Introduction
The United Nations High Commissioner for Refugees reported that there were over 15 million refugees worldwide in 2012. Refugees are vulnerable to various traumatic and stressful experiences before, during, and after evacuation, and these experiences may cause emotional disturbances, such as post-traumatic stress disorder (PTSD), depression, anxiety, and/or alexithymia (Keyes, Reference Keyes2000), which can last for up to 2 decades after resettlement in a host country (Marshall et al., Reference Marshall, Schell, Elliott, Berthold and Chun2005). The amygdala is a key neural substrate underlying emotional disturbances following traumatic or stressful events (Brown et al., Reference Brown, LaBar, Haswell, Gold, Workgroup, Beall, Van Voorhees, Marx, Calhoun and Fairbank2014) because it plays pivotal roles in the generation and expression of affect (Davis, Reference Davis1992). Furthermore, structural and functional alterations in the amygdala are associated with long-term exposure to stress (Jedd et al., Reference Jedd, Hunt, Cicchetti, Hunt, Cowell, Rogosch, Toth and Thomas2015).
Altered resting-state connectivity of the amygdala has been reported in individuals exposed to traumatic or stressful environments. Increased amygdala connectivity to the dorsolateral prefrontal cortex (dlPFC) was reported in late adolescents who experienced childhood maltreatment (Herringa et al., Reference Herringa, Birn, Ruttle, Burghy, Stodola, Davidson and Essex2013). PTSD patients with dissociative symptoms exhibit greater connectivity between the amygdala and prefrontal cortex compared with PTSD without dissociative symptoms (Nicholson et al., Reference Nicholson, Densmore, Frewen, Théberge, Neufeld, McKinnon and Lanius2015). Because the frontal cortex likely inhibits amygdala-related fear and anxiety (Banks et al., Reference Banks, Eddy, Angstadt, Nathan and Phan2007), strengthened frontal–amygdala connectivity after a traumatic experience might underlie difficulties in the identification or expression of emotion, i.e. dissociation or alexithymia.
However, because different traumatic experiences lead to different types of neural alterations (Boccia et al., Reference Boccia, D'Amico, Bianchini, Marano, Giannini and Piccardi2016), findings based on individuals who experienced childhood abuse or other trauma cannot be generalized to other groups, such as refugees (Liemburg et al., Reference Liemburg, Swart, Bruggeman, Kortekaas, Knegtering, Ćurčić-Blake and Aleman2012; Boccia et al., Reference Boccia, D'Amico, Bianchini, Marano, Giannini and Piccardi2016). To the best of our knowledge, no studies have investigated neural connectivity in a refugee sample (Liemburg et al., Reference Liemburg, Swart, Bruggeman, Kortekaas, Knegtering, Ćurčić-Blake and Aleman2012).
Several brain imaging studies have addressed alexithymia. Previous functional neuroimaging studies of alexithymia reported that alexithymia was associated with reduced activity of the limbic or paralimbic areas during emotional stimuli or imagery tasks, enhanced activity in the somatosensory or sensorimotor areas during exposure to stimuli with a physical context, and reduced activity in the insula or prefrontal cortex during social tasks (Moriguchi et al., Reference Moriguchi, Ohnishi, Lane, Maeda, Mori, Nemoto and Komaki2006; Moriguchi and Komaki, Reference Moriguchi and Komaki2013). Alexithymia has also been reported to be associated with structural brain changes, such as in gray matter volume in the insula or cingulate cortex (Goerlich-Dobre et al., Reference Goerlich-Dobre, Bruce, Martens, Aleman and Hooker2014a).
A change in functional connectivity during resting state has also been reported in alexithymia. An electroencephalography (EEG) study found that connectivity with the default mode network was decreased in alexithymia (Imperatori et al., Reference Imperatori, Della Marca, Brunetti, Carbone, Massullo, Valenti, Amoroso, Maestoso, Contardi and Farina2016). A functional magnetic resonance imaging (fMRI) study demonstrated increased resting-state functional connectivity between the default mode network and the prefrontal cortex in young healthy participants with alexithymia (Liemburg et al., Reference Liemburg, Swart, Bruggeman, Kortekaas, Knegtering, Ćurčić-Blake and Aleman2012). The association between functional connectivity and alexithymia may vary depending on the characteristics of the study participants. One fMRI study reported that increased prefrontal–insula connectivity was associated with a higher degree of alexithymia in smokers, but not in non-smokers (Sutherland et al., Reference Sutherland, Carroll, Salmeron, Ross and Stein2013). The correlation between alexithymia and resting-state functional connectivity of the default mode network also differed between patients with major depressive disorder and control subjects (Ho et al., Reference Ho, Wong and Lee2016). However, no studies have investigated resting-state functional connectivity and its relationship with alexithymia in refugees. Whereas previous studies on alexithymia focused on the default mode network or insula in relatively young healthy subjects, our study focused on the amygdala, which may be hyper-activated by the repeated traumas experienced by refugees.
