length was only available for the newly collected dataset of 66 odontocete specimens and 17 ichthyosaur specimens. For comparing the disparity between the groups, we used the coefficient of variation (CV).

Figure 3. Comparison between hyoid indexes for ichthyosaurs and toothed whales. A, Hyoid robustness, comparing hyoid length (HL) and width (HW). Coefficient of variation (CV) for odontocetes (n = 169) is 14.40, CV for ichthyosaurs (n = 29) is 8.33. Green points represent ichthyosaurs; filled squares represent Triassic taxa; dots represent Early and Middle Jurassic non-ophthalmosaurid taxa; triangles represent Late Jurassic–Cretaceous ophthalmosaurids. Colored dots represent odontocete families: red, Monodontidae; black, Delphinidae; violet, Phocoenidae; cadet blue, Iniidae; pink, Ziphiidae; light brown, Kogiidae; dark brown, Pontoporiidae; dark gray, Physetereiidae. B, HL compared with mandible length (ML). CV for odontocetes (black dots) (n = 66) is 14.93, CV for ichthyosaurs (green dots) (n = 17) is 16.03. Ichthyosaur silhouette by Esther van Hulsen; odontocete (dolphin) silhouette from Wikimedia Commons.
For discussing the shape of the ophthalmosaurid hyoid, the following morphological data were collected from personal observations and the literature: cross section in anterior end, midway, and in the posterior end; amount of curvature and the position of the ventralmost point of the curvature; whether the element had the same width throughout or the ends were expanded. Four specimens from the Late Jurassic–Early Cretaceous Slottsmøya Member at Spitsbergen were studied in detail: PMO 222.654 (Janusaurus lundi), PMO 222.669 (Palvennia hoybergeti), PMO 222.667 (Keilhauia sp.), and one Ophthalmosauridae indet. (Roberts et al. Reference Roberts, Druckenmiller, Sætre and Hurum2014; Delsett et al. Reference Delsett, Druckenmiller, Roberts and Hurum2018, Reference Delsett, Roberts, Druckenmiller and Hurum2019). Note that even if some taxonomic issues are not fully resolved, all are nested within Ophthalmosauridae (Delsett et al. Reference Delsett, Roberts, Druckenmiller and Hurum2019; Zverkov and Prilepskaya Reference Zverkov and Prilepskaya2019). Morphological data and the close-up study of four specimens are given in the Supplementary Material (table and text).
Internal Microstructure
For description of the internal microstructure, the four ichthyosaur specimens from the Slottsmøya Member were computed tomography (CT) scanned with a Nikon Metrology XT H 225 ST microfocus instrument at the Natural History Museum, University of Oslo, with voxel sizes from 50 to 75 μm. CT scanning is a nondestructive method that provides an overview of the internal structure in skeletal elements in all planes. However, the limits of voxel size mean that many small structures (e.g., many tissue types and osteocyte lacunae) are too small to be visible with conventional microCT, and either synchrotron scanning or classical thin sections must be used for mapping of such structures (Sanchez et al. Reference Sanchez, Ahlberg, Trinajstic, Mirone and Tafforeau2012).
One specimen (PMO 224.252) did not preserve any visible internal microstructure, whereas the remaining three provided clear data. After CT scanning, the inner cross section of one specimen (PMO 222.667) was investigated in more detail to confirm the composition of the apparent outer compact sheath. At a point determined by the CT scan where both the outer and inner zones were present, we destructively sampled a 2.5-mm-thick slice using a Struers Minitom precision cutoff machine, polished the sample, and scanned it on a flatbed scanner (regular light). This optical image could not be automatically matched to any certain position in the CT scan of the same hyoid, which meant it was necessary to understand the chemical properties of the infilling in the pores and the resulting patterns of X-ray attenuation and optical appearance. Thus, we conducted scanning electron microscopy–energy dispersive X-ray spectroscopy (SEM-EDS) element mapping of the slice with a Hitachi S–3600N SEM fitted with a Bruker XFlash 5030 energy dispersive X-ray detector at the Natural History Museum, University of Oslo.
