Attention-deficit/hyperactivity disorder (ADHD) is a diagnosis that is based on the presence of two symptom dimensions, inattention and hyperactivity/impulsivity (American Psychiatric Association, 2013). Although these symptom dimensions are highly correlated, they are considered to be dissociable. Consequently, ADHD presentations that are characterized primarily by one of these dimensions or by both of them are acknowledged. It is increasingly recognized that ADHD symptoms are continuous, with the full syndrome representing the extreme end of traits that are found in the general population (Coghill & Sonuga-Barke, Reference Coghill and Sonuga-Barke2012; Greven, Asherson, Rijsdijk, & Plomin, Reference Greven, Asherson, Rijsdijk and Plomin2011). In support of the dimensional view, studies have found the genetic factors that are related to ADHD as a diagnosis and to the broader phenotype to be highly similar (Demontis et al., Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo and Neale2019). Attention-deficit/hyperactivity disorder symptoms are associated with negative social and educational outcomes and psychiatric comorbidity across the symptom spectrum (Holmberg & Bölte, Reference Holmberg and Bölte2014; Vogel et al., Reference Vogel, Ten Have, Bijlenga, de Graaf, Beekman and Kooij2018). Therefore, studies of symptom dimensions rather than discrete diagnostic entities have been advocated (Cuthbert, Reference Cuthbert2014). Dimensional analyses allow researchers to examine how symptoms of ADHD are associated with cognitive and physiological markers across the whole phenotypic continuum.
Despite an extensive literature that documents cognitive impairment in ADHD, it is clear that ADHD symptomatology cannot be explained with reference to cognitive deficits alone (Brocki, Forslund, Frick, & Bohlin, Reference Brocki, Forslund, Frick and Bohlin2017; Castellanos, Sonuga-Barke, Milham, & Tannock, Reference Castellanos, Sonuga-Barke, Milham and Tannock2006; Martel, Reference Martel2009; Sjöwall, Roth, & Lindqvist, Reference Sjöwall, Roth and Lindqvist2013). Multiple pathway models of ADHD hypothesize that several factors including emotional and motivational processes and cognitive impairments contribute to the emergence of ADHD, but their relative importance and links to diagnostic presentations are debated (Castellanos et al., Reference Castellanos, Sonuga-Barke, Milham and Tannock2006; Martel, Reference Martel2009; Sergeant, Reference Sergeant2000). Attention-deficit/hyperactivity disorder overlaps substantially with externalizing disorders. Around 20% of diagnosed children fulfill the criteria for a comorbid diagnosis of oppositional defiant disorder (ODD) or conduct disorder (CD; Biederman, Reference Biederman2005; Jensen & Steinhausen, Reference Jensen and Steinhausen2015). A subgroup of children with ADHD also show a pattern of blunt affect and reduced concern for others, behaviors which are termed callous-unemotional (CU) traits (Frick & White, Reference Frick and White2008).
Although emotional disturbances are not part of the diagnostic criteria, many children with ADHD have difficulties within this area that severely affect their everyday functioning. Both the maladaptive expression of emotion and difficulties with emotion regulation are commonly described. For example, children with ADHD often react to disappointment with frustration and high levels of negative affect (Cole, Zahn-Waxler, & Smith, Reference Cole, Zahn-Waxler and Smith1994), and they are often perceived by peers and teachers as overly emotionally intense and intrusive (Diamantopoulou, Henricsson, & Rydell, Reference Diamantopoulou, Henricsson and Rydell2005; Gardner & Gerdes, Reference Gardner and Gerdes2015). These emotional disturbances have most consistently been linked to hyperactive/impulsive symptoms (Forslund, Brocki, Bohlin, Granqvist, & Eninger, Reference Forslund, Brocki, Bohlin, Granqvist and Eninger2016; Frick, Bohlin, Hedqvist, & Brocki, Reference Frick, Bohlin, Hedqvist and Brocki2018; Martel, Nigg, & Von Eye, Reference Martel, Nigg and Von Eye2009; Sjöwall, Roth, & Lindqvist, Reference Sjöwall, Roth and Lindqvist2013).
The causes and nature of emotional disturbances in ADHD are debated. A recent review identified three potential underlying mechanisms (Shaw, Stringaris, Nigg, & Leibenluft, Reference Shaw, Stringaris, Nigg and Leibenluft2014). At the most basic level, ADHD symptoms could be associated with atypical bottom-up driven reactivity to emotional stimuli such as faces with emotional expressions. This is a process that is supported by subcortical brain circuits with altered structure and function in ADHD including the amygdala and the orbitofrontal cortex (Hoogman et al., Reference Hoogman, Bralten, Hibar, Mennes, Zwiers, Schweren and Franke2017). Secondly, disrupted reward sensitivity could contribute to emotionality by enhancing attention to immediate rewards over long-term goals. Finally, emotional impairment may be driven by impaired top-down control that is linked to dorsolateral frontal brain regions. All of these processes could potentially manifest in increased arousal to emotionally salient stimuli.
Task-evoked pupil-dilation responses (i.e., increases in pupil size) provide a physiological index of arousal that is closely linked to activity in the locus coeruleus-noradrenergic (LC-NE) system. Pupil dilation is elicited by the LC-NE system through excitation of sympathetic and inhibition of parasympathetic activity (Joshi, Li, Kalwani, & Gold, Reference Joshi, Li, Kalwani and Gold2016; Reimer et al., Reference Reimer, McGinley, Liu, Rodenkirch, Wang, McCormick and Tolias2016; Samuels & Szabadi, Reference Samuels and Szabadi2008). The LC-NE system projects to wide-spread cortical areas and enhances attention to motivationally salient stimuli (Aston-Jones & Cohen, Reference Aston-Jones and Cohen2005). Consequently, pupil dilation responses are elicited by salient, novel, or emotionally arousing stimuli such as emotional faces (Kleberg, Hanqvist, Serlachius, & Högström, Reference Kleberg, Hanqvist, Serlachius and Högström2019; Laeng, Sirois, & Gredebäck, Reference Laeng, Sirois and Gredebäck2012). Threat-related stimuli such as faces with negative emotional expressions or words with negative valence typically elicit larger pupil dilation than do stimuli with neutral or positive valence (Bradley, Miccoli, Escrig, & Lang, Reference Bradley, Miccoli, Escrig and Lang2008; Hepsomali, Hadwin, Liversedge, & Garner, Reference Hepsomali, Hadwin, Liversedge and Garner2017; Kleberg et al., Reference Kleberg, Hanqvist, Serlachius and Högström2019; Price et al., Reference Price, Siegle, Silk, Ladouceur, McFarland, Dahl and Ryan2013; Silk et al., Reference Silk, Dahl, Ryan, Forbes, Axelson, Birmaher and Siegle2007). Increased pupil dilation to positive stimuli has also been found in response to positive compared with neutral stimuli (e.g., Bradley et al., Reference Bradley, Miccoli, Escrig and Lang2008; Oliva & Anikin, Reference Oliva and Anikin2018; but see Wang et al., Reference Wang, Naylor, Kramer, Zekyeld, Wendt, Ohlenforst and Lunner2018).