To explore the functional connectivity of the amygdala in a traumatized population, North Korean (NK) refugees living in South Korea, who had been exposed to a variety of stressful and traumatic situations, were recruited to this study. These individuals lived under the extremely oppressive NK political system before defection, and most experienced frequent life-threating situations during defection. They also experienced socioeconomic difficulties as they adapted to the developed South Korean (SK) society after settlement. Moreover, NK refugees have been reported to exhibit altered emotional processing (Park et al., Reference Park, Jun, Lee, Kim, Lee, Yoo and Kim2015; Lee et al., Reference Lee, Lee and Park2017), and they are more likely to be alexithymic than are native SKs. Thus, NK refugees may show altered frontal–amygdala connectivity in association with their alexithymia.
Therefore, the present study examined differences between NK refugees and native SK in terms of resting-state functional connectivity of the amygdala. Alexithymia can be defined as suppression of emotional recognition or expression, so in refugees it would likely be associated with compensatory hyper-regulation of the amygdala by the prefrontal cortex in an effort to avoid negative affect. It was hypothesized that the amygdala would show increased functional connectivity to the frontal cortex in NK refugees compared with native SK, and that the connectivity strength between the amygdala and frontal cortex would be positively associated with disturbances in emotional processing, such as alexithymia, in NK refugees.
Methods
Participants
NK refugees and native SK were recruited via advertisements. The exclusion criteria for all participants were as follows: (1) any metal or other implants that violated MRI safety standards and/or (2) a history of head injury, neurological disorders, untreated serious medical illness, and/or any neurodevelopmental disorders. SKs with a lifetime history of psychiatric disorder were screened and excluded. Six participants (five NK refugees and one native SK) were excluded because their MR images displayed artifacts. There were no significant demographic or clinical differences between the excluded and included participants. Ultimately, 45 NK refugees who were living in South Korea and 40 native SK controls were included in the present study. NK refugees in South Korea are predominantly female and middle-aged; NK refugees in the current study showed similar gender and age distributions. SK controls were matched for age and gender. For the NK refugees, the average time between their first departure from North Korea and participation in the present study was 9.55 ± 5.30 years; during defection they spent an average of 3.83 ± 4.29 years in countries other than South or North Korea, and their average length of residence time in South Korea was 5.40 ± 2.75 years. The research protocol of the present study was approved by the Institutional Review Board of Seoul National University Hospital and all participants provided written informed consent after receiving a comprehensive written and verbal description of the study.
Procedure
All participants were assessed using the Structured Clinical Interview for the DSM-IV (SCID) (First et al., Reference First, Gibbon, Spitzer and Benjamin1997), Beck Depression Inventory (BDI) (Beck et al., Reference Beck, Steer and Brown1996), and revised Toronto Alexithymia Scale (TAS) (Taylor et al., Reference Taylor, Bagby and Parker1992). For the NK refugee group, additional assessments were performed with the Clinician-administered PTSD Scale (CAPS) (Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Gusman, Charney and Keane1995) and a short interview after the clinical assessment, in which they were asked to briefly describe their life history and types of traumatic experiences. An MRI scanning session was carried out within 1–4 weeks of the clinical assessment. To protect against misunderstandings, the meaning of the questionnaire was fully explained, item by item, to the NK refugees.
The BDI is a widely used 4-point Likert-type self-report scale that assesses depressive symptoms. It consists of 21 items that measure most symptoms associated with depressive disorders; a higher BDI total score indicates greater symptom severity. The revised TAS is 23-item self-report questionnaire that assesses alexithymia symptoms and consists of three subscales: Difficulty Describing Feelings (DDF), which assesses the inability to verbally express emotional states; Difficulty Identifying Feeling (DIF), which evaluates the inability to consciously distinguish and recognize emotional states; and Externally Oriented Thinking (EOT), which measures the tendency to focus attention externally. In previous research on 343 community-dwelling subjects without psychiatric disorders, the mean total score on the Korean version of the TAS was 32.51 ± 11.07 (Lee et al., Reference Lee, Yu, Cho, Cho, Koh and Kim2010).
