INTRODUCTION
Diarrhoea is one of the major causes of mortality among children, accounting for 9% of all deaths among children under age 5 worldwide in 2015. This translates to over 1400 young children dying each day, or about 526 000 children a year [1]. Salmonellosis is one of the most common bacterial diarrhoeal illnesses among children and poses a significant public health burden worldwide [2]. Globally, the non-typhoidal members of the bacterial species Salmonella enterica are estimated to cause 93·8 million episodes of gastroenteritis each year, with 155 000 deaths [Reference Majowicz3]. In recent years, antimicrobial resistance among non-typhoidal Salmonella has increased worldwide, due to the widespread use of antimicrobial drugs in the human and veterinary sectors. This has limited the therapeutic options for treatment, raising global public health concern [Reference Abraham4].
Consumption of contaminated food and water are the two main sources of gastroenteritis caused by non-typhoidal Salmonella, although many routes of transmission have been recognized including contact with infected farm animals, pets, rodents, birds, reptiles and amphibians, environmental contamination, and direct transmission from person to person [Reference Mermin5]. However, the source and transmission of gastrointestinal non-typhoidal Salmonella infections in many developing countries are less well characterized [Reference Graham6].
In humans, salmonellosis is usually a self-limiting disease presented with symptoms such as diarrhoea, fever, vomiting, abdominal cramping, and other non-specific complaints such as myalgia, arthralgia, and headache [Reference Hohmann7, Reference Jones8]. Infants and young children are significantly more susceptible to the effects of non-typhoidal Salmonella infection compared with other age groups and are therefore also at higher risk of secondary complications [Reference Jones8]. The most common systemic infection is bacteraemia which occurs in 5–10% of invasive cases, with bacteraemic children having a high risk for the development of meningitis resulting in increased fatality rates [Reference Sirinavin, Chiemchanya and Vorachit9].
Developing countries bear the brunt of diarrhoeal illness burden in both mortality and morbidity. In South Asia and the Middle East, S. enterica Typhimurium has been particularly prominent, with the case-fatality as high as 30% in some outbreaks [Reference Nasrin10]. The strain in this geographic region is considerably more virulent than that found in northern Europe and North America [Reference Nic Fhogartaigh and Dance11]. The more virulent strains in the poor and middle-income socio-economic regions have shown to be also associated with extensive resistance to antibiotics [Reference Rahman12]. In the Middle East and North African regions, the prevalence of non-typhoidal Salmonella diarrhoeal infection among children under age 5 varies widely, ranging from 3% in Egypt [Reference Pazzaglia13] to 34% in Saudi Arabia [Reference al-Freihi14]. Obtaining local, accurate epidemiological data on enteric pathogens such as Salmonella is crucial to understand and combat diarrhoea in children under age 5.
In Iraq, diarrhoea is a major cause of morbidity and mortality in children under the age of 5 years [Reference Tawfeek, Najim and Al-Mashikhi15]. In general, child mortality rate of 13% for boys and 12% for girls was reported in Iraq in 2003 [Reference Ahmed16]. However, identification of the aetiological agents of diarrhoeal cases is handicapped by the lack of stool-culture characterization in routine diagnostic laboratories. In Baghdad, central Iraq, non-typhoidal Salmonella was the second most frequently reported cause of diarrhoea in children under age 5 [Reference Al-Kubaisy17]. In Mosul, northern Iraq, non-typhoidal Salmonella were detected in 15% of diarrhoeal cases in children [Reference Alrajab, Abdullah and Shareef18]. However, in the south of Iraq, there is no published data on the role of non-typhoidal Salmonella diarrhoeal infection in children <5 years old and its associated risk factors. The current study, therefore, aimed to determine the prevalence, clinical presentation, serotype and antimicrobial resistance profiles, and risk factors associated with non-typhoidal Salmonella infection in children in Thi-Qar Governorate, south-eastern Iraq.
METHODS
Study setting
Thi-Qar Governorate is located in south-eastern Iraq, and considered one of the least developed governorates in the country. The economy is largely rural and depends mainly on agriculture. Thi-Qar is the poorest governorate in Iraq. In 2011, 37·8% of the population lived below the poverty line of US$2·5 per day [19].
This study was conducted at two children's hospitals in central Thi-Qar: Bent Al-Huda Teaching Hospital and Mohammed Al-Mousawi Hospital. These are the two government referral hospitals that provide a full range of services to both inpatients and outpatients children who reside in urban and rural communities in Thi-Qar Governorate. Iraq has long summer with hot and dry months from March to September and the average temperatures in those months range higher than 40 °C.