Previous studies have shown that individuals with ADHD have reduced pupil dilation during cognitive task performance, suggesting difficulties with arousal regulation and effort regulation (Metin, Sonuga-Barke, Wiersema, Roeyers, & Vermeir, Reference Metin, Sonuga-Barke, Wiersema, Roeyers and Vermeir2017; Wainstein et al., Reference Wainstein, Rojas-Líbano, Crossley, Carrasco, Aboitiz and Ossandón2017). To our knowledge, no previous study has examined pupil dilation to emotional faces in relation to ADHD symptomatology.
It is not clear whether the disturbances of positive or negative emotion, or both, are most characteristic of ADHD. Most previous studies of the emotional processes that are related to the ADHD phenotype have examined negative emotionality, but a growing literature suggests that disrupted expression and regulation of positive affect is also involved (Beauchaine & Zisner, Reference Beauchaine and Zisner2017; Brocki et al., Reference Brocki, Forslund, Frick and Bohlin2017). At first sight, it might be counterintuitive that increased positive affect would be a developmental concern. However, intense positive affect could lead to diminished social reciprocity and difficulties with focusing on long-term goals (Beauchaine & Zisner, Reference Beauchaine and Zisner2017; Bunford, Evans, & Langberg, Reference Bunford, Evans and Langberg2018). Dysregulation of positive affect in ADHD may be part of the general difficulties with inhibiting strong approach motivation (Brocki et al., Reference Brocki, Forslund, Frick and Bohlin2017). A number of studies have reported concurrent and longitudinal links between ADHD symptoms and high levels of positive emotionality. For example, increased positive emotionality reported by parents has been found to predict ADHD symptoms longitudinally, even after controlling for cognitive functioning and negative emotionality (Forslund et al., Reference Forslund, Brocki, Bohlin, Granqvist and Eninger2016; Frick et al., Reference Frick, Forslund, Fransson, Johansson, Bohlin and Brocki2017; Sjöwall, Bohlin, Rydell, & Thorell, Reference Sjöwall, Bohlin, Rydell and Thorell2017).
Several studies have found evidence for enhanced responses to rewarding social stimuli such as smiling faces in children and adults with ADHD (Ichikawa et al., Reference Ichikawa, Nakato, Kanazawa, Shimamura, Sakuta, Sakuta and Kakigi2014; Passarotti, Sweeney, & Pavuluri, Reference Passarotti, Sweeney and Pavuluri2010; Shaw et al., Reference Shaw, Stringaris, Nigg and Leibenluft2014). For example, hyperresponsiveness to happy faces in individuals with ADHD has been found in the temporal cortical areas that are involved in face processing (Ichikawa et al., Reference Ichikawa, Nakato, Kanazawa, Shimamura, Sakuta, Sakuta and Kakigi2014). Other studies have found evidence for hyperreactivity in the brain areas that are involved in reward processing such as the striatum and medial prefrontal cortex as well as in the dorsolateral prefrontal areas that are linked to top-down regulation (Passarotti et al., Reference Passarotti, Sweeney and Pavuluri2010; Posner et al., Reference Posner, Maia, Fair, Peterson, Sonuga-Barke and Nagel2011). This suggests that ADHD is linked to both atypical top-down regulation of positive emotionality and enhanced bottom-up reactivity to positive emotional stimuli.
The symptoms of ADHD are also associated with disrupted negative emotionality (Bunford, Evans, & Wymbs, Reference Bunford, Evans and Wymbs2015; Graziano & Garcia, Reference Graziano and Garcia2016; Sobanski et al., Reference Sobanski, Banaschewski, Asherson, Buitelaar, Chen, Franke and Faraone2010). Consistent with these findings, atypical responses to cues of negative affect have been reported during the earliest stages of processing (Ichikawa et al., Reference Ichikawa, Nakato, Kanazawa, Shimamura, Sakuta, Sakuta and Kakigi2014; Romani et al., Reference Romani, Vigliante, Faedda, Rossetti, Pezzuti, Guidetti and Cardona2018). Recently, Flegenheimer, Lugo-Candelas, Harvey, and McDermott (Reference Flegenheimer, Lugo-Candelas, Harvey and McDermott2018) found atypical event-related potentials for fearful but not happy or angry faces in young children with ADHD. In contrast other studies have reported atypical responses to both positive and negative emotions (Alperin, Gustafsson, Smith, & Karalunas, Reference Alperin, Gustafsson, Smith and Karalunas2017; Tye et al., Reference Tye, Battaglia, Bertoletti, Ashwood, Azadi, Asherson and Mcloughlin2014).
Comorbid externalizing symptoms could potentially explain the observed link between ADHD and atypical reactivity to emotion, but this question remains largely unexplored. Like ADHD, comorbid externalizing disorders are likely to be linked to emotional problems. Some recent studies have suggested that negative emotionality and irritability are features that are shared between hyperactive/impulsive symptoms and externalizing disorders such as ODD and CD, and they are not disorder specific (Sobanski et al., Reference Sobanski, Banaschewski, Asherson, Buitelaar, Chen, Franke and Faraone2010; Steinberg & Drabick, Reference Steinberg and Drabick2015). In contrast, disrupted positive emotion processing may be uniquely associated with ADHD, particularly with the hyperactive/impulsive symptom dimension (Beauchaine & Zisner, Reference Beauchaine and Zisner2017; Martel et al., Reference Martel, Nigg and Von Eye2009).
Callous-unemotional traits may be linked to a different type of emotional reactivity than is represented in the larger externalizing symptom dimension (i.e., ODD and CD). Callous-unemotional traits have been linked to attenuated emotional arousal to cues of negative emotion in others (Dadds et al., Reference Dadds, Gale, Godbee, Moul, Pasalich, Fink and Hawes2016). This attenuated emotional response could lead to impaired socialization learning from signs of distress or anger in peers or parents in this group (Blair, White, Meffert, & Hwang, Reference Blair, White, Meffert and Hwang2013; Frick & White, Reference Frick and White2008). In contrast, ODD, in which aggression is primarily reactive, may instead be linked to a pattern of increased arousal to a wide range of emotional stimuli.
