It has been a decade since Patel & David’s review in Advances titled ‘Why aren’t depot antipsychotics prescribed more often and what can be done about it?’ (Reference Patel and DavidPatel 2005). Increasingly, the term ‘long-acting injectable antipsychotic medication’ (LAI) has been adopted in preference to the more stigmatising ‘depot’ and the latter’s association with limitations attributed to first-generation antipsychotics (Reference Patel, Taylor and DavidPatel 2009). The burgeoning range of LAIs has largely been one of reformulations of existing medications, rather than the development of novel agents, and the use of LAIs has continued to fall in many jurisdictions (Reference Patel, Haddad and ChaudhryPatel 2010). Poor adherence to maintenance antipsychotics, rather than patient preference, is still the primary indication for LAIs (Reference Patel, Taylor and DavidPatel 2009). The continuing challenges with LAIs include:
-
• adherence, even when suboptimal adherence is more overt than with oral agents
-
• variable prescribing by clinicians, with a focus mostly on long-standing rather than first-episode schizophrenia
-
• variable acceptance by patients and clinicians
-
• increasing concern regarding tolerability.
There is now greater emphasis on recovery-focused care and supported decision-making, rather than overly paternalistic relationships between clinicians and patients (Reference Chopra, Hamilton and CastleChopra 2009). This collaborative shift in the therapeutic dyad encompasses shared decision-making about LAIs (Reference Haddad, Lambert, Lauriello, Haddad, Lambert and LaurielloHaddad 2011) and hence joint discussion and consideration of medication options, and their benefits v. risks, as well as adherence. Although the risk of extrapyramidal side-effects (e.g. tardive dyskinesia), particularly with (first-generation) early LAIs, has continued (Reference Kane and Garcia-RiberaKane 2009), there is now a greater spotlight on cardiometabolic sequelae, including diabetes, obesity and hyperlipidaemia (Reference da Silva Freire Coutinho, Fenton and Quraishide Hert 2009). Accordingly, we review the rate of utilisation of ‘depot’ or LAI medication in the context of the changing clinical landscape.
Schizophrenia and adherence: are LAIs part of the answer?
Schizophrenia is the only widely researched indication for LAIs. The overall prevalence of suboptimal adherence among people with schizophrenia who are prescribed antipsychotic medication ranges from one- to two-thirds (Reference Patel, Taylor and DavidPatel 2009). Although there has been much early research and support for maintenance treatment with LAIs in patients with schizophrenia who exhibit poor medication adherence, use of this route in this clinical population in the USA has ranged from 19 to only 30% (Reference West, Marcus and WilkWest 2008). In a national sample of US psychiatrists, LAI prescribing was inversely related to the prior use of second-generation oral antipsychotics and other oral psychotropic medications (Reference West, Marcus and WilkWest 2008). In the UK, about 30% of patients with schizophrenia are prescribed LAIs (Reference Barnes, Shingelton-Smith and PatonBarnes 2009). However, the long-term outcome in schizophrenia is heterogeneous, whether or not maintenance antipsychotic medication is prescribed (Reference Harrow and JobeHarrow 2007). Although patients and clinicians alike often overestimate adherence (Reference Velligan, Wang and DiamondVelligan 2007), poor adherence with LAIs is more obvious than with oral medications.
First-episode psychosis
Irrespective of the route (oral or LAI), treatment adherence in people with schizophrenia is associated with fewer relapses and readmissions to in-patient care (Reference Staring, Van der Gaag and KoopmansStaring 2010; Reference Offord, Wong and MirskiOfford 2013). Although patients with first-episode schizophrenia are the least likely to be prescribed LAIs, they are also at substantial risk of the consequences of suboptimal treatment or poor adherence, including the impact of relapse. Consequently, consideration of the role of LAIs has broadened from relapse prevention in people with longstanding schizophrenia and poor adherence, to include people with first-episode psychosis. It has been argued that this might facilitate earlier treatment that is more effective in the longer term, rather than waiting for a person to enter a chronic relapsing phase of illness that is only partially responsive to medication (Reference Emsley, Oosthuizen and KoenEmsley 2008). Nevertheless, sufficient time to evaluate patients referred to early intervention services is required before either oral antipsychotics or LAIs are initiated, as a substantial proportion of referrals turn out not to have a first-episode psychotic disorder. Indeed, in one 4-year observational study, 41% of referrals to an early intervention service were ‘non-cases’: significantly, almost half had a major depressive or anxiety disorder (Reference O'Donoghue, Lyne and RenwickO’Donoghue 2012).