Odontocete stylohyals and thyrohyals were also CT scanned to map their internal structures and compare them with the ichthyosaur hyoids. One specimen of an adult Lagenorhynchus albirostris (NHMO-DMA-32381) and one juvenile Hyperoodon ampullatus (NHMO-DMA-29427) were scanned at the Natural History Museum, University of Oslo. Lagenorhynchus uses several feeding mechanisms (raptorial, crushing, suction), H. ampullatus is a suction-feeding ziphiid with a blunt head and reduced dentition, with a diet primarily of squid (Heyning and Mead Reference Heyning and Mead1996; Werth Reference Werth2006a; Berta and Lanzetti Reference Berta and Lanzetti2020).
Results
Size and Shape
The plot of log-transformed hyoid length versus width (Fig. 2A) shows little scatter around the linear regression line, with a slope a = 0.93 ± 0.04 (1σ). Although the slope is only barely significantly different from the isometric value of 1 (95% confidence interval: 0.89–1.01), we can infer a slight negative allometry, meaning that longer hyoid elements are relatively somewhat thinner (more gracile).
The hyoid lengths versus mandibular lengths (Fig. 2B) produce a regression slope of a = 0.85 ± 0.07, also a weakly significant negative allometry; that is, longer hyoids are paired with relatively shorter mandibles.
When we correct for phylogeny with PGLS (Fig. 2), the regression of HL versus HW remains practically unchanged, with slope a = 0.89 ± 0.03 (1σ). The phylogenetic signal lambda was estimated as 0.40. For HL versus ML, the magnitude and significance of any allometric relationship is slightly strengthened, with a = 0.79 ± 0.05. In this case, lambda was estimated as 0.0. However, it must be remembered that the expected variance of the parameter estimation is large because of uncertainties in both the topology and the branch lengths in the phylogenetic tree, especially considering the high number of unresolved taxa in the ichthyosaur phylogeny (Moon Reference Moon2017).
When the two indexes for ichthyosaurs and whales are compared (Fig. 3), odontocetes show a much larger disparity than the ichthyosaurs with regard to hyoid robustness. CV for the hyoid robustness for ichthyosaurs is 8.33, whereas for odontocetes it is 14.40. The relative length of the hyoid compared with the mandible could only be calculated for 66 odontocete specimens, with mostly Phocoena phocoena, Lagenorhynchus albirostris, and Delphinapterus leucas specimens, and 17 ichthyosaur specimens, distributed between ophthalmosaurids and non-ophthalmosaurids. For these subsets, CV is 14.93 for odontocetes, whereas for ichthyosaurs it is 16.03.
Internal Bone Microstructure
The ichthyosaur hyoid CT scans all display the same overall inner pattern, and we describe them together as follows. In medial/lateral view, the hyoid has an internal structure with two cones of cancellous bone, narrowing toward the middle of the element, surrounded by more compact, cortical bone (Fig. 4).

Figure 4. Late Jurassic ophthalmosaurid hyoids from Slottsmøya Member, Svalbard. One hyoid per taxon, in lateral view; see Fig. 1F for placement in skull. Palvennia hoybergeti (PMO 222.669) in A, photo; and B, computed tomography (CT) scan. Janusaurus lundi (PMO 222.654) in C, photo, and D, CT scan. Ophthalmosauridae indet. (PMO 224.252) in E, photo; and F, CT scan. Notice the absence of visible internal structure in this specimen. Keilhauia sp. (PMO 222.667) (incomplete anterior portion) in G, photo; H, flatbed scan of slice with transverse cross section in regular light; and I, CT scan of the entire hyoid. J–M, scanning electron microscopy (SEM) images of the slice of PMO 222.667 in H: J, SEM backscatter image; K, SEM–energy dispersive X-ray spectroscopy (SEM-EDS) element map of barite, noticeably within the pores; L, SEM-EDS element map of calcium, i.e., bone; and M, SEM-EDS element map of aluminum and silicon, i.e., clay infill in some pores. Scale bars, 10 mm (A, C, E, G).