In summary, although ADHD symptoms have been associated with disrupted emotional processes, questions remain about the relative importance of positive and negative emotions, the brain mechanisms involved, and the role of comorbid externalizing symptoms. The studies that have examined emotional processes in ADHD have mainly relied on group comparisons, rather than on dimensional analyses. We sought to examine the relationship between ADHD symptoms and pupil dilation to facial emotional expressions of both positive emotion (happiness) and negative emotion (anger and fear) while controlling for symptoms of externalizing disorders. Studies of pupil dilation have previously been conducted in examinations of various neurodevelopmental disorders as well as in typical development (Kleberg, del Bianco, & Falck-Ytter, Reference Kleberg, del Bianco and Falck-Ytter2018; Prehn-Kristensen et al., Reference Prehn-Kristensen, Molzow, Förster, Siebenhühner, Gesch, Wiesner and Baving2017; Sepeta et al., Reference Sepeta, Tsuchiya, Davies, Sigman, Bookheimer and Dapretto2012; Wainstein et al., Reference Wainstein, Rojas-Líbano, Crossley, Carrasco, Aboitiz and Ossandón2017), but to our knowledge, no previous study has examined pupil dilation to emotional stimuli with respect to symptoms of ADHD. To further characterize the correlates of the pupil dilation response, we examined its relationship to parental ratings of emotionality and emotion-regulation skills for the specific emotions that we studied.
In light of the previous literature, we hypothesized that ADHD and externalizing symptoms (ODD and CD) would be linked to higher pupil dilation to both positive (happy) and negative (angry and fearful) emotional expressions and that CU traits would be linked to lower pupil dilation to negative emotions specifically. Successful emotion regulation could potentially be linked to both increased pupil dilation (indicating mental effort) and decreased responses (indicating reduced bottom-up reactivity). Therefore, this hypothesis was undirected. We also examined the relationship between parental ratings of emotionality and pupil dilation, but we left this hypothesis undirected.
Method
Participants
The final sample consisted of 71 children (18 female) of which 26 had received a diagnosis of ADHD. Families of children with ADHD were contacted through outpatient clinics and advertising in newspapers and social media. In total, 33 children with ADHD and their families initially agreed to participate. Of these, two were excluded because of equipment failure, five because the child eventually did not want to take part in the experiment, and one because the parents did not hand in the symptom measures. Parents confirmed that the child had received a diagnosis of either ADHD, combined ADHD (ADHD-C), ADHD with primarily inattentive presentation (ADHD-PI), or ADHD not otherwise specified (ADHD-NOS) from a clinical psychologist or psychiatrist in regular care, and they specified the clinic and year of diagnosis. Of the included children, one had a diagnosis of ADHD-PI, one had ADHD-NOS, and 24 had the combined presentation. Parent ratings confirmed that the symptom levels were within the range of clinical concern according to the Swanson, Nolan, and Pelham Scale (SNAP-IV; Bussing, Fernandez, Harwood, Wei Hou, et al., Reference Bussing, Fernandez, Harwood, Wei Hou, Garvan, Eyberg and Swanson2008) in all but two cases. These children were included in the analyses, but we conducted exploratory analyses after excluding them. This did not change any of the results. Comorbid diagnoses according to medical records or parental report were dyslexia (n = 2), speech and language disorder (n = 1), and developmental coordination disorder (n = 1).
Eighteen of the children with ADHD were on medication for ADHD (metylphenidate: n = 12; guanfacine: n = 2; atomeoxetine: n = 1; dexamphetamine: n = 1; drug name not reported: n = 2). The families were asked to withdraw medication during the day of the experiment if possible, but four children had taken medication at the day of visit (guanfacine: n = 2; methylphenidate: n = 2). One additional participant was treated for insomnia with melatonin.
To control for the potential effects of medication, we coded medication status at the day of testing as a new binary variable. Participants who failed to washout from stimulant medication (n = 2), who were treated with long-acting nonstimulants (guanfacine or atomoxetine, n = 3), or who were treated with unknown substances (n = 2) were coded as on medication. We ran the exploratory analyses after removing the participants who were on medication (see Results).
In addition to the children with an ADHD diagnosis, a group of typically developing children was recruited. One thousand families in the local area with children who were 8 to 12 years of age were randomly selected from the population registry and contacted by mail. One hundred and sixteen interested parents responded to an online survey, and children who matched the diagnosed children on age, sex, and when possible socioeconomic status were invited and took part in the study. In total, 47 children were invited and tested. Of these, two children had no valid data due to equipment failure. None of the typically developing children had a psychiatric disorder according to parental report or questionnaires. However, teacher ratings on the Strength and Difficulties Questionnaire (SDQ; Goodman, Reference Goodman1997) and SNAP-IV were in the clinical range, defined as scores above the 90th percentile of the national norms (Malmberg, Rydell, & Smedje, Reference Malmberg, Rydell and Smedje2003), for three of them (ADHD-C, conduct and emotional problems: n = 1; ADHD-PI: n = 1; emotional problems: n = 1). Given that the purpose of the study was to examine symptom dimensions across the whole range, these participants were included in the analyses, but all of the results remained unchanged when they were excluded.
Parental education was graded on a scale from 1 (representing 9 years of schooling or less) to 6 (representing a master or doctoral degree). Income was also graded on a 6-point Likert-type scale, with 1 corresponding to an annual income that was less than approximately $10,500 USD in the local currency and 6 to approximately $52,500 USD or more). Socioeconomic status was operationalized as the mean of both parents’ education and income levels. An IQ score was estimated as the mean scaled score according to published norms (mean = 10; SD = 3) of two subtests, Block Design and Information, from the Wechsler Intelligence Scale for Children (WISC, 4th Edition; Wechsler, Reference Wechsler2003). These subtests were chosen because they had the highest loadings on full-scale IQ according to published Swedish norms. Data for IQ were missing for seven children with ADHD. As can be seen in Table 1, no significant group differences between children with and without ADHD were found for SES, IQ, age, or gender proportion.
