INTRODUCTION
Foodborne diseases are an important and continuing public health problem comprising a broad variety of illnesses [Reference Tauxe1–Reference Rocourt3]. Everyone is basically susceptible for these diseases, but young children, the elderly, pregnant women, and immunocompromised persons are considered to be particularly vulnerable [Reference Lund and O'Brien4]. With the ageing of populations in the European Union (EU), an increase in morbidity and mortality from foodborne diseases is expected [Reference Bouwknegt, van Pelt and Havelaar5]. Most foodborne diseases have a microbial aetiology and occur sporadically, i.e. without an apparent epidemiological link to other cases. According to the zoonosis directive [6], a foodborne disease outbreak (FBDO) is defined as ‘an incidence, observed under given circumstances, of two or more human cases of the same disease and/or infection, or a situation in which the observed number of human cases exceeds the expected number and where the cases are linked, or are probably linked, to the same food source’ [6, p. 33]. The causative (i.e. aetiological) agents in these outbreaks are mostly microbial agents or microbial toxins [Reference Scallan2]. FBDOs are common and responsible for a high burden of disease in populations and also lead to deaths in developing as well as in developed countries [Reference Tauxe1, 7]. In Europe, a total of 5363 FBDOs were recorded in 2012 [Reference Werber and Bernard8, 9], affecting 55 453 people of whom 5118 were hospitalized and 41 died [9].
FBDOs need to be systematically investigated to understand their epidemiology. Successful outbreak investigations provide important insights into the causative agent, the suspected food vehicle, as well as factors in food preparation or handling contributing to the outbreak [Reference Murphree10, 11]. The chance of identifying a suspected vehicle can be increased through the application of analytical epidemiological studies (principally cohort studies and case-control studies) [Reference Murphree10, Reference Palmer12]. However, these studies are not commonly applied in outbreak investigations [Reference Werber and Bernard8, Reference Murphree10], particularly not by local health departments, which usually represent the competent authority in local FBDOs, and most FBDOs are local [Reference Werber and Bernard8].
Foodborne outbreaks in the EU have to be investigated by the competent authorities of EU member states [6]. Member states have to report FBDOs to European Food Safety Authority (EFSA), which, in essence, constitutes the EU foodborne outbreak reporting system. EFSA lays down a set of technical specifications of how the results of investigations of FBDOs have to be reported. The results of these investigations are categorized according to the strength of evidence provided for a specific food vehicle. ‘Strong’ evidence requires convincing results provided by the epidemiological investigation (descriptive or through analytical study designs), microbiological investigation, or both [11].
The aim of this study was to identify characteristics of investigated FBDOs, reported to EFSA, in which analytical epidemiological studies contributed to strong evidence for the suspected vehicle(s), compared to non-analytical evidence (i.e. microbiological or descriptive epidemiological methods only).
MATERIALS AND METHODS
Data source
A dataset on FBDOs in Europe was provided by EFSA for analysis. It consisted of data of FBDOs from the member states for the years 2007–2011, in which the strength of the evidence for the suspected vehicle was considered strong as only those have to be reported in detail [11].
Variables
The nature of evidence for the suspected food vehicle, i.e. epidemiological, microbiological, or both, was reported. The outcome of interest was ‘analytical epidemiological evidence’, defined as ‘a statistically significant association between consumption of a food vehicle and being a case in an outbreak demonstrated by study designs such as a cohort study, a case-control study or similar studies’ [11, p. 5]. The outcome variable ‘analytical evidence’ was created with a binary outcome (‘yes’ – presence of analytical epidemiological evidence; ‘no’ – microbiological or descriptive epidemiological evidence only). Thus, the comparison group were FBDOs in which strong evidence for the suspected vehicle was provided by microbiological evidence, descriptive epidemiological evidence (since 2010), or both (since 2010).
We included the following eight reported characteristics of a FBDO as potential explanatory variables in the analysis (definitions based on EFSA [11]): (i) causative agent (bacterium, virus, parasite, or toxin that was detected and/or isolated in the course of the investigation and which is ‘considered to be the cause of the FBDO’), (ii) deaths (reported number of outbreak cases who died as a result of the FBDO), (iii) hospitalizations (known number of outbreak cases in the FBDO who were hospitalized), (iv) outbreak size (number of all persons meeting the outbreak case definition, including those who were hospitalized or who died as a result of the FBDO), (v) setting (location where the food was consumed), (vi) place of origin (place, other than setting, where the contamination or the mishandling of the implicated food occurred), (vii) severity of outbreak (severe if at least one person died as a result of the outbreak or at least ten persons or 25% were hospitalized; self-constructed variable), (viii) type of outbreak (general, i.e. affecting persons living in more than one household, vs. single household).