We validated this observation by flatbed scanning and SEM-EDS of the slice of PMO 222.667. SEM-EDS showed major presence of barite (Fig. 4J–M). SEM-EDS also confirmed that the high CT contrast is due to barite in the pores and that there is a higher barite content than what appears in the flatbed scan. Thus, in regular light, barite-filled areas can appear either white or brown-black, as in the outer zone. Through SEM, it can be observed that not all pores, notably in the outer sheath, are filled with barite, and thus they are invisible on CT. Thus, the actual porosity of the outer zone of the ichthyosaur hyoid is somewhat higher than it appears on the CT images (Fig. 4). However, as most of the pores are filled with barite, this does not change the main interpretation of the microstructural architecture of the hyoids; they have two relatively porous cones within a more compact sheath. Barite precipitation in pores has been proposed as the reason for preservation of three-dimensional marine reptile elements in the Slottsmøya Member, with barite possibly mobilized through the activity of cold methane seeps (Delsett et al. Reference Delsett, Novis, Roberts, Koevoets, Hammer, Druckenmiller and Hurum2016).
The ichthyosaur hyoids have no inner cavity; however, the most cancellous zone is situated at the narrowest and thus innermost portion of the cones. The two cancellous cones do not meet in the middle, which means that the middle section of the element, including the curve, only consists of cortical bone. The layer of cortical bone is thicker at the inner side of the curvature. At the anterior and posterior ends, the cancellous structure reaches the surface and is only covered by cortical bone in a few smaller patches.
In the two odontocetes, the stylohyals and thyrohyals all share an internal microstructure of cancellous bone throughout, thus lacking cortical bone as well as an inner cavity (Fig. 5). There are some slight differences between homologous elements: the thyrohyal of Hyperoodon ampullatus (NHMO-DMA-29427) and the stylohyals of L. albirostris (NHMO-DMA-32381) both have an inner architecture of two cones of cancellous bone surrounded by an outer layer with slightly less porosity. The stylohyal of H. ampullatus (NHMO-DMA-29427) has one inner cone, with the widest side at the end facing the basihyal, with larger pores and presumably more cancellous bone than the surrounding area. This element is slightly curved, with the inner side of the curvature less cancellous than the outer. In L. albirostris (NHMO-DMA-32381), the thyrohyal does not possess any cones, but exhibits a broad band throughout the middle of the element with an increased porosity.

Figure 5. Odontocete hyoid apparatus. Hyperoodon ampullatus (NHMO-DMA-29427, juvenile) in A, photograph of entire hyoid apparatus; B, stylohyal; and C, thyrohyal in CT scan showing internal architecture. Lagenorhynchus ampullatus (NHMO-DMA-32381) in D, photograph of entire hyoid apparatus; E, stylohyal; and F, thyrohyal in CT scan. The elements in the hyoid apparatus are connected via metal wires. Scale bars, 10 mm (A, D). Abbreviations: BH, basihyal; T, thyrohyal; S, stylohyal.
Morphology of the Ophthalmosaurid Hyoid
The majority of the ichthyosaur hyoids are curved in two planes: dorsoventrally and mediolaterally. The amount of curvature varies from strongly curved (approx. 45°) in Palvennia hoybergeti and Undorosaurus nessovi (Zverkov and Efimov Reference Zverkov and Efimov2019) to nearly straight (almost 0°) in the Ophthalmosauridae indet. specimen PMO 224.252. In most of the studied specimens, the maximum curve is situated approximately anteroposteriorly midway, whereas in two specimens it is situated in the posteriormost half (PMO 222.669 and PMO 224.252), and in Janusaurus lundi, in the anterior half.