Table 1. Demographic information, number of included trials, and clinical information
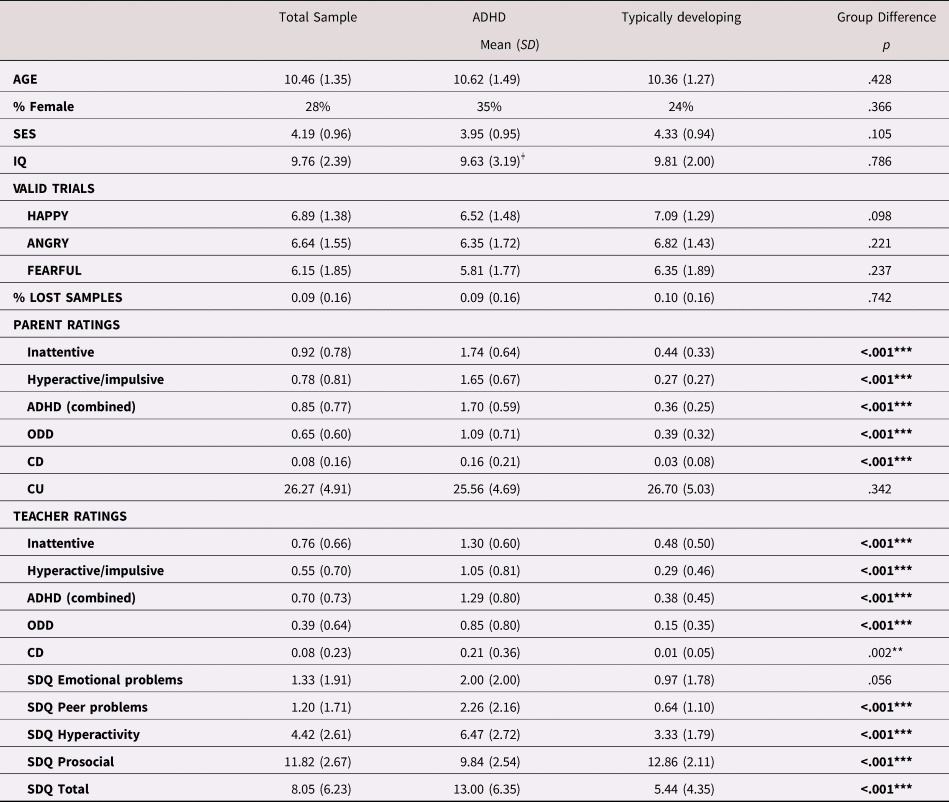
Note: ODD = Oppositional Defiant Disorder; CD = Conduct Disorder; CU = Callous/unemotional; SDQ = Strength and Difficulties Questionnaire. IQ scores represent the mean scaled scores of the block design and information subtests from the WISC-IV, according to published national norms (M = 10; SD = 3). ǂ IQ scores were missing for seven children with ADHD. **p < .1. ***p <.001.
Questionnaires
Symptoms of ADHD and ODD were measured with parental and teacher ratings on the SNAP-IV (Bussing, Fernandez, Harwood, Wei Hou, et al., Reference Bussing, Fernandez, Harwood, Wei Hou, Garvan, Eyberg and Swanson2008), which asks the informant to rate the degree of severity on each of the 18 ADHD symptoms and eight ODD symptoms that are listed in the DSM-5 criteria on a 4-point Likert-type scale. The SNAP-IV has good psychometric properties, with Cronbach α ranging between good and excellent (.79–.96) for the different subscales (Bussing, Fernandez, Harwood, Cynthia, et al., Reference Bussing, Fernandez, Harwood, Cynthia, Garvan, Eyberg and Swanson2008). The parents and teachers were also asked to indicate the presence of the DSM-5 CD symptoms on the same scale. Callous-unemotional traits were measured with the Inventory of Callous-Unemotional Traits (Essau, Sasagawa, & Frick, Reference Essau, Sasagawa and Frick2006).
The parents completed the Emotion Questionnaire (Rydell, Thorell, & Bohlin, Reference Rydell, Thorell and Bohlin2003), which asks the informant to rate the child's degree of emotionality and regulation efficiency for specific emotions. The questionnaire gives separate measures for emotionality (i.e., the degree of emotional reactivity) and regulation. Separate measures were calculated for specific emotions (i.e., fear, happiness, and anger). The questions were scored on a scale ranging from 1 to 5, with higher values indicating greater difficulties with emotion regulation and emotionality. A validation study reported high test–retest reliability and high construct validity with Cronbach α ranging between .87 and .93 (Rydell et al., Reference Rydell, Thorell and Bohlin2003). Here, ratings of regulation and emotionality of the emotions that were studied in the experiments were used.
Teachers completed the SDQ (Goodman, Reference Goodman1997), a screening measure for emotional symptoms, conduct and peer problems, and symptoms of hyperactivity/impulsivity. The SDQ also gives a total difficulties score, which is a composite measure for psychopathology. The SDQ scores were not used in the main analysis, but they served as a screening measure for undetected psychopathology (see Participants). All of the teacher ratings were missing for 16 children (7 with ADHD, 22.5% of the final sample), resulting in a sample size of 54 children (19 with ADHD) with valid teacher ratings.
Experimental Paradigm
The stimuli were images of emotional faces from a standardized database (Lundqvist, Flyckt, & Öhman, Reference Lundqvist, Flyckt and Öhman1998) that were shown one at a time in randomized order. The task was designed to rule out mental effort that is associated with explicit emotion recognition as a confounder, so stimuli that were likely to be easily identified were selected. This was confirmed, as the proportion of correctly identified images was close to the ceiling (93.2%). The identification rate was also plotted for each stimulus image separately. The analysis showed that the identification rate exceeded 80% for all of the images except one, which had an identification rate of 50%. The results did not change when this stimulus was removed, so it was retained. An example of the stimuli are shown in Figure 1.
Figure 1. Example of (a) happy, (b) fearful, and (c) angry stimuli used in the experiment.
The images were cropped to show only the inner regions of the face. In total, 32 images were shown to each participant, evenly distributed between four conditions: three emotional expressions (angry, happy, fearful) and neutral faces (n = 8). The same actors appeared once with each expression, meaning that the stimulus set contained eight unique actors (50% male, 50% female). The stimuli were presented for 4 s, and they were preceded by a fixation cross on a uniform gray screen for 1.5 s. Immediately after the offset of the stimulus, the participants were asked to identify whether the depicted person felt angry, happy, fearful, or emotionally neutral.
Data Recording
Gaze and pupil data were recorded with a corneal reflection eye tracker (Tobii TX120, Tobii, Danderyd, Sweden) at a sample rate of 60 HZ. The participants viewed the stimuli from an approximate distance of 60 cm on a 17-inch monitor. The testing took place in a quiet room at either the psychology department at Uppsala University or an outpatient clinic. For practical reasons, it was not always possible to run the experiment in the same rooms, and illuminance varied slightly between sessions. We attempted to establish an ambient illuminance of approximately 460 lux, but this was not always possible to attain. Therefore, illuminance was measured before each experimental session. Post hoc analyses showed no relationship between illuminance level and the pupil-dilation response (p = .35). This was expected because task-evoked changes in pupil dilation are largely independent of baseline pupil size (Beatty & Lucero-Wagoner, Reference Beatty, Lucero-Wagoner, Cacioppo, Tassinary and Berntson2002).