The CAPS is a 30-item structured interview assessing current and lifetime diagnoses of PTSD based on the DSM-IV. The re-experience, avoidance, arousal, and dissociative subtypes are evaluated in terms of symptom frequency, intensity, and severity (the sum of symptom frequency and intensity); the current and lifetime CAPS scores used in the present study were the sum scores of current and lifetime symptom severity, respectively. The Trauma Exposure Check List for NK Refugees was used to evaluate the types of traumatic events that NK refugees had experienced during residency in North Korea, or in the process of escape (Jeon et al., Reference Jeon, Hong, Lee, Kim, Han and Min2005). This checklist assesses 23 common traumatic events experienced by NK refugees, including torture, violence, arrest, imprisonment, human trafficking, witnessing public executions, and other life-threatening incidents.
fMRI data acquisition
Functional and structural images were acquired using a 3T MRI system (Trio Tim, Siemens; Erlangen, Germany) with a 12-channel birdcage head coil. For resting-state fMRI image acquisition, T2*-weighted echo-planar imaging (EPI) was used with the following parameters: TR/TE/flip angle = 3500 ms/30 ms/90°, slice thickness = 3.5 mm, in-plane resolution = 1.9 × 1.9 mm2, field of view = 240 mm, and matrix size = 128 × 128. For structural image acquisition, T1-weighted, 3D magnetization-prepared rapid gradient echo (3D MPRAGE) was used with the following parameters: TR/TE/TI/flip angle = 1670 ms/1.89 ms/900 ms/9°, slice thickness = 1.0 mm, in-plane resolution = 1.0 × 1.0 mm2, field of view = 250 mm, and matrix size = 256 × 256. A certified psychologist and an MRI engineer were present to supervise the entire procedure during the scanning sessions. During the resting-state scan (6 min, 58 s), participants were instructed to stay awake and stare at the black monitor screen with their eyes open. During structural image acquisition (3 min, 54 s), participants were instructed to stay relaxed.
fMRI data preprocessing
The resting-state fMRI data were preprocessed using SPM12 software (Wellcome Trust Centre for Neuroimaging, London, UK). All images were checked via visual inspection for gross motion artifacts due to the continuous movement of the patient throughout the scans, and for susceptibility artifacts due to metallic foreign bodies, allowing removal of data possibly corrupted due to artifacts. Six motion correction parameters obtained from the pre-processing procedure, and outliers obtained from ART-based scrubbing, were also removed (the 97th percentile in the normative sample was used to detect outliers). The coordinate origin-of-input image was set to the anterior commissure prior to preprocessing, functional volumes were realigned, and differences in slice timing were corrected. The functional images were co-registered with anatomical images and then spatially normalized to Montreal Neurological Institute (MNI) space using a transformation matrix derived from the T1 anatomical image segmentation; the obtained functional images were 3 × 3 × 3 mm3. Finally, functional images were spatially smoothed using a Gaussian kernel with a full-width at half-maximum (FWHM) of 6 mm.
Functional connectivity analysis
The CONN functional connectivity toolbox v16b (http://www.nitrc.org/projects/conn) was used to perform the resting-state functional connectivity analyses (Whitfield-Gabrieli and Nieto-Castanon, Reference Whitfield-Gabrieli and Nieto-Castanon2012). All data were bandpass filtered (0.008–0.09 Hz) and physiological, and other spurious sources of noise in the blood oxygenation level-dependent signal were removed by component-based noise correction (CompCor) (Behzadi et al., Reference Behzadi, Restom, Liau and Liu2007). Six motion correction parameters obtained from the pre-processing procedure were also removed. The seed-to-voxel analysis was performed using the bilateral amygdala as the seed region, which was predefined using the Harvard-Oxford atlas in FSL (fMRIB, Oxford, UK) (Smith et al., Reference Smith, Jenkinson, Woolrich, Beckmann, Behrens, Johansen-Berg, Bannister, De Luca, Drobnjak and Flitney2004). The mean time course was calculated from each seed; then, Pearson's correlation analyses were performed including all other voxels in the cerebral region. For the group level analysis, Pearson's correlation coefficients were converted to z-scores using Fisher's r-to-z transformation to improve normality, and a second-level general linear model was carried out with the independent t test used to compare the mean z-scores of the NK refugees and native SK. Entire functional clusters whose connectivity with the bilateral amygdala differed significantly between groups were extracted. The mean z-values of the connectivity between the amygdala and functional clusters that showed group differences were calculated, and were regarded as indicating the strength of functional connectivity. The reported results of the seed-to-voxel correlation analyses were thresholded at a family-wise error rate (FWE) corrected cluster level of p < 0.05, and an uncorrected peak level of p < 0.001 for each seed.
Statistical analysis
To compare the demographic and clinical data of the two groups, independent t tests were used to analyze continuous values and chi-square tests were performed for analysis of categorical values. Two-sample t tests were performed to identify significant between-group differences in functional connectivity according to the z-maps of the bilateral amygdala of the two groups. Additionally, Pearson's correlation analyses of the relationship between connectivity strength (mean z-value) and the clinical data were performed within the NK refugee group to investigate the associations of functional connectivity with clinical symptom severity.