In addition to the ambivalence of treating clinicians about treatment recommendations, the limited use of LAIs in first-episode psychosis is likely compounded by the views of carers and families, including their attitudes and beliefs about mental illness, their level of acceptance of it, and whether any perceived risks outweigh benefits (Reference Kane and Garcia-RiberaKane 2009).
Schizophrenia and comorbid substance use
Although LAIs have been prescribed to people with schizophrenia and comorbid substance use (Reference Green, Drake and BrunetteGreen 2007; Reference Koola, Wehring and KellyKoola 2012), particularly when there are concerns about medication adherence, the supporting evidence for such use is limited and pertains to case reports and open-label studies of flupentixol, zuclopenthixol, risperidone (Reference Koola, Wehring and KellyKoola 2012) and paliperidone (Reference Vázquez Vázquez, González-Rodríquez and Sanz AsinVázquez Vázquez 2012). Two studies suggesting a favourable effect of LAI risperidone in this population (Reference Rubio, Martinez and PonceRubio 2006; Reference Rosenheck, Krystal and LewRosenheck 2011) require replication and evaluation via randomised controlled trials (Reference GreenGreen 2012).
Recommendations in clinical guidelines
Most clinical guidelines for the treatment of psychotic disorders – including those of the National Institute for Health and Care Excellence (NICE 2014), the American Psychiatric Association (Reference Lehman, Lieberman and DixonLehman 2010), the Canadian Psychiatric Association (Reference Addington, Bouchard and GoldbergAddington 2005), the Schizophrenia Patient Outcomes Research Team (PORT) (Reference Kreyenbuhl, Buchanan and DickersonKreyenbuhl 2010) and Texas Medication Algorithms (Reference Argo, Crismon and MillerArgo 2007) – pay regard to patient preference and recommend, albeit vaguely, that LAIs be prescribed for patients with recurrent relapses linked to suboptimal adherence or non-adherence. However, these guidelines have not recommended that LAIs be used in patients with schizophrenia who favour oral medications and have been consistent in their adherence to these, nor in patients who have experienced significant poor tolerability or response to LAIs (Reference Kane and Garcia-RiberaKane 2009).
The evidence that LAIs enhance adherence and outcomes
First-generation LAIs v. oral antipsychotics
An observational study of patients who had been prescribed oral or LAI haloperidol or fluphenazine showed that those prescribed LAIs were twice as likely to remain in treatment and had a longer period until all-cause medication discontinuation (Reference Zhu, Ascher-Svanum and ShiZhu 2008). In patients with first-episode schizophrenia or schizoaffective disorder, there were lower rates of readmission to hospital and discontinuation of LAI perphenazine compared with oral haloperidol (Reference Tiihonen, Wahlbeck and LonnqvistTiihonen 2006).
A synthesis of Cochrane systematic reviews of first-generation LAIs found a slight benefit on global functioning in comparison with oral antipsychotics, but there were no differences between individual LAIs (Reference Adams, Fenton and QuraishiAdams 2001). These findings were probably limited by an underrepresentation of individuals with poor adherence to oral antipsychotics in randomised controlled studies (Reference Adams, Fenton and QuraishiAdams 2001; Reference Tiihonen, Haukka and TaylorTiihonen 2011). A Cochrane review comparing zuclopenthixol decanoate with other first-generation LAIs in schizophrenia revealed superior efficacy in delaying or preventing relapse, but at the expense of more adverse side-effects (Reference da Silva Freire Coutinho, Fenton and Quraishida Silva Freire Coutinho 2009).
A 3-year international observational study of first-generation LAIs compared with oral olanzapine favoured the latter (Reference Haro, Suarez and NovickHaro 2007). However, a nationwide cohort study in Finland found that, compared with oral antipsychotics, LAIs (first generation and risperidone) were associated with reduced risk of readmission (Reference Tiihonen, Haukka and TaylorTiihonen 2011).