The categories of the variable setting were collapsed, where deemed plausible, for ease of analysis and interpretation. All categories with n < 10 were combined into one category. The continuous variables outbreak size and hospitalization were categorized on the basis of quartiles (Table 1).
Table 1. Frequency of outbreak characteristics of foodborne disease outbreaks (FBDOs) reported to European Food Safety Authority (EFSA) 2007–2011, in which the evidence for the suspected vehicle was ‘strong’*
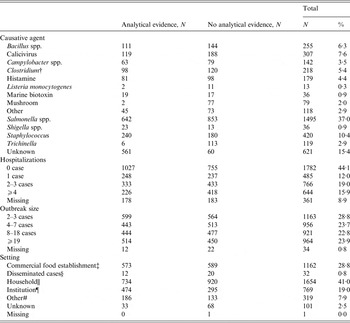
* Univariable significant variables (analysis adjusted for reporting country and year of notification).
† Clostridium perfringens and Clostridium botulinum.
‡ Restaurant/café/pub/bar/hotel, mobile retailer/market/street vendor, takeaway or fast-food outlet, temporary mass catering (fairs, festivals).
§ Geographically dispersed outbreaks.
|| Household, domestic kitchen, camp, picnic.
¶ Canteen or workplace catering, hospital/medical care facilities, residential institution (nursing home, prison, boarding schools), school, kindergarten.
# Other, farm (primary production) (n < 10), aircraft/ship/train (n < 10).
The following three variables concerning the suspected food vehicle were excluded from analysis because they were a result (an effect) of the FBDO investigation and thus could not influence the preceding kind of FBDO investigation and the nature of the evidence (definitions based on EFSA [11]) provided (reverse causation): (i) vehicle (food considered to have been the vehicle of the causative agent or its toxins, reported in categories), (ii) vehicle information (detailed information on kind of food) and (iii) vehicle origin (food vehicle originated from domestic market, from intra-EU trade, or was imported from outside EU).
Statistical analysis
In univariable analyses, the relationship between the presence of analytical epidemiological evidence and the eight outbreak characteristics was evaluated using binary regression analysis. We used the likelihood ratio test to assess statistical significance. Variables with a P value <0·05 were considered candidate variables for multivariable analysis. Multicollinearity was investigated by determining the variance inflation factor with a threshold value of ⩽3.
Characteristics associated with analytical evidence in univariable analysis were further assessed using a multivariable binary logistic regression model. Candidate variables were offered to the model using a forward stepwise selection approach with a threshold value of P < 0·05. Goodness of the model fit was evaluated by computing the pseudo-coefficient of determination, i.e. Nagelkerke's R 2. Year of reporting and reporting country were forced into the model for adjustment. These adjustments were required for two reasons: first, in 2009 a change in the technical reporting specifications occurred. From 2007 to 2009, according to EFSA's technical specifications, strong evidence could only be provided by FBDOs in which the suspected vehicle was identified by use of an analytical epidemiological study or in which the causative agent was detected in the suspected food vehicle. From 2010 onwards, strength of evidence, judged by the investigators, was based on all available evidence allowing for more lines of evidence to contribute to strong evidence for a suspected food vehicle than before [11]. Second, the frequency of investigations of FBDOs with strong evidence varied widely with a few countries accounting for most investigations of FBDOs with strong evidence from analytical study designs.
RESULTS
Descriptive results
The EFSA dataset consisted of 4102 foodborne outbreaks with strong evidence for a suspected food vehicle. We excluded aggregated information on more than one outbreak (n = 38) and outbreaks where <2 cases were involved (n = 26). This reduced the dataset to 4038 FBDOs. The presence of analytical epidemiological evidence was reported in 2012 (50%) of these 4038 outbreaks.
The comparison group consisted of FBDOs in which microbiological evidence was reported as the only line of evidence (73%) or in combination with descriptive epidemiological evidence (6%) or in which only descriptive epidemiological evidence was present (19%); in 2% the nature of evidence was not reported. The proportion of investigations of FBDOs with analytical epidemiological evidence for the suspected vehicle decreased from 2010 onwards (Fig. 1).