In all the studied specimens except J. lundi, the posterior end of the hyoid was expanded dorsoventrally, whereas the shape and outline of the anterior ends were more variable. Overall, the cross section at most points in most ophthalmosaurids is oval (mediolaterally compressed), but in many specimens varies through the element. The surface texture of most hyoids is smooth. There are longitudinal striations in the outer bone layer in the posteriormost portion in PMO 222.654, PMO 222.669, and PMO 224.252. These might be related to potential tracheal ossifications, which have been observed in the Cretaceous ophthalmosaurid Platypterygius australis (Kear Reference Kear2005).
None of the ophthalmosaurids are preserved with a hyoid corpus. However, in a stratigraphically older parvipelvian, a presumed mature specimen of Ichthyosaurus sp. (CAMSM J 35189), we have observed what we interpret as an ossified hyoid corpus (Fig. 6). The proposed hyoid corpus is close to the assumed in vivo position, as are the two hyoids. The element does not resemble any braincase elements of Ichthyosaurus, and instead is similar to the hyoid corpora in Hauffiopteryx and Stenopterygius. The hyoid corpus has a rounded triangular outline, as in these two taxa (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013; Maxwell and Cortés Reference Maxwell and Cortés2020; Miedema and Maxwell Reference Miedema and Maxwell2022). It differs from Hauffiopteryx in not showing two distinct posterior indentations and from Stenopterygius in being slightly more triangular (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013; Maxwell and Cortés Reference Maxwell and Cortés2020; Miedema and Maxwell Reference Miedema and Maxwell2022). The Ichthyosaurus hyoid corpus has a delineated flat surface posteriorly, at the base of the triangle, and it bulges anteriorly, as in Stenopterygius (Miedema and Maxwell Reference Miedema and Maxwell2022).

Figure 6. Presence of a hyoid corpus in a Jurassic Ichthyosaurus sp. (CAMSM J 35189). A, An entire cranium in ventral view; see Fig. 1F for orientation. Box shows area with the hyoid apparatus enlarged in B. C, Line drawing of the hyoid apparatus with an ossified hyoid corpus present in this specimen. D, Enlarged photo of the hyoid corpus. Abbreviations: aa, atlas–axis pleurocentra; cb, ceratobranchial (1?); HC, hyoid corpus; pt, pterygoid. Scale bars, 50 mm (A, B).
Discussion
Although ophthalmosaurid ichthyosaurs and extant odontocetes possess crania composed of different osteological elements, the comparisons that we undertook were useful for understanding convergence, because we suspected that the hyoid apparatus—and its connection to underwater feeding—might reflect the levels of convergence observed between the body shapes of these two groups. While the hyoid apparatus is fundamental to the feeding cycle, our work highlights important differences between these groups. The odontocetes possess two pairs of elongated ossified rods and an often-ossified midline element, whereas ophthalmosaurids possess one pair of slenderer, ossified rods with a more compact microstructure, likely connected to a midline hyoid corpus. Differences can result from the different ancestry, as the hyolingual apparatus in whales must accommodate suckling milk when they are young (Heyning and Mead Reference Heyning and Mead1996; Werth Reference Werth2007). It is unknown whether ichthyosaurs also could have used their hyoid apparatus in sound production (Cozzi et al. Reference Cozzi, Huggenberger and Oelschläger2017), thermoregulation (Werth Reference Werth2007), or breathing (Wahl Reference Wahl2011).
Among extant reptiles, the architecture of the hyoid apparatus is highly variable. The evolution of a single pair of ossified rods in the hyoid apparatus might have happened in basal archosaurs and is retained by many taxa (e.g., most archosaurs), whereas it is usually more complex in lepidosaurs (Li and Clarke Reference Li and Clarke2015). Ichthyosaurs were diapsids, but with a presently unresolved relationship and divergence time with archosaur–lepidosaur clades (Simões et al. Reference Simões, Caldwell, Tałanda, Bernardi, Palci, Vernygora, Bernardini, Mancini and Nydam2018, Reference Simões, Kammerer, Caldwell and Pierce2022; Martínez et al. Reference Martínez, Simões, Sobral and Apesteguía2021). Moreover, the homology of hyoid elements with a shared tetrapod ancestor is unknown, even if the ossified hyoid elements in ichthyosaurs likely represent the CB1 (or possibly CB2), the most commonly ossified elements in reptiles (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013).