Study design and cases enrolment
All children <5 years old with complaints of acute diarrhoeal disease admitted to the outpatient clinics in both hospitals from March to August 2016 were eligible for participation in the present study. The World Health Organization (WHO) definition for diarrhoea was used for case inclusion. A diarrhoea case was defined as the passage of three or more watery or loose stools (within 24 h) [20]. A Salmonella infection case was defined in this study as a diarrhoeal infection with stool culture-positive for non-typhoidal Salmonella spp. Children were excluded if they had been pre-treated with antimicrobials in the preceding week, had multiple complications unrelated to diarrhoeal disease or if the caregivers (parents or guardians) refused to provide information about their children. The children were evaluated for eligibility for the study by paediatricians after the initial clinical examination.
Specimen collection and questionnaire
Fresh stool specimens were collected from each study participant in sterile screw-capped containers. This was facilitated by the nursing staff and laboratory technologists on the day of admission to hospital. All samples were immediately placed in Amies transport media with charcoal (COPAN, Italy), stored, refrigerated (4 °C) and then transported to the Microbiology Laboratory, University of Thi-Qar where testing started on the same day.
Treating paediatricians were asked to complete a simple form for information on clinical features and duration of disease. In addition, a questionnaire was administered to the child's parent or guardian by the research team to gather information on basic demographic, socio-economic indicators, and information on potential risk factors for infection. To ensure the reliability of information, all the questionnaires were administered by native Arabic speakers and were checked for completeness and consistency every day.
Microbiology
Isolation and identification of Salmonella from stool specimens were performed as recommended by the Global Foodborne Infections Network laboratory protocol [21]. Briefly, approximately 1 g of faeces was suspended in 9 ml of Buffered Peptone Water and incubated at 37 °C for 24 h. Then, 100 µl of pre-enriched suspension was added to 10 ml of Rappaport–Vassiliadis Broth (Oxoid, England) and incubated at 42 °C for 24 h. In parallel, 1 ml of the same suspension was added to 10 ml of tetrathionate broth (Oxoid, England) and incubated for 24 h at 42 °C. The sample from these two enrichment broths was cultured by streaking onto xylose lysine deoxycholate agar, brilliant green agar and Salmonella–Shigella agar (Oxoid, England) and the plates were incubated at 37 °C for 18– 24 h. Presumptive Salmonella colonies were then confirmed biochemically using the API-20E kit (bioMerieux, France). In addition, confirmation was performed by one-step PCR for the S. enterica gene invA (521 bp amplicon) using primers invA-F 5′–TTGTTACGGCTATTTTGACCA–3′ and invA-R 5′–CTGACTGCTACCTTGCTGATG–3′ as previously described by Swamy et al. [Reference Swamy22].
Serotyping of Salmonella isolates was performed by the Iraqi National Centre for Salmonella, at the Central Public Health Laboratories in the Baghdad's Centre for Disease Control and Prevention. Finally, antimicrobial susceptibility testing of Salmonella isolates was performed using the disc diffusion method on Mueller–Hinton agar plates according to the guidelines of the Clinical and Laboratory Standards Institute [23]. The following 12 antibiotics were used: ampicillin (10 µg), amoxicillin + clavulanic acid (20 µg + 10 µg), ceftriaxone (30 µg), cefotaxime (30 µg), ciprofloxacin (5 µg), chloramphenicol (30 µg), gentamicin (10 µg), nalidixic acid (30 µg), streptomycin (10 µg), azithromycin (15 µg), tetracycline (30 µg), and trimethoprim sulfamethoxazole (1·25–23·75 µg) (Mast Diagnostics Ltd, Merseyside, UK). Escherichia coli ATCC25922 strain was used as a quality control organism in susceptibility test. Isolates resistant to three or more different classes of antimicrobials were classified as multi-drug resistant (MDR).