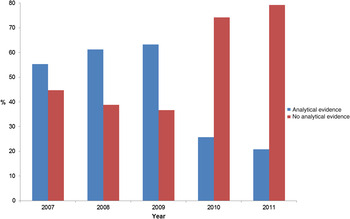
Fig. 1. Proportion of foodborne disease outbreaks (FBDOs) with ‘strong’ evidence for a food vehicle reported to European Food Safety Authority (EFSA) from 2007–2011, which reported use of an analytical epidemiological study, by reporting year. Number of FBDOs by reporting year: 2007 (1556), 2008 (690), 2009 (766), 2010 (493), 2011 (533).
The median number of affected persons in FBDOs, reported to EFSA from 2007 to 2011, was 7 [interquartile range (IQR) 3–18, min-max 2–20 000]. In around half (47%) of the FBDOs, at least one person was reported to be hospitalized per outbreak (median 1, IQR 0–3, min-max 0–11 352); 143 deaths were reported in 49 (1%) outbreaks. Salmonella spp. was the most frequently reported causative agent (37%). Most reported FBDOs occurred in households (41%), followed by those in commercial food establishments (29%). FBDOs in institutions were often (62% of all institutional outbreaks) associated with analytical evidence (Table 1).
Multivariable results
Four variables were selected for multivariable analysis: causative agent, hospitalization, outbreak size, and setting. All remained in the final model with a Nagelkerke's R 2 of 0·472. In total 3665 outbreaks were considered in the multivariable analysis (Table 2).
Table 2. Characteristics of foodborne disease outbreaks (FBDOs) reported to European Food Safety Authority (EFSA) 2007–2011 associated with evidence from analytical epidemiological study designs, among outbreaks, in which the evidence for the suspected vehicle was considered ‘strong’*
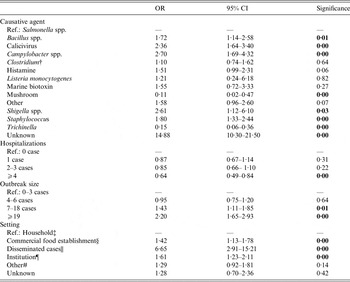
OR, Odds ratio; CI, confidence interval.
* All ORs adjusted for reporting country and year of notification.
† Clostridium perfringens and Clostridium botulinum.
‡ Household, domestic kitchen, camp, picnic.
§ Restaurant/café/pub/bar/hotel, mobile retailer/market/street vendor, takeaway or fast-food outlet, temporary mass catering (fairs, festivals).
Geographically dispersed outbreaks.
¶ Canteen or workplace catering, hospital/medical care facilities, residential institution (nursing home, prison, boarding schools), school, kindergarten.
# Other, farm (primary production) (n < 10), aircraft/ship/train (n < 10).
Causative agent
Most notably, the odds of being an outbreak, where the causative agent was not reported (vs. outbreaks caused by Salmonella spp.), was 15 times higher in investigations with analytical evidence than without. Further, causative agents associated with analytical epidemiological evidence were Bacillus spp., calicivirus, Campylobacter spp., histamine, Shigella spp., and Staphylococcus.
Hospitalization
The number of hospitalized persons was negatively associated with FBDO investigations with analytical epidemiological evidence.
Outbreak size
There was a positive association between an outbreak with analytical epidemiological evidence and the outbreak size.
Setting
Most notably, the odds of being an outbreak with disseminated (i.e. geographically dispersed) cases (vs. household cases) was almost seven times higher in outbreaks with analytical evidence than the odds of being an outbreak with disseminated cases (vs. household cases) in FBDOs with microbiological/descriptive epidemiological evidence only. Furthermore, an association with investigations with analytical epidemiological evidence was noted for FBDOs in institutional and commercial settings (Table 2).
DISCUSSION
Timely investigation with the aim of controlling FBDOs is an important public health task [Reference Palmer13]. Targeted interventions rely on identifying the contaminated food, i.e. on investigations that provide strong (convincing) evidence for a food vehicle. We analysed data on 4038 such investigations reported in Europe from 2007 to 2011 to better understand the characteristics of FBDOs, where use of analytical study designs (vs. microbiological investigations/descriptive epidemiology only) contributed to the strong evidence implicating a particular food vehicle. Characteristics associated with the presence of analytical epidemiological evidence in investigations of FBDOs were the causative agent, number of hospitalized cases, outbreak size, and setting. We identified statistical associations of analytical epidemiological study designs with geographically diffuse FBDOs, FBDOs occurring in institutional settings, and with FBDOs in which a pathogen had not (yet) been identified. Furthermore, analytical epidemiological evidence was associated with an increasing number of outbreak cases.