Feeding Modes
Our data on the hyoid robustness (HW:HL) and the HL:ML ichthyosaurs show that ophthalmosaurid ichthyosaurs from the Jurassic and Cretaceous possess similar values to those of earlier, non-ophthalmosaurid taxa (Fig. 2). The greatest variation is found among Triassic taxa, with regard to both the shape and size of the hyoid. This pattern is consistent with the Triassic–Jurassic extinction acting as a bottleneck for ichthyosaur diversity and disparity (Thorne et al. Reference Thorne, Ruta and Benton2011; Moon and Stubbs Reference Moon and Stubbs2020) and observed diversity in dentition among Triassic ichthyosaurs (Sander et al. Reference Sander, R, de Villar, Furrer and Wintrich2022). Even though recent research has shown that there was substantial taxic diversity in the Late Jurassic and Cretaceous (Fischer et al. Reference Fischer, Bardet, Benson, Arkhangelsky and Friedman2016), we interpret the strong allometry between ichthyosaur hyoids as a signal that there was no major shift in feeding mode within Ophthalmosauridae. However, hyoid length shows a weak significant negative allometry with mandible length (Fig. 2B), which means that specimens with long hyoids have relatively shorter mandibles. Four out of the five specimens with the longest hyoids in this study belong to the Ophthalmosauridae.
In general, discussion about feeding modes in ichthyosaurs has centered around whether the group includes suction feeders or not. For example, Motani et al. (Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013) compared hyoid data for non-ophthalmosaurids (Triassic–Middle Jurassic) with those for other marine vertebrates, including extant species, and concluded that ichthyosaurs were not suction feeders, because their hyoids were too small and slender. By comparison, our calculations indicate that ichthyosaurs never evolved suction feeding, something that can also be supported by the presence of teeth throughout the entire ichthyosaur evolutionary history, as odontocetes using raptorial feeding actively use their teeth (when present) to catch their prey (Werth Reference Werth2007).
If ichthyosaurs did not use suction feeding, but ate large quantities of cephalopods, they must have used some form of raptorial feeding. Our study shows that hyoids in ophthalmosaurids display a mixture of traits found within suction feeders and others. The hyoids were curved, to a variable degree. The CT data (Fig. 4) show that internal trabecular architecture is preserved in specimens with curved bones, confirming that the curvature is a real feature and not a taphonomic artifact, as has been suggested for other taxa (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013). Curved hyoids are common in reptiles, usually with the longer side ventrally, which is also assumed here. This study shows that the maximum bend in most of the specimens is situated approximately midway, whereas it is situated in the posterior portion in two taxa and anteriorly in one. A placement of the bend farther toward the posterior end is the case in Alligator (Li and Clarke Reference Li and Clarke2015). Johnston and Berta (Reference Johnston and Berta2011) found a significant correlation between a curved stylohyal and suction feeding in whales and hypothesized this was for a larger area for muscle attachment. However, as the Hyperoodon ampullatus in the present study shows (Fig. 5A,B), stylohyals can also be straight in suction feeders.
A complete lack of suction feeding among ichthyosaurs is surprising, as suction feeding is the most common feeding method in the oceans, and common for vertebrates that consume cephalopods. This feeding mechanism is not dictated by the hyoid apparatus alone but as a result of its interaction with other parts of the skull. Among odontocetes, different taxa can generate suction with different head shapes, and many use a combination of suction feeding and other modes of feeding (Werth Reference Werth2007; Johnston and Berta Reference Johnston and Berta2011; Peredo et al. Reference Peredo, Pyenson, Marshall and Uhen2018). Suction feeding in odontocetes is often correlated with a large basihyal and thyrohyal, a small mouth opening, a short and broad head, few or nonfunctional teeth, and a diet based on squid (Reidenberg and Laitman Reference Reidenberg and Laitman1994; Werth Reference Werth2006b; Johnston and Berta Reference Johnston and Berta2011). Johnston and Berta (Reference Johnston and Berta2011) failed to find a correlation between the basihyal/thyrohyal surface area and suction feeding, but found that an ankylosed, and thus steady, basihyal–thyrohyal was important.