First- v. second-generation LAIs and oral antipsychotics
A meta-analysis of first- and second-generation LAIs found benefits over oral agents in non-randomised observational, but not in randomised, studies (Reference Kirson, Weiden and YermakovKirson 2013). Other systematic reviews of first- and second-generation LAIs compared with oral antipsychotics (Reference Leucht, Heres and KaneLeucht 2011; Reference Zhornitsky and StipZhornitsky 2012) found them to be associated with a reduced rate of relapse. Also, a 2-year multicentre randomised study comparing risperidone LAI with equivalently dosed oral second-generation antipsychotics demonstrated reduced readmission rates, but no difference in terms of discontinuation of treatment (Reference Rosenheck, Krystal and LewRosenheck 2011).
A multicentre randomised controlled study comparing haloperidol with paliperidone LAI in patients with schizophrenia or schizoaffective disorder found ‘efficacy failure’ (defined as any of: increased out-patient visits, crisis stabilisation, in-patient admission or inability to discontinue oral antipsychotics within 8 weeks of LAI commencement) in a third of participants (Reference McEvoy, Byerly and HamerMcEvoy 2014). Although the study did not find haloperidol or paliperidone to differ in the rate of ‘efficacy failure’ over 2 years, haloperidol was associated with a greater rate of akathisia, and paliperidone with greater weight gain and elevated serum prolactin.
Cost-effectiveness
In general, second-generation LAIs are considerably more expensive than first-generation LAIs. Such considerations, which are particularly relevant to healthcare services, need to be balanced against the effectiveness, differential side-effect profile and tolerability of medications in individual patients, thus affording more personalised medicine. A systematic review of 28 economic evaluations of LAIs for schizophrenia found that second-generation LAIs, particularly risperidone, were likely to be a more cost-effective initial management strategy than haloperidol LAI and other LAI or oral formulations. However, the review was limited by utilisation of qualitative (not quantitative) assessment and the unavailability or exclusion of possibly relevant papers and conference abstracts (Reference Achilla and McCroneAchilla 2013).
Acceptability of LAIs
Validated recovery approaches in psychiatry emphasise:
-
• the centrality of the patient’s goals
-
• psychoeducation, cognitive–behavioural strategies to support treatment adherence, relapse prevention plans, and social and coping skills training
-
• the therapeutic relationship to identify strengths, resources and goals (Reference Chopra, Hamilton and CastleChopra 2009).
Consequently, patients’ expectations and acceptance of LAIs need to be considered in the context of the quality of the patient–clinician relationship (Reference McCabe, Bullenkamp and HanssonMcCabe 2012). Using a collaborative recovery approach when discussing the benefits and risks of LAIs with patients emphasises their sense of self, social inclusion and relationships, instilling hope rather than focusing mainly on symptoms and impairments. The compulsory administration of LAIs under mental health legislation because the patient is at risk of harming themselves or others does not mitigate the onus on the clinician to take a recovery-based approach: patients who perceive coercion in their contact with mental health services are less likely to be engaged in care (Reference Stanhope, Marcus and SolomonStanhope 2009).
Not surprisingly, the acceptability of LAIs to people with schizophrenia is also influenced by their previous experience of them (Reference Heres, Schmitz and LeuchtHeres 2007), and side-effects adversely affect adherence (Reference Dibonaventura, Gabriel and DupclayDibonaventura 2012). A survey of patients’ views about LAIs found that two-thirds were not given sufficient information by their clinicians. Concerns about losing autonomy and about pain secondary to injections fuelled negative views about this treatment (Reference Jaeger and RosslerJaeger 2010). However, the majority of patients who had actually received LAIs had positive attitudes towards them (Reference Walburn, Gray and GournayWalburn 2001; Reference Heres, Schmitz and LeuchtHeres 2007; Reference Waddell and TaylorWaddell 2009), whereas only a minority of patients who had not tried them found the suggestion of LAIs acceptable (Reference Heres, Schmitz and LeuchtHeres 2007).