Results
The demographic characteristics of the 45 NK refugees and 40 native SK are summarized in Table 1. There was only a marginal difference in TAS scores (p = 0.074). Of the three TAS subscales, NK refugees had higher scores on the DIF than the native SK (p = 0.001). Additionally, based on the SCID and CAPS, current Axis-I psychiatric disorders were diagnosed in 16 NK refugees; however, psychotropics were prescribed to only three NK refugees at the time of the study. Correlation between TAS, BDI, CAPS and number of traumatic experiences were described in the online Supplementary Table S1.
Table 1. Demographic characteristics of the study participants (N = 85)
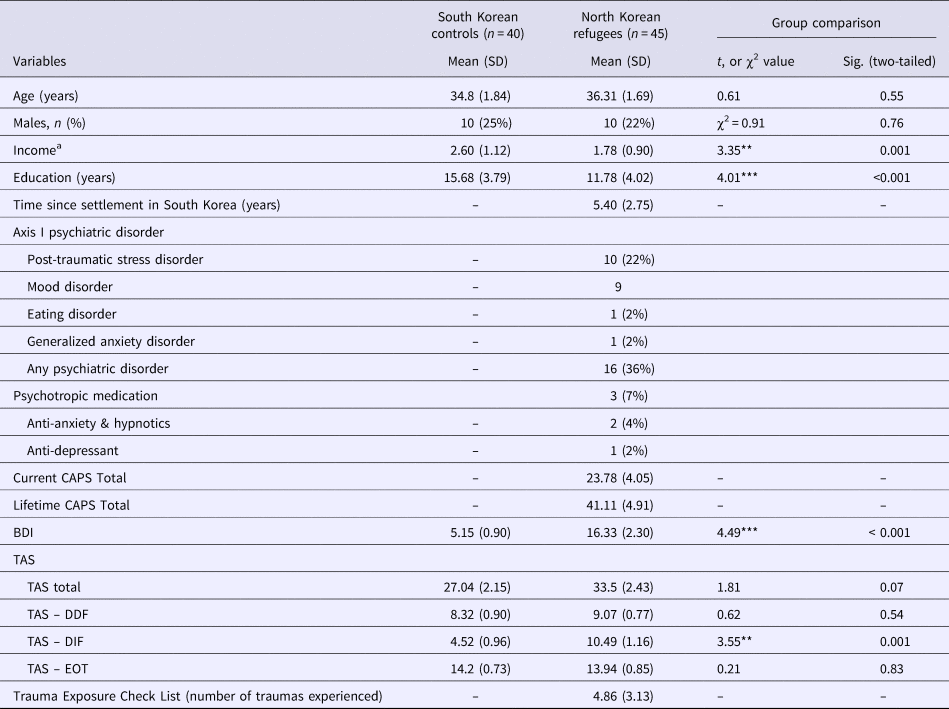
CAPS, Clinical-administered PTSD Scale; BDI, Beck Depression Inventory; TAS, Toronto Alexithymia Scale; TAS-DDF, TAS-Difficulty Describing Feelings; TAS-DIF, TAS-Difficulty Identifying Feeling; TAS-EOT, TAS-Externally Oriented Thinking.
a Monthly income was classified into five levels (1: no income; 2: under 1 million KRW; 3: 1–2 million KRW; 4: 2–3 million KRW; 5: over 3 million KRW)
**p < 0.01, ***p < 0.01.
Differences in functional connectivity of the amygdala between NK refugees and native SK
Compared with native SK, NK refugees showed greater functional connectivity between the left amygdala and left dlPFC [Brodmann area (BA) = 10, k = 115, z = 4.608, corrected p = 0.047] and right dlPFC (BA = 10, k = 216, z = 4.002, corrected p = 0.003; Table 2, Fig. 1). Additionally, in NK refugees, the left amygdala showed higher connectivity to the dorsal anterior cingulate cortex (dACC) than in native SK (BA = 32, k = 163, z = 4.646, corrected p = 0.008). In contrast, the functional connectivity of the right amygdala seed did not differ between the two groups. There were no significant differences in functional connectivity between NK refugees with and without current Axis-I psychiatric disorders, or between NK refugees taking and not taking psychotropic medication. Even after excluding NK refuges with current Axis-I psychiatric disorders and those taking psychotropic medication, the functional connectivity strength for all three connections (i.e. left amygdala–right dlPFC, left amygdala–left dlPFC, and left amygdala–dACC) was significantly higher in NK refugees than in native SK. After controlling for education and income, the group differences in functional connectivity strength remained significant.

Fig. 1. Regions showing significant increases in positive functional connectivity in North Korean (NK) refugees compared with South Korean (SK) controls (left amygdala seed). (a) Bilateral dorsolateral prefrontal cortex (dlPFC) [Montreal Neurological (MNI) coordinates right: x = 26, y = 44, z = 25, left: x = −30, y = 56, z = 30] and (b) dorsal anterior cingulate cortex (dACC) (MNI coordinates: x = 12, y = 26, z = 34).