Our results are plausible and may provide an evidence base for recommendations for their successful use. For example, in institutional settings the list of potential suspects is confined to food vehicles served by the institution, allowing for targeted and timely exposure assessment and hypothesis generation. In addition, institutions usually keep records of their food purchases and food from the same lots or leftovers are often available. Thereby, many lines of inquiries can be followed to identify the suspected food in this setting. Consistent with another study, the use of epidemiological studies was associated with increasing outbreak size [Reference O'Brien14]. Analytical epidemiological studies are based on comparisons between groups of persons, thus requiring a number of cases that can be studied. The larger the outbreak size, the easier (logistically) an epidemiological study can be conducted and the higher the likelihood of distinguishing true effect sizes, thus reducing the likelihood of false-negative results. A minimum numeric threshold for conducting analytical studies cannot be derived from our analysis, because this decision depends on many factors (e.g. severity and dynamic of the outbreak). Notwithstanding, FBDOs in institutions (e.g. restaurant or canteen outbreaks) of a certain size, call for analytical epidemiological studies conducted by local health departments. Simple tools for data entry and analysis, e.g. the linelist tool [Reference Werber and Bernard8], are available for basic epidemiological comparisons.
In geographically diffuse FBDOs, spanning over more than one jurisdiction, the exact place of exposure is unknown for most or all cases, reducing the possibility of obtaining microbiological evidence. In this situation, analytical epidemiological studies, usually conducted by regional or national public health authorities, are often crucial in identifying the suspected food vehicle.
Likewise, if the causative agent is unknown, epidemiological evidence, besides product tracing, is the only means for implicating a particular food vehicle. Similarly, it is difficult to obtain microbiological evidence by end-product testing if the causative agent has a low tenacity (e.g. Campylobacter spp.) or the contaminated food has a short durability (such as fresh produce). This may partially explain why the Campylobacter spp. outbreaks, mainly transmitted by quickly perishable meat or milk products [Reference Forsythe15, 16], were associated with analytical epidemiological evidence. Furthermore, the incubation period of usually 2–5 days [16] prolongs the time span between exposure and outbreak detection, further reducing the chance of finding viable organisms in food products [Reference O'Brien14].
Our study is subject to a number of limitations. First, we have elucidated FBDO characteristics associated with analytical epidemiological evidence (compared to evidence from microbiological investigations and/or descriptive epidemiology only) by analysing ‘successful’ investigations, i.e. those that provided strong evidence for a suspected food vehicle. Although we believe the results are plausible, the study design is not suited to infer that we have identified FBDO characteristics where analytical studies should be employed. To this end, FBDO investigations that provided strong evidence for a food vehicle should be compared with those that provided ‘weak’ or no evidence for a food vehicle. Unfortunately, details of the latter are not routinely collected at the European level. Second, the number of reported investigations of FBDOs in which analytical epidemiological studies have been performed appears unexpectedly high (and have been reported even for outbreaks with ⩽3 cases). This warrants cautious interpretation of the results. Furthermore, the number varied markedly by country, which necessitated analytical control by reporting country. From 2010 onwards, the unexpectedly high number of investigations of FBDOs with analytical epidemiological evidence for the suspected vehicle decreased substantially (Fig. 1), likely indicating a commendable improvement in the quality of the reported data. Third, the high number of incomplete FBDO reports limits the validity of the results. One variable (place of origin) had to be excluded from analysis due to more than 50% of missing information.
Analysis of the existing (reported) evidence for the suspected food vehicle elucidates where successful investigations have been conducted in relation to different settings, sizes, causative agents, and other characteristics. This, however, requires careful collection of this information at the local level, surveillance of this information at the national level and, thus far lacking, data quality assurance measures with reference to the foodborne outbreak reporting system at the European level (e.g. automated plausibility checks when uploading data to EFSA). Although epidemiological study designs are indispensable tools in the investigation of FBDOs, barriers exist as they are resource-intense and not suited for every FBDO [Reference Werber and Bernard8]. Our analysis is the first of its kind and may provide guidance when their use is warranted.
ACKNOWLEDGEMENTS
The authors thank EFSA for providing the dataset on FBDOs in Europe.
DECLARATION OF INTEREST
None.