Preregistration and analysis plan
The analysis plan and hypotheses were preregistered in the Open Science Framework (link: https://osf.io/vhj6q/registrations).
Data Processing
The data were analyzed by using custom scripts that were written in MATLAB (Mathworks, Inc.). A linear interpolation was used over gaps in the pupil data that were shorter than 200 ms. The pupil signal was then smoothed with a moving median filter with a window size corresponding to 200 ms. The pupil-dilation response was defined as the median pupil size during a 750–4,000-ms time window after stimulus onset, and it was expressed as the proportion of baseline pupil size. Baseline pupil size was estimated for each participant as the median pupil size during the 0–250-ms time window for all valid trials. This time window was chosen to exclude an initial decrease in the pupil dilation curve that is caused by the changes in luminance that occur directly after stimulus onset (see Figure 2). The gaze coordinates were filtered by using a dispersion-based fixation filter (Tobii fixation filter) with the velocity and dispersion threshold set to 1o of the visual field. Individual samples were removed if the gaze coordinates were outside of the face. Trials were rejected if less than 25% of the samples were valid (7.3% of all trials) or if the participant failed to identify the emotional expression (6.8% of all trials), as misinterpretation of the emotional expression could potentially affect the pupil response. Finally, data from individuals that contributed less than three valid trials in a condition were removed from that condition (Angry: n = 2; Fearful; n = 4; Happy: n = 1).
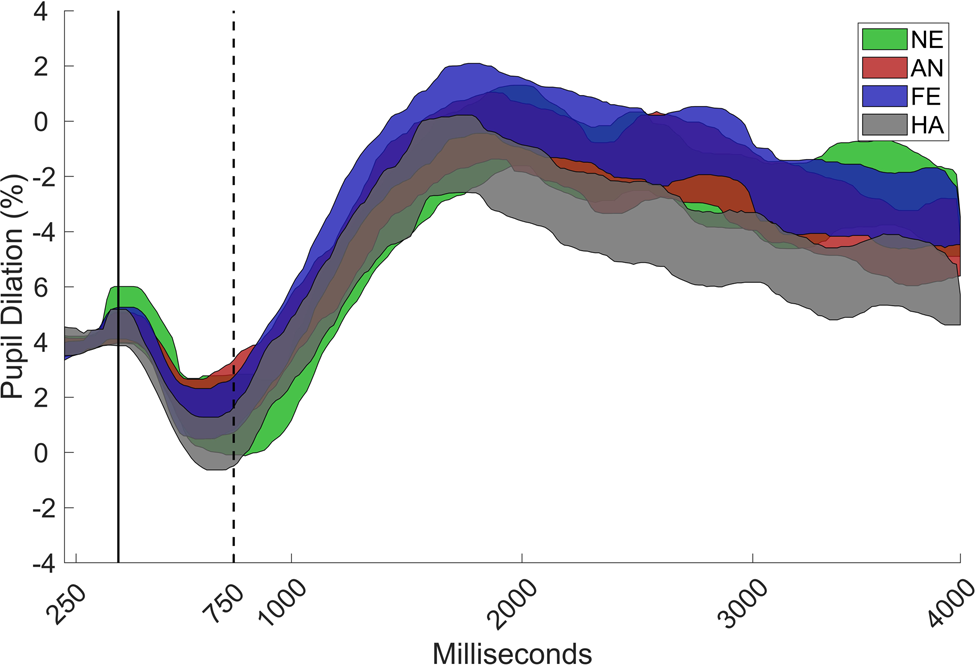
Figure 2. Grand mean of the pupil curve with 95% confidence interval for angry (AN), fearful (FE), happy (HA), and neutral (NE) faces, as proportion of baseline (0–250 ms) for the total sample (N = 71). The pupil dilation response was defined as the increase in pupil size during the 750–4000 ms interval.
Statistical Analysis
The data were analyzed with linear mixed effects models (LMMEs) including random intercepts for individual effects (i.e., treating multiple observations from one individual as repeated measures), trial effects (i.e., accounting for potential effects of trial order), and the influence of the actor that was displaying the emotion. The random intercept for “actor” served to control for minor visual idiosyncratic features of the included images. The pupil-dilation response was the dependent variable in all of the analyses.
Fixed effects for emotion, hyperactive/impulsive, inattentive, ODD, CU, and CD symptoms were added together to examine the link between these variables and the pupil dilation response. Two-way interaction terms between symptom measures, sex, and emotion were initially included but dropped from the final models if they were statistically nonsignificant (p > .05). To analyze the link between emotion regulation impairment and pupil dilation, separate LMMEs were fitted for each emotion with parental ratings of the ability to regulate the emotion in question and emotionality of the emotion in question.
The analyses were conducted by using the ‘fitlme’ function in MATLAB. Linear mixed effects models are preferable to general linear models in data sets with multiple trials per individual because they can account for both inter- and intraindividual variance and do not require listwise deletion of individuals with invalid data from some but not all conditions (Baayen, Davidson, & Bates, Reference Baayen, Davidson and Bates2008). The significance of the individual predictors was tested by comparing the models with and without the predictor in question via likelihood-ratio tests, which examine whether the model fits the data better if the predictor is included. The omnibus significance of the models with multiple predictor variables was tested against a null model that included only the intercepts.
All of the variables were z-transformed prior to analysis for ease of interpretation. The effect sizes are reported in the unit of these z-transformed values. These values represent the slope of the linear relationship for the continuous variables and pairwise post hoc contrasts in the categorical comparisons (Δb). Sex and age were included as covariates in all of the analyses. The collinearity diagnostics showed that the variance inflation factors were < 4 for all of the included predictor variables in the models, suggesting that there was no problem with multicollinearity.
The main analysis was dimensional, meaning that we examined the associations between the symptom dimensions and the pupil-dilation metrics across the spectrum of symptoms. We examined the validity of this approach by testing the interaction terms between group (ADHD, typically developing), emotion, and symptoms in the initial LMME model. Significant interaction effects would mean that the relationship between symptoms and pupil-dilation metrics would differ as a function of group, in which case the dimensional approach would not be valid (see also Preliminary Analysis).