Table 2. Brain regions showing significant differences in functional connectivity with the bilateral amygdala between NK refugees and SK controls

MNI, Montreal Neurological Institute; FWE, family-wise error rate; (R), right; (L), left; dlPFC, dorsolateral prefrontal cortex; dACC, dorsal anterior cingulate cortex.
We conducted an additional whole-brain comparison of connectivity with the amygdala between NK refugees and native SK, after excluding NK refugees with current Axis-I disorders and those taking psychotropic medications, and after controlling for education and income. After excluding those with Axis I disorders, the left amygdala showed higher connectivity to the right dlPFC, the right dACC, and the left fusiform cortex in NK refugees (online Supplementary Table S2 and Supplementary Fig. S1). After excluding those taking psychotropic medication, the left amygdala also showed higher connectivity to the right dlPFC, the right dACC, and the left fusiform cortex in NK refugees (online Supplementary Table S3 and Supplementary Fig. S2). After controlling for income and education, higher connectivity between the right amygdala and the bilateral insula and between the left amygdala and the right insula, was found in NK refugees (online Supplementary Table S4 and Supplementary Fig. S3).
Correlation between alexithymia and connectivity strength of the amygdala
The functional connectivity strength (i.e. z value) between the left amygdala and right dlPFC had a positive association with TAS total score in the NK refugees (r = 0.309, p = 0.047; Fig. 2) and, among the TAS subscales, DIF was correlated with the strength of functional connectivity between the left amygdala and right dlPFC (r = 0.332, p = 0.032). DDF and EOT were not significantly correlated with this left amygdala–right dlPFC connectivity strength (r = 0.293, p = 0.060 and r = 0.212, p = 0.177, respectively). The association between amygdala–dlPFC connectivity and DIF total score became non-significant after additionally controlling for DDF and EOT (r = 0.262, p = 0.129). DIF was a significant mediator of differences in left amygdala–right dlPFC connectivity between SKs and NK refugees (Sobel test statistic = 2.291, p = 0.022). However, after controlling for BDI score, the mediation effect was no longer significant.
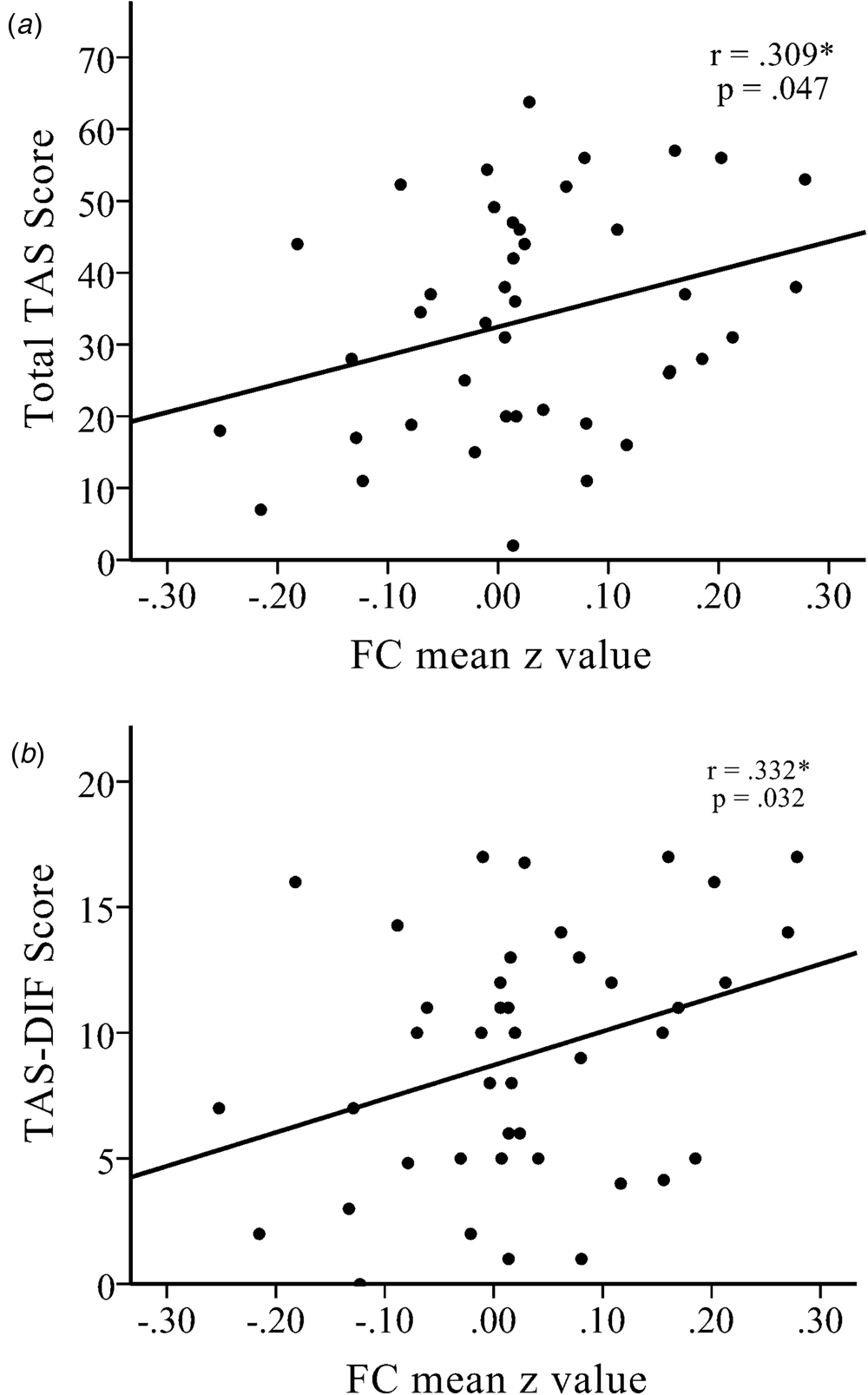
Fig. 2. There were significant positive correlations between increased functional connectivity between the left amygdala seed and the right dlPFC with (a) the total Toronto Alexithymia Scale (TAS) score and (b) the TAS-Difficulty Describing Feelings (TAS-DIF) score in NK refugees. *p < 0.05, **p < 0.01, ***p < 0.01.
The associations between amygdala–dlPFC connectivity and TAS total score remained significant even after controlling for CAPS score, BDI score, and the number of traumatic experiences of NK refugees (left amygdala–right dlPFC, r = 0.396, p = 0.015; left amygdala–left dlPFC, r = 0.390, p = 0.017). CAPS scores for NK refugees were not significantly correlated with left amygdala–right dlPFC connectivity (r = 0.032, p = 0.833), left amygdala–left dlPFC connectivity (r = 0.132, p = 0.387), or left amygdala–dACC connectivity (r = 0.190, p = 0.211). NK refugees’ BDI scores were also not significantly correlated with left amygdala–right dlPFC connectivity (r = 0.061, p = 0.709), left amygdala–left dlPFC connectivity (r = 0.092, p = 0.571), or left amygdala–dACC connectivity (r = 0.097, p = 0.551).
