Mycophenolate mofetil (MMF) has become a widely used immunosuppressive agent owing to its potent cytostatic effect on lymphocytes.Reference Shipkova, Armstrong, Oellerich and Wieland 1 It is commonly used to prevent graft rejection in transplant patients and as a steroid-sparing therapy for a variety of autoimmune conditions, including connective tissue diseases and inflammatory myopathies. Mycophenolate mofetil has the advantage over other immunosuppressants of having a favorable safety profile, lacking nephrotoxicity and hepatotoxicity.Reference Shipkova, Armstrong, Oellerich and Wieland 1 Its main adverse effects relate to gastrointestinal disturbances, such as diarrhea and nausea, occurring in as many as 30% of patients.Reference Shipkova, Armstrong, Oellerich and Wieland 1 Of particular note, MMF is traditionally considered one of the least neurotoxic immunosuppressive drugs, occasionally causing tremor and mild psychiatric symptoms. 2 Serious neurological adverse effects of MMF, such as progressive multifocal leukoencephalopathy and central nervous system (CNS) lymphoproliferative disorders, have nonetheless been recognized. 2 , Reference O'Neill, Vernino, Dogan and Giannini 3 Post-marketing surveillance data additionally suggest that encephalopathy and seizures might be related to MMF use in transplant patients concomitantly treated with corticosteroids and cyclosporine. 2 An observational study of neurological complications following liver transplant also reported on frequent occurrence of encephalopathy and seizures in patients on combined MMF and calcineurin inhibitor (CNI) therapy.Reference Derle, Kibaroglu and Ocal 4 It must nonetheless be emphasized that CNIs are highly neurotoxic drugs, such that the potential contribution of MMF to the development of those adverse CNS effects remains unclear. There is also one case report of a patient who suffered generalized tonic–clonic seizures while on MMF and acyclovir following a penetrating keratoplasty.Reference Lee, Kim, Wee and Lee 5 Acyclovir has an independent neurotoxic potential, and when co-administered with MMF the pharmacokinetics of both drugs are altered such that their serum concentrations increase simultaneously, which was the presumed causative mechanism of seizures in that case. Herein, we report a patient with probable MMF-induced encephalopathy and status epilepticus.
A 61-year-old Inuit female from a northern Québec community was transferred to our institution for assessment of altered mental status. She had been diagnosed with statin-associated necrotizing autoimmune myopathy at our center 5 weeks earlier, based on positive serum anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMGCR) antibodies and a compatible muscle biopsy. For 6 weeks before the diagnosis, she had been treated with high-dose corticosteroids for presumed polymyositis. When the diagnosis of necrotizing autoimmune myopathy was made, MMF (1 g twice daily) and monthly intravenous immunoglobulins (IVIg) were added. The patient was also known to have insulin-dependent diabetes mellitus type 2, hypertension, dyslipidemia and non-alcoholic fatty liver disease without cirrhosis.
Three weeks after MMF was started, the patient developed behavioral changes, confusion and psychiatric symptoms, including visual hallucinations and delusional thoughts, over a 2-week period. There were no other neurological or systemic symptoms, nor any history of preceding febrile illness or of toxin use. On arrival at our center, the neurological examination was remarkable for decreased alertness, psychomotor retardation, confused verbal responses, eye opening to speech and inconsistency in following simple motor commands. The remainder of the examination was essentially normal. The initial workup revealed no metabolic, toxic or infectious abnormalities. The patient was subsequently admitted for further investigation. Preliminary CSF analysis, including Gram stain, was unremarkable, except for mildly elevated protein at 0.61 g/L (normal 0.15-0.45 g/L). Subsequent CSF bacterial culture was negative, as were syphilis, tuberculosis and human immunodeficiency virus testing. Gadolinium-enhanced head MRI showed only non-specific microvascular changes.