It seems that clinicians often view LAIs as a last-resort treatment to mitigate the risk of relapse (Reference Waddell and TaylorWaddell 2009). Prescribing practices vary not only within but also between countries. Clinicians in the USA in particular tend to hold negative attitudes towards LAIs (Reference Patel, Taylor and DavidPatel 2009). A survey of US psychiatrists found that less than one-fifth of patients were prescribed LAIs for poor adherence to oral medications (Reference West, Marcus and WilkWest 2008). Among psychiatrists surveyed in the UK, 38% thought that LAIs were less effective in patients with first-episode psychosis and a third thought that patients preferred oral medications (Reference Patel, Haddad and ChaudhryPatel 2010). Despite their view that LAIs were associated with superior adherence compared with oral medications, half of the surveyed psychiatrists had reduced their prescribing of LAIs in the preceding 5 years. A survey of psychiatrists, patients and relatives in Switzerland found that fewer than one in ten psychiatrists prescribed an LAI following a first episode of psychosis (Reference Jaeger and RosslerJaeger 2010). This study also found that patients were more negative about LAIs than their relatives or psychiatrists, mostly because of the above-mentioned concerns about their autonomy being restricted and experiencing pain as a result of the injections.
Antipsychotic polypharmacy
Antipsychotic polypharmacy is more likely in patients treated with LAIs (Reference Barnes, Shingelton-Smith and PatonBarnes 2009) and in those with suboptimal response to a single LAI (Reference Patel, Taylor and DavidPatel 2009). A study of patients in the USA found that they were less likely to be prescribed LAIs together with adjunctive oral antipsychotics than their counterparts in Europe (Reference Olfson, Marcus and Ascher-SvanumOlfson 2007). Polypharmacy may also contribute to clinicians’ views about less favourable tolerability of LAIs compared with oral medications (Reference Patel, Taylor and DavidPatel 2009).
Prescribing guidelines
Key points on antipsychotic prescribing in the updated NICE (2014) clinical guideline on psychosis and schizophrenia are summarised in Box 1.
BOX 1 Summary of key NICE guideline points on antipsychotic medication for psychosis and schizophrenia in adults
-
• Do not offer antipsychotic medication to people at increased risk of developing psychosis, or to decrease the risk of, or prevent, psychosis.
-
• Before starting antipsychotic medication: check weight, waist circumference, pulse and blood pressure; fasting blood glucose, HbA1c, blood lipid profile and prolactin levels; assess for any movement disorders; assess nutritional status, diet and level of physical activity; offer electrocardiogram (ECG) if specified in the drug’s Summary of Product Characteristics, or physical examination has identified specific cardiovascular risk or history of cardiovascular disease, or on in-patient admission.
-
• Regularly throughout treatment, especially during titration, monitor and record: response to treatment, including changes in symptoms and behaviour; side-effects of treatment; emergence of movement disorders; weight, weekly for 6 weeks, at 12 weeks, at 12 months then annually (plotted on a chart); waist circumference, annually (plotted on a chart); pulse and blood pressure at 12 weeks, at 12 months, then annually; fasting blood glucose, HbA1c and blood lipid levels at 12 weeks, at 1 year and then annually; adherence; and physical health.
-
• For initial episodes, if the person wishes to try psychological interventions alone, advise that these are more effective in conjunction with antipsychotic medication. If the person still wishes to try psychological interventions alone: offer family intervention and cognitive–behavioural therapy (CBT); agree to a time (≤1 month) to review treatment options, including introducing antipsychotic medication; and continue regular monitoring of symptoms, distress, impairment and level of functioning, including in education, training and employment.
-
• For subsequent acute episodes, offer oral antipsychotic medication and psychological interventions (family intervention and individual CBT).
-
• The choice of antipsychotic medication should be decided between the person and healthcare professional, taking into account the views of the carer if the patient agrees.
-
• Provide information and discuss the likely benefits and possible side-effects of each drug, including: metabolic (including weight gain and diabetes); extrapyramidal (akathisia, dyskinesia and dystonia); cardiovascular (including prolonging QT interval); hormonal (including increasing plasma prolactin); and other (including unpleasant subjective experiences).