The correlation analysis performed using the CONN toolbox was employed for whole-brain analysis of NK refugees, to detect any brain areas whose connectivity with the amygdala was significantly correlated with TAS score. Connectivity with the amygdala was correlated with TAS total scores in a wide range of brain areas, including the prefrontal and cingulate areas (online Supplementary Fig. S4).
Discussion
The present study found differences between NK refugees and native SK in terms of resting-state functional connectivity of the amygdala. Specifically, NK refugees had heightened connectivity between the amygdala and frontal–cortical regions, including the bilateral dlPFC and dACC. Additionally, the stronger connectivity between the amygdala and dlPFC was associated with a greater degree of alexithymia, independent of depression, PTSD, and the number of traumatic experiences. To the best of our knowledge, this study is the first to investigate resting-state connectivity in a refugee sample and show differences from the general population.
Consistent with our first hypothesis, NK refugees showed enhanced connectivity between the left amygdala and bilateral dlPFC during resting state. Previous studies have observed neural coupling between the left amygdala and frontal–cortical regions during emotion regulation tasks, especially when participants are provided with cognitive strategies to downregulate the emotional response to negative stimuli (Goldin et al., Reference Goldin, McRae, Ramel and Gross2008). In the present study, functional connectivity was measured during resting state. This suggests that refugees may have increased prefrontal down-regulation of the amygdala even in the absence of negative stimuli. The increased connectivity may also reflect compensation for altered amygdala activity during the resting condition. A prolonged state of heightened negative affect in refugee populations may require negative feedback to achieve down-regulation of the prefrontal cortex. NK refugees also exhibited enhanced coupling between the amygdala and dACC. The ACC is typically engaged during conflict monitoring and error processing, during conscious efforts to divert attention from a threat, or during the modulation of subcortical systems that generate emotional responses (Bantick et al., Reference Bantick, Wise, Ploghaus, Clare, Smith and Tracey2002; van Veen and Carter, Reference van Veen and Carter2002; Etkin et al., Reference Etkin, Büchel and Gross2015). Taken together, the present and previous findings indicate that increased frontal–limbic connectivity in NK refugees was associated with down-regulation of emotion or compensation after emotional appraisal by the dlPFC. However, considering the varied roles of the dlPFC, the exact mechanism of increased prefrontal–amygdala connectivity in refugees remains unclear.