Statistical analysis
The binary results of stool-culture testing (Salmonella negative = 0/Salmonella positive = 1) and variables on demographic, socio-economic indicators, and information on potential risk factors were recorded for all cases enrolled in this study. The data were stored in Excel spreadsheet and analysed using STATA version 11.0 for Windows. Descriptive analysis of categorical variables was based on frequency summary and using the χ 2 test. The analyses of Salmonella diarrhoeal infection risk factors were conducted in two steps. Firstly, the association between potential predictor factors (independent variables) and dependent variable (stool-culture positive for Salmonella spp.) was initially assessed using univariate logistic regression analysis to quantify the strength of association between the independent variables and Salmonella positivity. The second stage in the analysis consisted of building a multivariable logistic regression model based on potential risk factors identified from the univariate analysis with P-value ⩽0·25. The most appropriate final model was selected using the backward stepwise selection approach. The associations between stool-culture positivity for Salmonella spp. and risk factors were assessed by odds ratio (OR), and 95% confidence intervals (CIs) were considered significant at P-value ⩽0·05. All pairwise interactions between the variables in the final model were examined for significance. Goodness of fit of the final model was assessed using the Hosmer–Lemeshow test.
Ethical consideration
The study protocol was approved by the Murdoch University Human Research Ethics Committee (Project No. 2015/224). Permission to conduct the study was also obtained from the Ministry of Health, Iraq (Permit No.11/5/393) and the children's hospitals in Thi-Qar Governorate (Permit No.1/4/26885). As the study subjects were children under age 5, informed verbal consent was obtained from their caregivers (parents/guardians) before enrolment. The objectives and an importance of the present study were explained to all caregivers and confidentiality of their information was confirmed.
RESULTS
A total of 320 children with acute diarrhoea were enrolled between March and August 2016 from the two defined hospitals in Thi-Qar, south-eastern Iraq. For 33 (10·3%, 95% CI 8·4–12·4) diarrhoea cases, patient stool culture yielded S. enterica on the day of hospital admission and therefore met the criteria for a Salmonella case. Of these, 18 (54·5%) were serotyped as S. Typhimurium. Other serotypes included S. Muenchen (4/33), S. Hato (4/33), S. Hadar (3/33), S. Enteritidis (3/33), and S. Mbandaka (1/33).
Responses from the questionnaire administered to the parents/guardians were evaluated to determine socio-demographic characteristics among the subjects identified with diarrhoea throughout the study period (Table 1). Of 320 children with diarrhoea, 180 (56·3%) were males and 140 (43·7%) were females. Children aged between 1 and 2 years showed a significantly (P < 0·05) higher rate (38·2%) of acute diarrhoea; also, a significantly (P < 0·05) higher rate (64·7%) was evident among children from rural areas. Almost one-third (28·4%, 91/320) of the caregivers of children admitted with diarrhoea were educated to primary education level only, and the majority (86%, 275/320) were unemployed. With regard to water supply and sanitation, in 76·5% (245/320) of the cases the caregivers reported not boiling their water prior to drinking. Approximately 30% (98/320) of children with diarrhoea were reported by caregiver to be exclusively bottle fed for the first 6 months and were from households containing domestic animals. More details are presented in Table 1.