Significant interaction effects between emotion and symptom dimensions were followed up with separate LMMEs for each emotion. Bonferroni corrections for multiple comparisons were used in all of the follow-up tests. In the analyses that were related to parent-rated emotionality and emotion regulation, Bonferroni corrections for multiple comparisons were applied at the level of each emotion (for two comparisons). The residual plots indicated normally distributed residuals, so the LMMEs were fitted with an “identity” link function.
To control for a potential effect of gaze allocation on the pupil-dilation response, the eye–mouth ratio was added as a covariate in all of the analyses. The eye–mouth ratio is defined as the proportion of the total time spent looking at the eyes and mouth of the time spent looking only at the eyes, so it is an index of the relative distribution of gaze to the core regions of the face.
Power Analysis
We conducted a power analysis by using Monte Carlo-based simulation in the SIMR package (Green & MacLeod, Reference Green and MacLeod2016), which we implemented in R (R Core Team, 2013). This analysis showed that the data set had ~ 75% power to detect a relationship between the continuous symptom measures and pupil-dilation responses, with an effect size corresponding to 0.4 standard deviations of the symptom variable. This corresponds to approximately 0.3 points on the SNAP-IV rating scale (see Table 1), and was considered to be a meaningful effect. The power analysis was repeated for a sample size of n = 54, which is equal to the number of children with valid data from teacher ratings only. With this sample size, the power to detect a meaningful effect was below 55%.
To examine the generalizability of the results, we conducted additional analyses including (a) both parent and teacher ratings of symptoms and (b) teacher ratings alone. Because a large proportion of the participants did not have teacher ratings and these analyses were not prespecified, they are reported in the Supplementary Materials.
Results
Preliminary Analyses
A dimensional analysis would be problematic in the presence of significant interaction effects between group, symptom measures, and pupil dilation because these interaction effects would suggest that the relationship between symptoms and pupil dilation differs depending on the group. Therefore, interaction terms between the group and symptom measures and three-way interaction terms between group, symptom measures, and emotion were included in the initial model. These results are described in the supplementary materials. As can be seen, no significant interaction effects that involved group and inattentive, hyperactive/impulsive, ODD, or CU symptoms were found. Unexpectedly, a three-way interaction was found between CD symptoms, group, and emotion (p = .01). However, no follow-up tests survived the Bonferroni correction for multiple comparisons (see Supplementary Materials). There was also no Group × Emotion interaction, χ2 = 2.76, p = .599. Also, ADHD symptoms were not significantly related to baseline pupil size, χ2 = 0.23, p = .631, b = −0.03, SE = 0.06, or to the proportion of correctly identified faces, χ2 = 3.09, p = .079, b = −0.02, SE = .01.
Main Analysis
Effects of emotion
A main effect was found for emotion, χ2 = 22.32; p < .001. Happy faces elicited lower pupil dilation than did angry, χ2 = 11.64, p = .004, Δb = 0.23, SE = 0.07, fearful, χ2 = 13.24, p = .002, Δb = 0.24, SE = 0.07, and neutral faces, χ2 = 11.42, p = .004, Δb = 0.22, SE = 0.06, but no significant difference between angry and fearful faces was found, χ2 = 0.04, p > .50, b = 0.02, SE = 0.07. There were also no differences between angry and neutral, χ2 = 0.70, p > .50, Δb = -0.05, SE = 0.06, or fearful and neutral faces, χ2 = 1.30, p > .50, Δb = -0.08, SE = 0.06 (Figure 2).
Relationship between pupil dilation and symptom measures
As can be seen in Table 2, no main effects for sex, age, or any of the symptom dimensions were found. However, the interaction between hyperactive/impulsive symptoms and emotion was significant, χ2 = 9.37, p = .025. Bonferroni corrected follow-up analyses showed that the higher levels of hyperactive/impulsive symptoms predicted larger pupil dilation to happy faces, χ2 = 9.98, p = .008, b = 0.30, SE = 0.09, but they were not linked to pupil dilation to angry, χ2= 3.17, p = .30, b = −0.15, SE = 0.09, fearful, χ2 = 0.42, p > .50, b = 0.06, SE = 0.09, or neutral faces, χ2 < .01, p > .50, b = −0.01, SE = 0.09. These results are shown in Table 2 and Figure 3. No significant interaction effects were observed between emotion and the inattentive, ODD, CD, or CU symptom dimensions.
Table 2. Relationship between pupil dilation response and symptoms
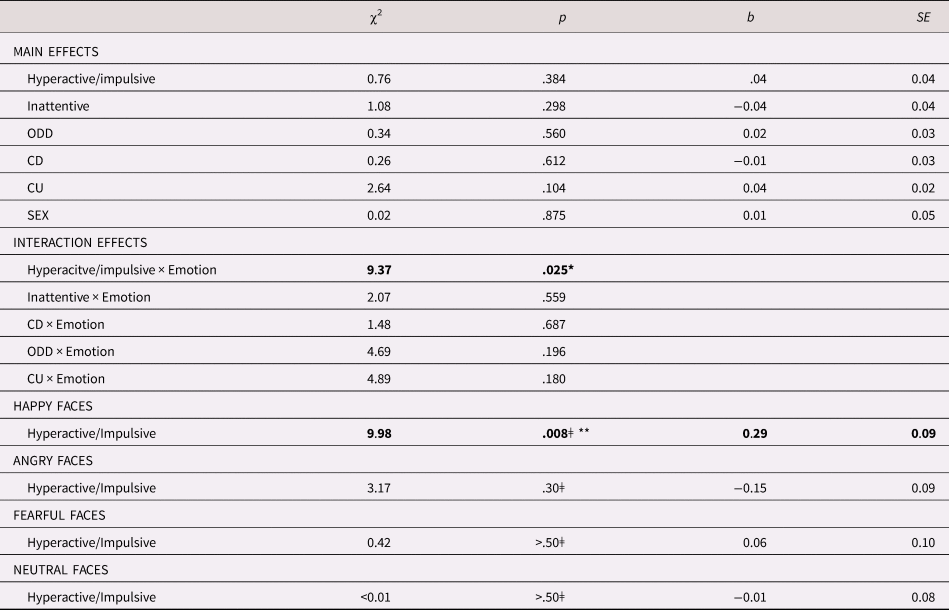
Note: ǂ Bonferroni corrected for three comparisons. ODD = Oppositional Defiant Disorder; CD = Conduct Disorder; CU = Callous/unemotional; *p < .05; **p < .01.