Interestingly, significant differences in connectivity between the NK refugees and native SK were observed only in the left amygdala, with the right amygdala seed showing no group differences in connectivity. This is somewhat consistent with previous findings showing that the left amygdala is a key region in the generation and processing of emotion (Banks et al., Reference Banks, Eddy, Angstadt, Nathan and Phan2007), especially cognitive representations of fear (Phelps et al., Reference Phelps, O'Connor, Gatenby, Gore, Grillon and Davis2001). Furthermore, high alexithymia scores are associated with alterations in the volume of the left amygdala, which is in turn associated with emotional perception (Goerlich-Dobre et al., Reference Goerlich-Dobre, Lamm, Pripfl, Habel and Votinov2015). Alexithymia was associated with a blunted amygdala response to emotional speech prosody tasks (Goerlich-Dobre et al., Reference Goerlich-Dobre, Witteman, Schiller, van Heuven, Aleman and Martens2014b). As the amygdala involves bottom-up attention to emotional stimuli (Vuilleumier, Reference Vuilleumier2005), reduced activation of the amygdala may be related to the reduced automatic focus on emotional stimuli in alexithymia (van der Velde, Reference van der Velde, Gromann, Swart, Wiersma, de Haan, Bruggeman, Krabbendam and Aleman2015). The present results revealed an association between alexithymia and the connectivity strength between the left amygdala and right dlPFC in NK refugees. Alexithymia is a disorder of emotional processing characterized by difficulties in verbalizing and identifying emotion, and a tendency to focus on external events (Taylor et al., Reference Taylor, Bagby and Parker1999). The present findings indicate that increased amygdala–dlPFC connectivity in NK refugees may underlie their inability to consciously identify their emotional states.
The dlPFC is a key brain area for attention, working memory, and executive functions. With respect to emotional processing, the dlPFC plays roles in regulating emotion via deploying attentional resources or cognitive reappraisal (Ochsner et al., Reference Ochsner, Silvers and Buhle2012). The dlPFC plays central roles in alexithymia and emotional regulation (Walker et al., Reference Walker, O'Connor and Schaefer2011; Liemburg et al., Reference Liemburg, Swart, Bruggeman, Kortekaas, Knegtering, Ćurčić-Blake and Aleman2012). An EEG study reported that individuals with a lesser degree of alexithymia exhibit lower dlPFC activation during the reappraisal of emotional stimuli (Pollatos and Gramann, Reference Pollatos and Gramann2012). Conversely, an fMRI study found that, in individuals with a greater degree of alexithymia, functional connectivity between the default mode network and prefrontal cortex was stronger during resting state (Liemburg et al., Reference Liemburg, Swart, Bruggeman, Kortekaas, Knegtering, Ćurčić-Blake and Aleman2012), which suggests that the neural systems involved in emotion regulation may be active even in the absence of emotional stimuli in individuals with alexithymia. Alexithymic individuals are also more likely to suppress rather than cognitively reappraise emotion (Swart et al., Reference Swart, Kortekaas and Aleman2009), which typically involves activation of the right lateral prefrontal area (Goldin et al., Reference Goldin, McRae, Ramel and Gross2008). Combined with previous findings, the present results suggest that the dlPFC downregulated neural systems to inhibit prolonged experience of the negative affect that may underlie alexithymia in refugees.
Amygdala–dlPFC connectivity might indicate an association between the emotional reactivity of the amygdala and the emotional regulation of the dlPFC. The present findings indicate that increased amygdala–DLPFC connectivity may underlie the alexithymia seen in NK refugees, especially their inability to identify their emotional states. Avoidance during fear conditioning was reported to activate the dlPFC and modulate the amygdala (Delgado et al., Reference Delgado, Jou, Ledoux and Phelps2009). Our findings suggest that over-regulation of the amygdala by the dlPFC may produce alexithymia as an avoidance strategy in traumatized refugees.
There may be some issues regarding the direction of the association between alexithymia and amygdala–dlPFC connectivity. Alexithymia has been regarded as a form of emotional dysregulation, and emotional dysregulation has been suggested to be associated with weakened prefrontal–amygdala connectivity, especially in PTSD. However, the current study found that alexithymia in refugees was related to enhanced prefrontal–amygdala connectivity. Our study suggests that not only hypo, but also hyperconnectivity between the dlPFC and the amygdala can produce emotional disturbances such as alexithymia. While prefrontal hyporegulation of the amygdala may be related to fear or anxiety, prefrontal hyperregulation may be related to emotional suppression, as seen in alexithymia.