On the second day of admission, the patient’s neurological status worsened, with no localization to noxious stimuli, no eye opening to speech and absent verbal responses. We opted to start empiric therapy with acyclovir and to discontinue MMF. Later during the night she experienced two episodes of brief generalized tonic–clonic seizures, which were treated with intravenous lorazepam and phenytoin. An emergent electroencephalogram (EEG) performed while the patient was not clinically seizing showed bifrontally predominant generalized epileptiform activity, consistent with non-convulsive status epilepticus (Figure 1A). She was then urgently transferred to the neurological intensive care unit for sedation with propofol and continuous EEG telemetry. Levetiracetam was subsequently added for persistent electrographic epileptiform activity. Further testing for infectious and immunological causes was negative, including CSF anti-N-methyl-D-aspartate receptor antibodies, anti-neuronal cell surface antibodies, herpes simplex virus and enterovirus assays, as well as serum anti-onconeuronal antibodies. A whole-body CT scan did not reveal evidence of an underlying malignancy. Following titration of the antiepileptic agents, the patient steadily improved, and after 4 days she was transferred back to the ward on a regimen of phenytoin and levetiracetam. She experienced no recurrence of clinical seizures, and a repeat EEG a week later showed only mild-to-moderate diffuse persistent slowing (Figure 1B). After this event, she did not suffer any residual neurological deficit. She was eventually discharged home on levetiracetam and high-dose corticosteroids. Monthly IVIg was continued for treatment of her myopathy. Two months after discharge, the patient returned to her previous level of functioning without recurrence of seizures.
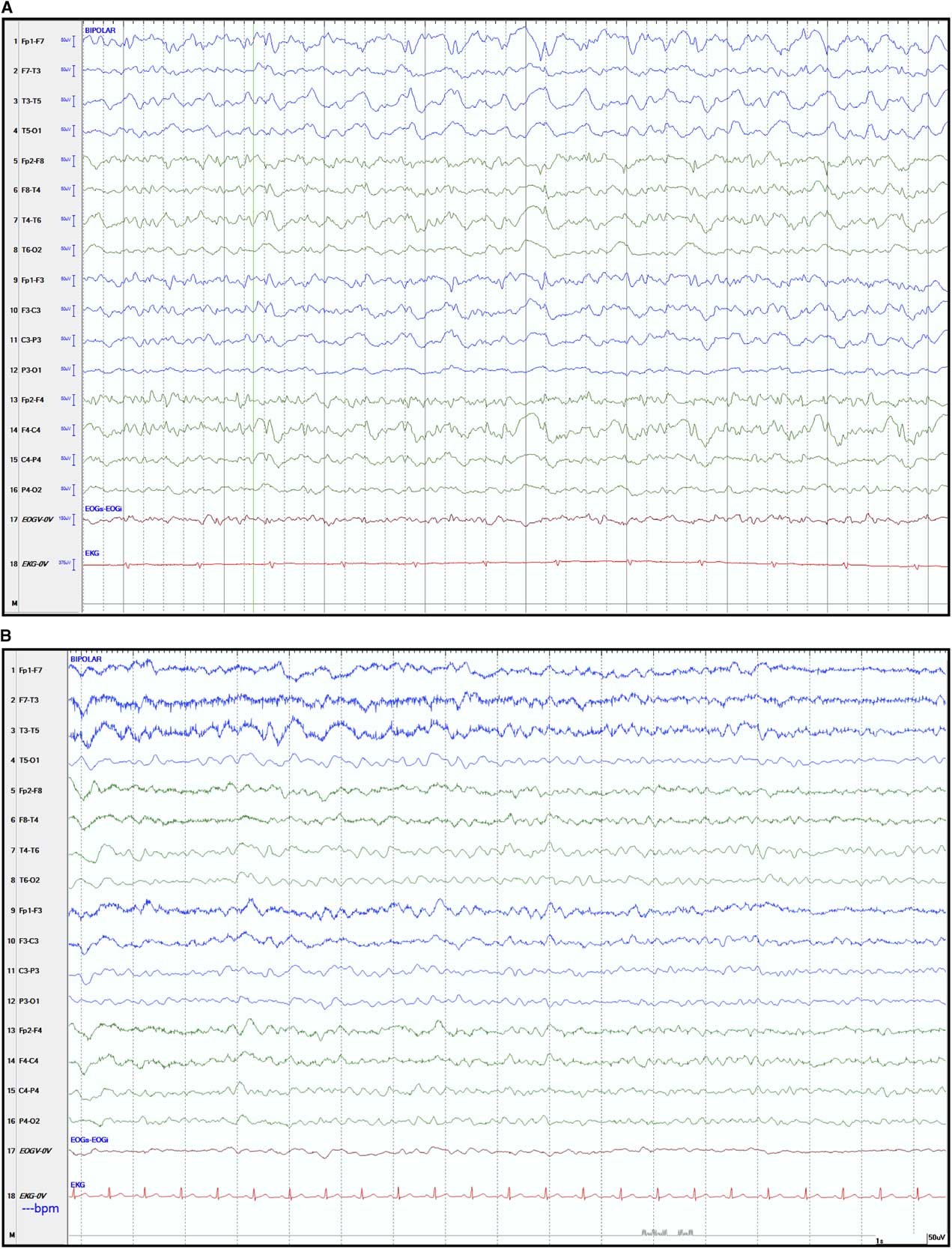
Figure 1 Electroencephalographic recordings during non-convulsive status epilepticus and after its resolution. (A) Electroencephalogram (EEG) tracings showing diffuse rhythmic fast epileptiform activity (>2.5 Hz low amplitude repetitive spikes) seen mostly over the right hemisphere and maximal in the anterior head regions. No clinical ictal phenomena were recorded during the electrographic seizure. The EEG meets the Salzburg working criteria for non-convulsive status epilepticus in this patient without prior epileptic encephalopathy.Reference Beniczky, Hirsch and Kaplan 9 (B) Follow-up EEG recorded after resolution of the non-convulsive status epilepticus, showing mild diffuse persistent but non-specific slowing of cerebral activity. The EEG showed widespread trains of 2-4 Hz delta waves intermingled with 4-6 Hz theta waves. The background activity consisted of poorly sustained 6-7 Hz theta rhythm. Standard EEG display scales of 50 µV height and 1 second width are shown.