Odontocetes experienced a trend toward mandibular bluntness, evolving from ancestors with relatively longer jaws (Werth Reference Werth2006a), and adaptations for suction feeding evolved separately several times within the group. The pattern in ichthyosaur evolution is less clear. The early, possibly amphibious ichthyosauromorph Cartorhynchus lenticarpus had a short snout and might have used suction feeding, possibly in combination with durophagy, whereas Chaohusaurus, also from the Early Triassic, had a relatively longer snout (Motani et al. Reference Motani, Jiang, Chen, Tintori, Rieppel, Ji and Huang2014; Jiang et al. Reference Jiang, Motani, Huang, Tintori, Hu, Rieppel, Fraser, Ji, Kelley, Fu and Zhang2016; Huang et al. Reference Huang, Motani, Jiang, Tintori, Rieppel, Zhou, Ren and Zhang2019, Reference Huang, Motani, Jiang, Ren, Tintori, Rieppel, Zhou, Hu and Zhang2020). Later ichthyosaurs, including ophthalmosaurids, had long skulls with elongated, narrow snouts. A large, lateral gape probably meant less negative pressure and not the circular mouth opening associated with suction feeding (Werth Reference Werth2007), thus more similar to Delphinus than to Phocoena (Werth Reference Werth2006b). The actual size and shape of the gape in life is hard to determine without preserved soft tissue from ophthalmosaurid skulls, but has been estimated to be up to 75° (Cortés et al. Reference Cortés, Maxwell and Larsson2021). Our data indicate that hyoid length might have increased more than mandible length in late ichthyosaurs. The extent of the mandibular symphysis likely also contributes to feeding mode in cetaceans (Johnston and Berta Reference Johnston and Berta2011; Cooper et al. Reference Cooper, Hieronymus, Vinyard, Bajpai, Thewissen, Hembree, Platt and Smith2014) and should be investigated systematically for ichthyosaurs. Some early ichthyosauromorphs had a short and weak symphysis (Huang et al. Reference Huang, Motani, Jiang, Ren, Tintori, Rieppel, Zhou, Hu and Zhang2020), whereas it is extensive in many parvipelvians (McGowan and Motani Reference McGowan and Motani2003; Cortés et al. Reference Cortés, Maxwell and Larsson2021).
The lack of suction feeding in ichthyosaurs has been supported by the absence of an ossified hyoid corpus across Ichthyosauria, except for Hauffiopteryx typicus (Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013; Maxwell and Cortés Reference Maxwell and Cortés2020). A cartilaginous hyoid corpus situated at the midline of the skull has been suggested (Kiprijanoff Reference Kiprijanoff1881; Kear Reference Kear2005; Motani et al. Reference Motani, Ji, Tomita, Kelley, Maxwell, Jiang and Sander2013). However, the discovery of ossified hyoid corpora at different ontogenetic stages in Stenopterygius (Miedema and Maxwell Reference Miedema and Maxwell2022) and in Ichthyosaurus (Fig. 6) might indicate that ichthyosaurs did possess a hyoid corpus after all. No preserved hyoid corpora are known from ophthalmosaurids, which is unsurprising if they were cartilaginous; only a couple of ophthalmosaurid specimens possess any soft tissue, none of them from the skull (Delsett et al. Reference Delsett, Friis, Kölbl-Ebert and Hurum2022). The few documented hyoid corpora might be because not all taxa had them, but also because the element is easily obscured in laterally or dorsally preserved slab specimens; it can be lost due to the lack of articulation with other elements; or overlooked because it is small. Thus, additional ichthyosaur taxa might preserve hyoid corpora with subsequent (re)assessments of specimens.