The positive association between alexithymia and amygdala–dlPFC connectivity in refugees has theoretical implications, including experiential avoidance theory (Kashdan et al., Reference Kashdan, Barrios, Forsyth and Steger2006) or constructed emotion theory (Barrett, Reference Barrett2006, Reference Barrett2017). When NK refugees are forced to suppress their emotions under traumatic or oppressive environments, to avoid harmful consequences, experiential avoidance may result from the down-regulation of negative affect. However, the excessive tendency to down-regulate affect may also lead to disturbances in the integrated construction of emotion. Insufficient or distorted construction of emotion may also be associated with alexithymia. Future research assessing experiential avoidance, reappraisal, and suppression under diverse emotional stimuli would be helpful. In the current study, the positive association between alexithymia and amygdala–dlPFC connectivity was found only in NK refugees, and the alexithymia scores of participants were not particularly high. Future studies investigating other populations, including those with severe alexithymia, may be also needed.
In the present study, the association between alexithymia and amygdala–dlPFC connectivity in NK refugees was independent of PTSD, depressive symptoms, and the number of traumatic experiences. Moreover, depression and PTSD symptoms were not related to amygdala connectivity. Taken together, these findings suggest that the association between alexithymia and amygdala–dlPFC connectivity in NK refugees may be related to their sociocultural experiences; i.e. the extremely oppressive NK society may discourage the expression of negative affect, which in turn could cause frontal–amygdala coupling and alexithymia.
Among the three factors of alexithymia, only DIF was higher in NK refugees. DIF in NK refugees was correlated with amygdala–dlPFC connectivity. A previous study found that the association between alexithymia and dysphoric affect among refugees was mainly explained by DIF (Söndergaard and Theorell, Reference Söndergaard and Theorell2004). The increased DIF of NK refugees, which was correlated with amygdala–prefrontal connectivity, suggests that their heightened negative affect may need to be controlled or warded off by increasing amygdala–dlPFC connectivity as a compensatory strategy.
These findings suggest that decoupling of the amygdala and the frontal cortices might reduce alexithymia. However, weakened frontal control over the amygdala may result in a hyperactive amygdala and aggravation of fear and anxiety. In most psychological therapies for trauma victims, the participants are encouraged to identify and express emotions, even though the risk of an anxiety attack or re-traumatization during the session may be high. Electronic or magnetic stimulation of amygdala-to-frontal cortex functional connections might modulate emotional processing, which would be helpful for enhancing therapeutic effects or reducing the adverse effects of treatment in trauma victims or refugees.
The present study had several limitations that should be noted. First, the cross-sectional design of the study did not allow for determination of the precise time-course or causal relationships between the experiences of refugees, their alexithymia, and the altered functional connectivity in the amygdala. Longitudinal studies investigating the functional connectivity of the amygdala may be more helpful in this regard. Second, approximately one-third of the NK refugees in the present study had current psychiatric disorders, the results which may have affected the results. However, psychiatric disorders are common in refugees and the arbitrary exclusion of such individuals may have resulted in a non-representative refugee sample. Moreover, stronger connectivity between the amygdala and right prefrontal/cingulate cortex remained significantly higher in NK refugees, even after excluding those with Axis I disorders. Third, due to the difficulties in recruitment, no native SK with traumatic experiences similar to those of NK refugees, in terms of severity or length of trauma, were included in the present study. Fourth, the alexithymia scores were not high in either group in the current study. Mild alexithymia may represent a personality type or coping strategy in response to emotional stressors, whereas severe alexithymia may indicate psychopathology underlying disturbances of emotional processing. It is possible that the neural connectivity associated with mild alexithymia differs from that in severe alexithymia. Finally, the present findings should be generalized to other refugee populations with caution because different results may be observed in refugees who did not experience an extremely oppressive and isolated sociocultural society. Thus, the present findings may be more relevant to citizens who grew up under very oppressive regimes rather than to refugees in general.
In conclusion, NK refugees exhibited heightened frontal–amygdala connectivity that was correlated with alexithymia. The present results suggest that increased frontal–amygdala connectivity in refugees might produce alexithymia to avoid experiencing negative affect.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719000175
Author ORCIDs
Seog Ju Kim, 0000-0003-2467-5451
Acknowledgement
This research was supported by the Brain Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT & Future Planning (No. 2016M3C7A1904336) and National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2016R1A2B4011561).
Author contributions
Seog Ju Kim conceived and planned the experiments. Seog Ju Kim and Yu Jin Lee supervised the project. Inkyung Park, Jin Yong Jun, So Young Yoo, So Hee Lee, Soohyun Kim, Juhyun Park and Ah Reum Gwaq, carried out the experiments and collected data. Nambeom Kim, Inkyung Park, Kyung Hwa Lee, Sehyun Jun and Hang-Keun Kim analyzed the data and contributed to the interpretation of the results. Inkyung Park & Nambeom Kim took the lead in writing the manuscript with input from all authors. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Conflict of interest
The authors declare that they have no conflict of interest including relevant financial interests, activities, relationships, and affiliations.