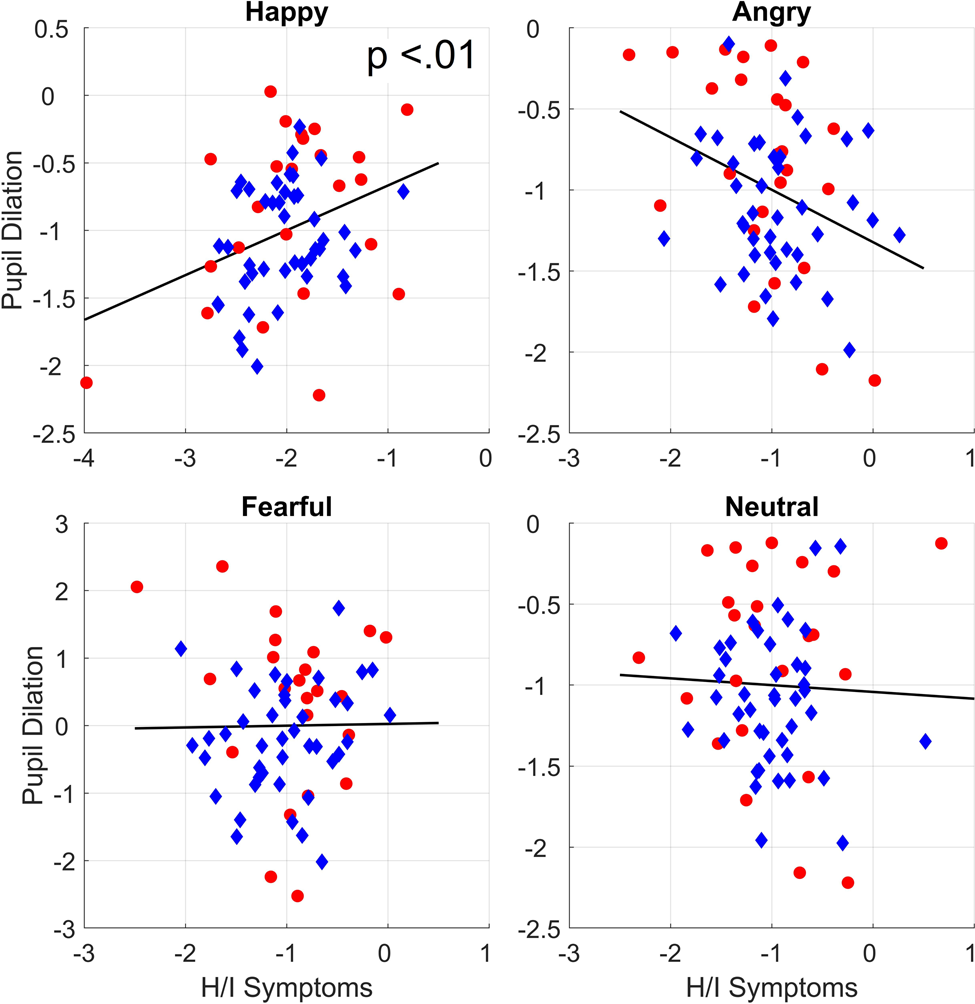
Figure 3. Marginal plots showing the relations between pupil dilation and hyperactive/impulsive symptoms after controlling for covariates. Circles represent children with ADHD. Diamonds represent children with typical development.
Relationships between pupil dilation, parental ratings of emotionality, and emotion regulation
The analyses of the relationships between emotion regulation, emotionality, and pupil dilation were conducted separately for each emotion. Pupil dilation to happy faces was linked to higher parental ratings of emotionality for happiness, χ2 = 5.26, p = .044, b = 0.12, SE = 0.05, but not emotion regulation, χ2 = 1.73, p = 0.39, b = 0.07, SE = 0.05. No significant relationships were found between pupil dilation to angry faces and emotionality, χ2 = 0.65, p > .50, b = −0.03, SE = 0.04, or regulation, χ2 = 0.10, p > .50, b = −0.01, SE = 0.04. Pupil dilation to fearful faces was also not significantly related to parent-rated emotionality, χ2 = 0.12, p > .50, z = 0.0163, SE = 0.05, or regulation, χ2 = 0.41, p > .50, b = −0.03, SE = 0.05.
Teacher Ratings
All of the significant results remained when the means of teacher and parent ratings were used as dependent variables. However, these effects were not significant in the analyses that were conducted with teacher ratings only in the subset of participants with available teacher ratings (see the Supplementary Materials). As described above, statistical power was limited in the analysis of teacher ratings because data was available from only a subset of the sample (n = 54).
Tests for medication effects
Pupil dilation to happy faces was still significantly related to hyperactive/impulsive symptoms when the children who were on medication at the day of testing (n = 7) were removed, χ2 = 6.97, p = .008, b = 0.26, SE = 0.10. In contrast, the relationship between parent-rated emotionality for happiness and pupil dilation to happy faces was only borderline significant after removing these participants, χ2 = 3.69, p = .055, b = 0.10, SE = 0.051.
Discussion
Attention-deficit/hyperactivity disorder and comorbid externalizing conditions are associated with emotional disturbances, but there is an ongoing debate about the nature of these impairments. In the current study, pupil dilation responses to emotional faces were studied in a group of school-age children that were oversampled for individuals with a diagnosis of ADHD. In light of recent studies that have shown that the symptoms of ADHD form a continuous phenotype (e.g., Demontis et al, Reference Demontis, Walters, Martin, Mattheisen, Als, Agerbo and Neale2019; Larsson et al., Reference Larsson, CHang, D'Onofrio and Lichtenstein2014), dimensional analyses were conducted.
Our results suggest that hyperactive/impulsive symptoms are uniquely linked to increased pupil dilation induced by happy faces beyond the influence of inattentive and externalizing symptoms. No relationship was found between responses to faces with negative expressions (anger and fear) and symptoms of ADHD or externalizing disorders. Pupil dilation to happy faces was also positively linked to parental ratings of positive emotionality, indicating that the observed increased reactivity to happy faces is related to everyday behavior. To the best of our knowledge, this study is the first to have examined pupil dilation to emotional stimuli with respect to symptoms of ADHD.