The lack of evolutionary change and variation in hyoid shape in ichthyosaurs over 140 Myr seems to contrast the large variation in this apparatus in cetaceans, acquired over a much shorter time span in the oceans (less than 40 Myr; Pyenson Reference Pyenson2017), where diet and feeding have been important factors behind cranial evolution (Coombs et al. Reference Coombs, Felice, Clavel, Park, Bennion, Churchill, Geisler, Beatty and Goswami2022) Thus, even though the Bauplans of later ichthyosaurs and toothed whales have many similarities, these organisms did not follow a parallel evolutionary trajectory. A similar divergence can also be observed in the widely different evolution of the pelvic girdle and hind limb (Delsett et al. Reference Delsett, Roberts, Druckenmiller and Hurum2017) and in the rates of body-size evolution, wherein ichthyosaurs reached gigantic sizes much earlier in their history compared with whales (Sander et al. Reference Sander, Griebeler, Klein, Juarbe, Wintrich, Revell and Schmitz2021).
Internal Microstructure Architecture
Our results describe the internal microstructure of ophthalmosaurid hyoids for the first time since the pioneer work by Kiprijanoff (Reference Kiprijanoff1881), who studied the cross section of an incomplete Cretaceous Platypterygius hyoid. In that study, no longitudinal section was examined, and the exact anatomical location of the cross section is not known. In general, however, the observations match ours. To our knowledge, this is also the first time that the hyoid apparatuses of the two odontocete species have been CT scanned to map the internal structure.
The hyoid elements from the two odontocetes consist of cancellous bone (Fig. 5), which has previously been observed in sperm whales (James and Soundararajan Reference James and Soundararajan1981). The cancellous structure is interesting in the context of the structural specializations of the odontocete skull, which is highly variable. In many ziphiids, the rostral elements (especially the premaxilla) are among the densest and hardest skeletal elements among all vertebrates, with an unknown function (de Buffrénil and Lambert Reference de Buffrénil and Lambert2011). In contrast to the majority of ziphiids, however, H. ampullatus appears to possess cancellous maxillary crests (Lambert et al. Reference Lambert, de Buffrénil and de Muizon2011) in addition to the cancellous hyoid apparatus described in this study. Interestingly, the same overall structure is found in the other odontocete studied here, Lagenorhynchus albirostris. In one ichthyosaur, a Lower Jurassic Stenopterygius, rostral elements (dentary and premaxilla) were more compact than any of the sampled postcranial elements (Anderson et al. Reference Anderson, Druckenmiller, Erickson and Maxwell2018).
The ichthyosaur and odontocete hyoids differ significantly in that the ichthyosaur hyoids all have an outer sheath of cortical bone surrounding inner cones of more cancellous bone, whereas the odontocete hyoid elements are cancellous throughout, albeit with a slightly denser outer zone, especially in the stylohyals, which are the suspensory parts. For evaluating whether ichthyosaurs could suction feed in the same way as some odontocetes do, the main question is the degree to which their hyoids were moving ventrally, as this is how suction pressure is created in odontocetes (Werth Reference Werth2006b). However, the most important influence on the distribution of cortical and cancellous bone is most commonly mechanics, and thus the movement of the bone (Main et al. Reference Main, Simons, Lee, Buffrénil, Ricqlès, Zylberberg and Padian2021). Strain measurements are not commonly recorded for mammal hyoid elements, and the properties of vertebral bones in relation to mechanical function are not well understood (Main Reference Main, Buffrénil, Ricqlès, Zylberberg and Padian2021), especially for cranial elements (Bailleul et al. Reference Bailleul, O'Connor and Schweitzer2019).