-
• Treatment with regular and ‘as required’ antipsychotic medication should be as follows: discuss and record side-effects that the person is most willing to tolerate; record indications, expected benefits and risks, and expected time for a change in symptoms and appearance of side-effects; at start of treatment, give a dose at lower end of the licensed range (shown in the British National Formulary or Summary of Product Characteristics) and slowly titrate upwards within the dose range given in those publications; if doses above the licensed range are used, document the reasons; record rationale for continuing, changing or stopping medication, and the effects of such changes; and carry out a trial of medication at optimum dose for 4 to 6 weeks.
-
• Do not use a loading dose of antipsychotic medication.
-
• Do not initiate regular combined antipsychotic medication, except for short periods.
-
• Offer clozapine to people who have not responded adequately to treatment despite sequential use of at least two different antipsychotics. At least one of the drugs should be a non-clozapine second-generation antipsychotic. If response to clozapine is inadequate, consider further review, including measuring therapeutic drug levels, before adding a second antipsychotic to augment treatment with clozapine. Choose a drug that does not potentiate the common side-effects of clozapine. A trial of augmentation may need to continue for 8–10 weeks.
-
• Consider depot/long-acting injectable antipsychotic medication for people who would prefer this treatment after an acute episode, or where avoiding covert non-adherence (either intentional or unintentional) is a clinical priority; initially use a small test dose as set out in the British National Formulary or Summary of Product Characteristics.
Choosing specific LAIs
Early LAIs were esterified preparations in an oil solution, administered every 1 to 6 weeks.
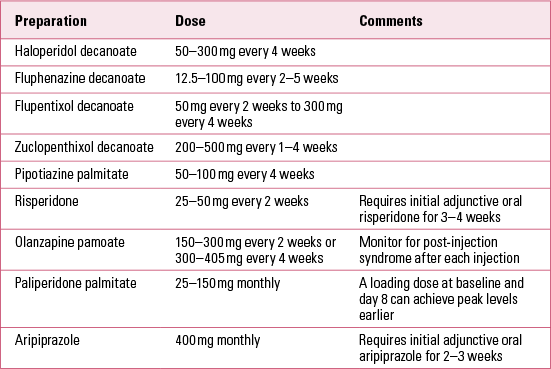
The preparation types have broadened since the advent of risperidone LAI, which is delivered via microspheres. In recent years, newer LAI preparations have been delivered via nanoparticles in aqueous suspension for paliperidone palmitate and olanzapine pamoate, and via lyophilised powder reconstituted in sterile water for aripiprazole monohydrate (Reference Gopalakrishna, Aggarwal and LaurielloGopalakrishna 2013). A list of available LAIs is presented in Table 1. Notwithstanding risk–benefit analyses for individual patients, LAIs have a range of advantages, but also pitfalls compared with their oral counterparts. Some of these considerations are summarised in Box 2.
BOX 2 Some advantages and disadvantages of LAIs over oral preparations
Advantages
-
• No first-pass metabolism; improved bioavailability
-
• More consistent delivery of antipsychotic with more stable plasma levels; minimized side-effects and reduced variations in symptom control
-
• Wider window of opportunity to re-engage assertively with patient if LAI refused
-
• Injection; plasma levels take longer to decline than with oral formulations
-
• Earlier detection of non-adherence, which is more overt and can be followed up quickly; potential reduction in relapse rates
-
• Reduced risk of accidental or intentional self-harm by overdose
Disadvantages
-
• Pain, erythema, swelling at the site of injection; nodule formation, particularly with oil-based injections
-
• Risk of damage to nerves, arteries or veins
-
• If side-effects occur they will be prolonged until the plasma level falls
-
• Patient may be allergic to the oily vehicle, so a test dose of oil-based LAIs is needed
-
• Need to confirm efficacy and tolerability of the oral formulations of the LAIs where required and practical
-
• Can take several weeks for plasma levels to reach steady state
-
• Potential logistical difficulties in administering injections to a patient who is in employment
-
• Possible stigma
-
• Patient dislike or even a phobia of needles
(After Feetam 2014: p. 12)
TABLE 1 LAI preparations

First-generation LAIs
For first-generation LAIs such as haloperidol, fluphenazine, flupentixol, zuclopenthixol and pipotiazine, it may take 2–3 months to achieve steady-state plasma concentrations (Reference TaylorTaylor 2009).