The absence of an alternative explanation for our patient’s condition prompted us to consider the possibility of a neurological complication induced by MMF. Our hypothesis is further supported by previous data suggesting a neurotoxic potential of MMF, 2 , Reference Derle, Kibaroglu and Ocal 4 – Reference Nasr, Hocker and Wijdicks 6 by the temporal relationship between the introduction of MMF and the onset of her encephalopathy, and her clinical improvement after its withdrawal.
Several features of this case argue against a diagnosis of idiopathic new-onset refractory status epilepticus,Reference Costello, Kilbride and Cole 7 including sub-acute presentation, complete neurological recovery, as well as the absence of a preceding febrile illness, CSF pleocytosis or MRI abnormalities. Furthermore, as the patient received only a single dose of acyclovir while on MMF and while already experiencing encephalopathy, neurotoxicity secondary to the combination of MMF and acyclovir seems unlikely.
The mechanism by which MMF may cause neurotoxicity is unknown. Mycophenolate mofetil may exert a direct toxic effect on vascular endothelium, akin to CNIs,Reference Rodrigues-Diez, Gonzalez-Guerrero and Ocana-Salceda 8 which would disrupt the blood–brain barrier (BBB). Inhibition of nitric oxide production in endothelial cells induced by MMFReference Shipkova, Armstrong, Oellerich and Wieland 1 could further contribute to BBB dysfunction. Subsequent accumulation of systemic toxins and MMF neurotoxins in the CNS milieu could thereby interfere with neuronal function and lead to encephalopathy and seizures.
In conclusion, although adverse neurological effects of MMF are uncommon, our patient illustrates its potential to cause more serious neurological problems. We suggest that MMF should be added to the list of drugs that may potentially induce encephalopathy and seizures.
Acknowledgments We are grateful to the patient for allowing us to publish this case report, and to Dr. Martin Veilleux for helping with preparation of the figure.
Disclosures
DP, KS, TM, CHC and LG have nothing to disclose.
Statement of Authorship
DP contributed the majority of the manuscript preparation and background research. KS contributed to manuscript preparation and background research. TM, CHC and LG contributed to manuscript preparation and reviewing. All authors read and approved the manuscript.