The current results are consistent with a number of recent studies that have linked ADHD, and hyperactive/impulsive symptoms in particular, to dysregulation of positive emotion (Brocki et al., Reference Brocki, Forslund, Frick and Bohlin2017; Forslund et al., Reference Forslund, Brocki, Bohlin, Granqvist and Eninger2016; Musser, Backs, Measelle, & Nigg, Reference Musser, Backs, Measelle and Nigg2011; Sjöwall, Roth, Lindqvist, & Thorell, Reference Sjöwall, Roth and Lindqvist2013), and this has important implications for our understanding of emotional disturbances that are linked to the ADHD phenotype. Strong positive affect and exuberance may contribute to everyday impairment by enhancing focus on short-term goals and rewards. Extreme levels of positive affect could also lead to inappropriate social behavior, eventually leading to peer rejection (Bunford et al., Reference Bunford, Evans and Wymbs2015). High levels of positive affect and exuberance may also be a longitudinal predictor of hyperactive/impulsive symptoms (Forslund et al., Reference Forslund, Brocki, Bohlin, Granqvist and Eninger2016; Frick et al., Reference Frick, Bohlin, Hedqvist and Brocki2018). Taken together, this and previous studies suggest that more adaptive regulation and expression of positive emotionality may be a promising target for interventions that are directed at children with hyperactive/impulsive symptoms.
The results of the main analyses remained when the mean of the parent and teacher ratings were used as predictors but not when teacher ratings alone were used. Because it was only possible to conduct the analysis of teacher ratings in a relatively small subgroup of children with valid data (particularly among children with ADHD), these results must be interpreted with caution. It is possible that the relationship between hyperactive/impulsive symptoms and arousal to faces is better reflected in parent than in teacher ratings, as increased positive affect and approach behaviors may be more visible at home than in a classroom setting. However, it is also possible that the null finding for teacher ratings only results from a lack of statistical power due to data loss.
Phasic pupil dilation is caused by activity in both branches of the autonomic nervous system. It is modulated by subcortical brain structures, particularly by the LC-NE system (Bast, Poustka, & Freitag, Reference Bast, Poustka and Freitag2018; Laeng et al., Reference Laeng, Sirois and Gredebäck2012; Reimer et al., Reference Reimer, McGinley, Liu, Rodenkirch, Wang, McCormick and Tolias2016). Therefore, our results suggest that these mechanisms may be involved. Interestingly, studies that have used other methodologies have also linked emotion dysregulation in ADHD to atypical autonomic functioning (e.g., Musser et al., Reference Musser, Backs, Measelle and Nigg2011, Reference Musser, Lugo, Ward, Tenenbaum, Morris, Brijmohan and Martinez2018). Our results support the use of pupil dilation as a feasible method for measuring the atypical emotional processing and autonomic reactivity that is linked to ADHD symptoms across the continuous phenotype. The method is noninvasive and relatively inexpensive, so it may be applicable in a wide range of research settings.
Contrary to our hypotheses, no relationships were found between pupil dilation to emotional expressions and the externalizing symptom dimensions of ODD, CD, and CU traits. This is at odds with previous literature that has linked these symptoms to disrupted processing of negative affect in others (Bunford et al., Reference Bunford, Evans and Wymbs2015; Shaw et al., Reference Shaw, Stringaris, Nigg and Leibenluft2014). It is possible that a restricted range of externalizing symptoms explains this null finding. None of the participating children had a formal diagnosis of ODD or CD, and the level of CU symptoms was not significantly higher among the children with ADHD than among those in the typically developing group.
Pupil dilation to happy faces was related to higher parental ratings of emotionality for happiness as well as to hyperactive/impulsive symptoms. An interesting question for future studies is to examine whether pupil dilation metrics in conjunction with parental ratings of specific behaviors can help to identify specific subgroups within the ADHD phenotype that is characterized by disrupted positive emotionality (e.g., Karalunas et al., Reference Karalunas, Gustafsson, Fair, Musser and Nigg2019).
Higher pupil dilation was observed in response to faces that displayed negative emotions (anger and fear) than to those that showed positive emotions (happiness). This is consistent with previous research that has reported higher pupil dilation in response to potentially threatening than to nonthreatening stimuli (e.g., Kleberg et al, Reference Kleberg, Hanqvist, Serlachius and Högström2019; Price et al, Reference Price, Siegle, Silk, Ladouceur, McFarland, Dahl and Ryan2013; Silk et al, Reference Silk, Dahl, Ryan, Forbes, Axelson, Birmaher and Siegle2007). Somewhat surprisingly, neutral faces also resulted in higher pupil dilation than happy faces did. The reason for this is not clear. One possibility is that it was more cognitively demanding to recognize the neutral faces than it was to recognize the emotional faces. This could have resulted in an increased pupil dilation reflecting cognitive load rather than emotional arousal (Laeng et al., Reference Laeng, Sirois and Gredebäck2012). A second possibility is that neutral faces, although correctly identified, could have been perceived as having negative emotional valence (e.g., Cooney, Atlas, Joormann, Eugène, & Gotlib, Reference Cooney, Atlas, Joormann, Eugène and Gotlib2006).
A limitation is that a small number of children were on stimulant and nonstimulant medication for ADHD, which is known to affect noradrenergic neurotransmission, at the day of testing. The results were highly similar when children who were on medication who either failed to washout, were on nonstimulant medication, or on unknown medication (total n = 7) were removed. However, it should be noted that the study did not have sufficient statistical power to formally test for differences between children with and without medication.
An interesting venue for future studies is to examine whether pupil dilation responses can measure treatment effects on emotional impairments. A second limitation is that the diagnoses of the children with ADHD were not independently confirmed. Although it is possible that some of the included children may not have reached the diagnostic threshold at an independent assessment, this is not likely to have affected the results of the dimensional analyses. Importantly, parent and teacher symptom ratings indicated a wide range of symptoms, which supports the use of a dimensional analysis. Parents of children with ADHD were asked to report comorbid diagnoses. However, because an independent clinical assessment was not conducted, it is possible that some comorbid disorders or causes for inattention hyperactivity other than ADHD may have gone unnoticed.
It should be noted that although dimensional studies can be informative about the mechanisms that underlie ADHD symptomatology, they do not directly examine ADHD as a clinical diagnosis. Because the analysis in the present study was dimensional, future studies are needed to determine to what extent the results apply to ADHD understood as a categorical construct. Studies including larger samples of children with a clinical diagnosis of ADHD would also have better statistical power to examine the nonlinear relationships between ADHD symptoms and pupil dilation such as interactions between diagnosis, symptom level, and pupil dilation. Finally, it should be noted that although a relationship between parental ratings of emotionality and pupil dilation to happiness was found, the effect was relatively modest and needs replication in future studies.
In conclusion, we found that hyperactive/impulsive symptoms were uniquely related to increased pupil dilation to happy faces after controlling for inattentive and comorbid externalizing symptoms. This finding contributes significantly to our understanding of the emotional processes that are linked to ADHD symptoms.
Supplemental Material
The supplementary material for this article can be found at https://doi.org/10.1017/S0954579420000036.
Acknowledgments
We thank Mozaffar Hessami, MD, and the staff at Kista BUMM for help during data collection.