The efficacy and tolerability of first-generation LAIs have been reviewed by Reference Adams, Fenton and QuraishiAdams et al (2001), including their side-effect profiles in comparison with oral antipsychotics. It should be noted that Reference Patel and DavidPatel & David (2005) raised the caveat that most of the randomised controlled studies included in that review were probably too short to optimise relapse prevention. This said, fluphenazine and haloperidol LAIs have higher rates of extrapyramidal side-effects such as tardive dyskinesia, dystonias and Parkinsonism compared with the second-generation LAIs risperidone, paliperidone, olanzapine and aripiprazole (Reference Gopalakrishna, Aggarwal and LaurielloGopalakrishna 2013).
Second-generation LAIs
Risperidone
Patients prescribed risperidone LAI require adjunctive oral risperidone for 3–4 weeks, as the biodegradable microspheres require several weeks for peak release. The medication also requires a special preparation technique and refrigeration before injection. The main tolerability problems with risperidone LAI are weight gain and hyperprolactinaemia (Reference Gopalakrishna, Aggarwal and LaurielloGopalakrishna 2013).
Paliperidone
Paliperidone LAI, based on the active metabolite of risperidone (9-hydroxyrisperidone), is available in prefilled syringes, does not require refrigeration before injection and can be administered monthly (as opposed to fortnightly for risperidone LAI); it is released as early as the first day and plasma levels peak at day 13 (an initial loading dose, followed by another at day 8, facilitates peak levels earlier), obviating the need for oral supplementation (Reference Gopalakrishna, Aggarwal and LaurielloGopalakrishna 2013). Paliperidone LAI is readily metabolised and thus has less potential for medication interactions than risperidone, and may be safer for patients with hepatic impairment (Reference Álamo and López-MuñozÁlamo 2013). There are no randomised head-to-head studies comparing paliperidone LAI with risperidone LAI.
Olanzapine
Olanzapine LAI reaches peak plasma levels in under a week and without the need for adjunctive oral medication. Although there is the option of monthly injections, fortnightly administration is recommended. Apart from side-effects akin to those of oral olanzapine (namely, weight gain, sedation and hyperlipidaemia), a post-injection syndrome, which can occur irrespective of treatment duration, has been the main and concerning limitation with this medication. The post-injection syndrome is characterised by sedation, delirium or altered conscious state, and speech and gait disturbance (Reference Gopalakrishna, Aggarwal and LaurielloGopalakrishna 2013). Presentations of this syndrome require monitoring and supportive care for several days, generally in intensive care, and there have been reported deaths (Reference KuehnKuehn 2013), possibly secondary to cardiac arrhythmia or cardiopulmonary arrest. Accordingly, patients must be monitored for 2–3 hours after each injection.
Aripiprazole
Aripiprazole LAI is a lyophilised powder that requires reconstitution in sterile water. Monthly administration of aripiprazole LAI has been found to be effective at doses of 300 and 400 mg. Although peak plasma concentration occurs 5–7 days after injection, a fortnight of adjunctive oral aripiprazole has been recommended to optimise levels. Reported side-effects include tremor, sedation, headache and increased QTc interval (Reference Gopalakrishna, Aggarwal and LaurielloGopalakrishna 2013).
LAIs in clinical practice
The available evidence indicates that LAIs are underutilised in clinical practice for people with schizophrenia (including the first episode) and possibly those with comorbid substance use disorders. Their utility and use in this context are affected by patient, clinician and service barriers that can work against best practice and evidence-based care.
Although adherence, albeit more discernible with LAIs, is a prime factor in considering these agents, the answer does not lie in a dichotomous ‘oral v. LAI’ approach to prescribing. If we are to learn from history, the therapeutic success or otherwise of LAIs essentially depends on the quality of follow-up care (Reference JohnsonJohnson 2009). Patients prescribed LAIs may relapse, even when adherent to these medications, and may also experience significant side-effects. Consequently, continued LAI use may not be appropriate for all patients, even if oral medication adherence is a significant concern. Also, differential costs of newer LAIs compared with older and cheaper agents should be balanced with individually tailored care, including considerations of tolerability.