Increased bone curvature, as seen in ophthalmosaurid ichthyosaurs, increases bone strain from movement. In long bones, curvature is nonetheless very common, possibly because despite its increased exposure to strain, the curvature causes the bone to move in a predictable and advantageous direction (Main et al. Reference Main, Simons, Lee, Buffrénil, Ricqlès, Zylberberg and Padian2021), a feature that could be valuable for a hyoid element. In curved elements from both groups (all ophthalmosaurid hyoids and the odontocete stylohyals), the inner side of the curvature is more compact than the outer side. Cortical bone is stronger in compression than in tension. A thicker cortex on the inner side could therefore mean that the bone was subject to bending forces in the direction of straightening of the bone, which would give compression on the outer side (i.e., thinner cortex required there) and tension on the inner side. The outer sheath of more compact bone likely reflects the types of loads that the hyoid experienced, different from those in odontocetes. This observation fits the finding that ichthyosaur hyoids were probably too slender (Figs. 2, 3) to create a large suction pressure as in suction-feeding odontocetes. There is no clear direction of the cancellous bone indicating any clear directional stress in the hyoids in any of the groups (Bishop et al. Reference Bishop, Hocknull, Clemente, Hutchinson, Farke, Beck, Barrett and Lloyd2018).
Neither the ophthalmosaurids nor the odontocetes in this study possess an open inner cavity, similar to the majority of skeletal elements in marine tetrapods. Marek et al. (Reference Marek, Moon, Williams and Benton2015) found that the hyoid of an Early Jurassic Hauffiopteryx was tubular and hollow. Our results show that ophthalmosaurid hyoids were not hollow, but rather that the inner portion was cancellous. This means that the observed empty space was probably not a real biological feature, but that these portions degraded faster, as seen in plesiosaur propodials (Liebe and Hurum Reference Liebe and Hurum2012), and thus appear “empty” or filled with sediment on a CT scan.
Conclusion
Documented examples of convergent evolution provide opportunities to investigate general rules in biology, as they represent repeated phenomena (Agrawal Reference Agrawal2017). Feeding is a central activity for marine tetrapods, investigated here thorough comparing specimens from groups with a convergent outer body shape: ophthalmosaurids and two odontocete whales. Ichthyosaurs are often portrayed as evolving an increasingly “dolphin-like” body, which should mean that Ophthalmosauridae, which include the last ichthyosaurs, were the closest parallel to today's toothed whales. Our study sought to show whether other skeletal elements followed broad convergences in Bauplans.
In odontocetes, suction feeding evolved several times, but based on hyoid shape, it seems to never have evolved in ichthyosaurs, which is at odds with suction feeding being extremely common, especially for cephalopod eaters. However, as ichthyosaurs might have possessed a hyoid corpus (based on observations in different Early Jurassic taxa), the possibility of some ichthyosaurs using a certain degree of suction in their feeding method is not out of the question, especially as odontocetes generate suction with a variety of head shapes. It is, however, most important to note that extant vertebrates often use a combination of feeding methods, and that the criteria for assigning a taxon to a certain feeding mode are not clear-cut, and that multiple lines of evidence should be used to understand feeding. For ichthyosaurs, future studies need more soft tissue to assess head shape, more on teeth and stomach contents, and an understanding of the other components in their ecosystems. This study shows the benefit of combining traits from inner and outer skeletal components.
Acknowledgments
The authors wish to thank V. Fischer, A. Henrici, R. Bennion, R. Vanis, B. Kear, A. J. Roberts, and I. Paparella for pictures and size data on ichthyosaur hyoids. A. Berta, N. Kelley, and R. Motani are thanked for sharing cetacean data. M. Riley, CAMSM, M. Perillo in Bonn, H. Meijer, University museum in Bergen, P. R. Møller, Natural History Museum in Copenhagen, and L. E. Johannessen, NHM-UiO, are thanked for collection access. For assistance with sampling, N. Castro and B. Lund are warmly thanked. K. Osen is thanked for discussions on feeding underwater. This work was funded by the Norwegian Research Council (project no. 335111) and research grants from EAVP and Fulbright-Norway to L.L.D.
Declaration of Competing Interests
The authors declare no competing interests.
Data Availability Statement
Data available through the Dryad Digital Repository: https://doi.org/10.5061/dryad.n02v6wx20.