Clinician and nurse education, training and support
In translating the evidence and clinical guidelines (such as NICE 2014) about LAI use into readily accessible, individually tailored and recovery-focused care, concomitant support for clinician education and supervision and quality improvement processes across primary and specialist care are vital. This need is evidenced by the influence of clinicians’ attitudes, which may be discordant with current guidelines, as well as the extent of awareness and vigilance about medication adherence and its marked impact on patient outcomes and relapse prevention.
Building on the essential framework of a recovery-informed approach, nurses administering LAIs have a key role in communication (in primary care and specialist settings) with other clinicians involved in patient care. This role requires engaging patients in broader discussions about their health (e.g. diet, exercise and smoking), as well as monitoring outcome measures, including tolerability (e.g. using The Liverpool University Neuroleptic Side Effect Rating Scale (LUNSERS); Reference Day, Wood and DeweyDay 1995). Nurses in this role, irrespective of the setting in which they are working, require initial mentoring and supervision, as well as relevant knowledge of psychopharmacology, anatomy and physiology germane to the medication. Advance choice (advance decision) conversations with the patient about the benefits and risks of treatment options such as LAIs can facilitate therapeutic engagement and care within a recovery framework (Reference Gray, Spilling and BurgessGray 2009). The Royal College of Psychiatrists (2014) has produced a patient leaflet on LAIs, and Box 3 lists questions that may be helpful in assessing a patient’s attitudes towards LAIs and oral antipsychotics.
BOX 3 Ascertaining the patient’s attitudes towards LAIs v. oral antipsychotics
The following questions are helpful:
-
• Would you rather have medication by LAI or by tablet?
-
• Do you think there is a difference between taking tablets daily or having an injection every few weeks?
-
• How do you compare your current medication (LAI) with your previous medication?
-
• How do you feel about the medication you have in the form of injections compared with earlier treatment with tablets or other injections?
(Adapted from Reference Walburn, Gray and GournayWalburn 2001)
Conclusions
The beliefs and attitudes that patients and clinicians hold about antipsychotic medication, as well as the quality of their recovery-focused relationship, are key factors in adherence. LAIs have a place in addressing non-adherence, and arguably should be part of the choices discussed with any patient requiring long-term treatment, even early in the illness course. However, LAIs are currently underused in schizophrenia. There is a need for better education of clinicians, including improved knowledge of guidelines such as those from NICE, and for strategies to address the stigma associated with ‘depot’ antipsychotics and to change beliefs that are contrary to evidence-based care.
MCQs
Select the single best option for each question stem
-
1 Long-acting injectable antipsychotics (LAIs):
-
a are generally better tolerated than oral antipsychotics
-
b prevent relapse in more than 95% of patients with schizophrenia
-
c are less subject to covert non-adherence than oral antipsychotics
-
d are administered at least every 4 weeks
-
e are more efficacious than similar oral antipsychotics.
-
-
2 Treatment adherence for LAIs:
-
a is to a small degree dependent on the quality of the patient’s relationship with the clinicians
-
b is worse than for oral antipsychotic medications
-
c is occasionally related to the patient’s personal opinion regarding their susceptibility to relapse
-
d is generally predicted by a patient’s verbal report of their adherence behaviour
-
e is significantly influenced by the side-effect profile.
-
-
3 Reasons for LAI underutilisation do not include:
-
a prescriber knowledge regarding these medications
-
b family or carer views
-
c patients’ concerns about pain and side-effects
-
d sufficient information provided to the majority of patients
-
e the cost of medications.
-
-
4 Advantages of LAIs compared with oral antipsychotics include:
-
a easier early detection of relapse
-
b better cardiometabolic tolerability in a proportion of patients
-
c less need to focus on a recovery-based approach to care
-
d markedly reduced hospital readmission rates
-
e reduced risk of self-harm with antipsychotic medication.
-
-
5 Regarding the NICE clinical guidelines for psychosis and schizophrenia in adults:
-
a physical health evaluation is sometimes required before initiation of LAIs
-
b LAIs should be considered when avoiding covert non-adherence is a clinical priority
-
c LAIs should be avoided in people after a first episode
-
d clinicians’ views are more important than patients’ views about LAIs
-
e LAIs delay the need for clozapine.
-
MCQ answers
| 1 | c | 2 | e | 3 | d | 4 | e | 5 | B |



eLetters
No eLetters have been published for this article.