Table 1. Univariable associations of potential risk factors of Salmonella diarrhoeal infection among children <5 years old admitted in two hospitals in Thi-Qar Governorate, Iraq
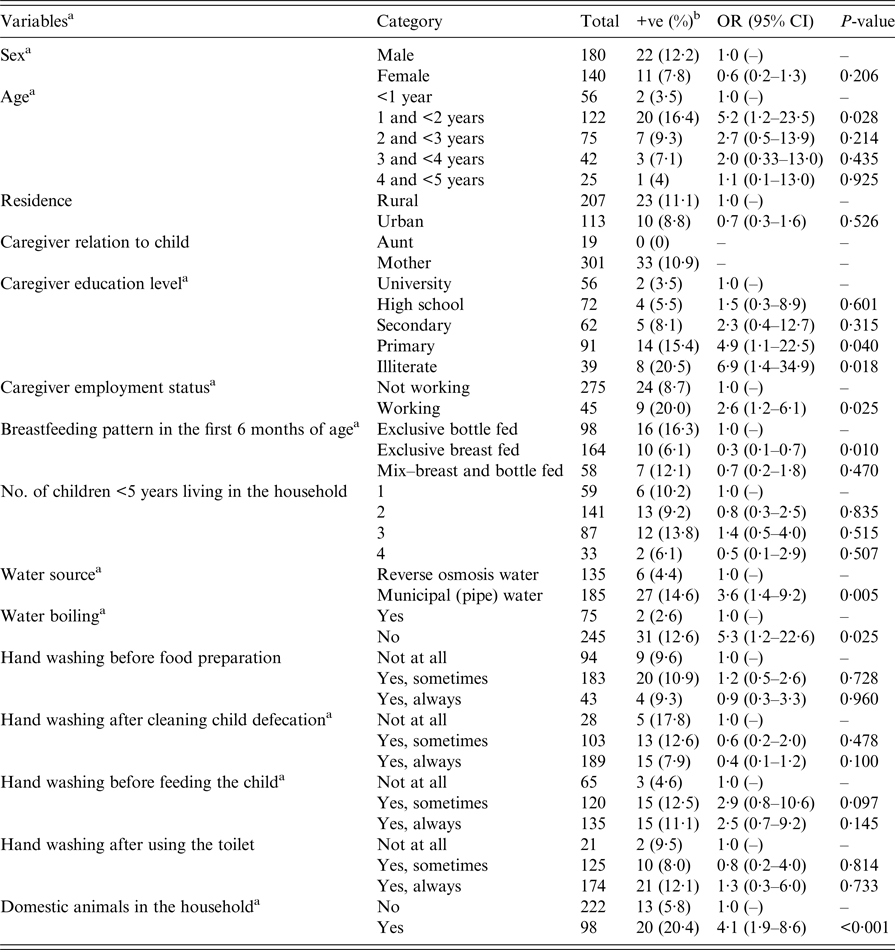
OR, odd ratio; CI, confidence interval.
a Variables with P < 0·25; risk factor offered to the final multivariable logistic model.
b +ve: Salmonella stool-culture positive; %: percentage.
Univariable logistic regression analysis was used to compare potential risk factors between Salmonella stool-culture positive and negative diarrhoea cases in Thi-Qar (Table 2). Sex and age of children, caregiver education level and employment status, breastfeeding, water source and water boiling, reported hand washing after cleaning child defecation and before feeding the child, and presence of domestic animals in the household showed a P-value <0·25 and were considered as potential risk factors in the univariable logistic regression model (Table 1). Out of these 10 potential risk factors, eight remained independently associated with Salmonella infections in the final multivariable logistic regression model (Table 2). The estimated ORs and their 95% CIs are presented in Table 2. None of the two-way interactions between variables were statistically significant (P > 0·05). The Hosmer–Lemeshow goodness-of-fit test suggested no evidence of lack of fit of the final model (Hosmer–Lemeshow χ 2 = 1·26, P = 0·9960).
Table 2. Multivariable logistic regression model of risk factors significantly associated with Salmonella diarrhoeal infection among children <5 years old admitted in two hospitals in Thi-Qar Governorate, Iraq

OR, odd ratio; CI, confidence interval; s.e., standards error.
These results (Table 2) suggest that, among the cases enrolled in this study, Salmonella diarrhoeal infection was more likely to occur among boys than girls (OR 8·4, 95% CI 2·8–24·9). Children aged between 1 and 2 years had significantly higher odds of Salmonella infection compared with those <1 year old (OR 8·0, 95% CI 2·9–22·1). Among the present study subjects, the likelihood of Salmonella infection in children from households supplied by pipe water was 4·7 (95% CI 1·6–13·9) times higher compared with those supplied with (purchased) reverse osmosis-treated water. Similarly, children from households with domestic animals were found to have a greater odds (OR 10·5; 95% CI 3·8–28·4) of being Salmonella stool-culture positive. The odds of Salmonella infection were higher (OR 3·9; 95% CI 1·0–6·4) among children belonging to a caregiver with only a primary-level education, compared with those with university-level education. Among the cases enrolled in this study, lower odds (OR 0·4; 95% CI 0·1–0·9) of Salmonella infection were associated with children exclusively breast fed compared with those exclusively bottle fed. According to the model results, the odds of Salmonella infection in children belonging to caregivers who reported always washing hands after cleaning child defecations was three times lower (95% CI 0·1–0·7) compared with those belonging to caregivers who did not wash hands at all (Table 2).
The main significant clinical feature associated with Salmonella diarrhoeal infection among children investigated during this study was abdominal pain (P = 0·001). Other features like fever, vomiting, duration, and consistency of diarrhoea did not differ significantly between Salmonella stool-culture positive and negative cases (Table 3).
Table 3. Clinical features associated with Salmonella diarrhoeal infection among children <5 years old admitted in two hospitals in Thi-Qar Governorate, Iraq

a +ve = stool-culture positive for Salmonella.
b −ve = stool-culture negative for Salmonella.
The results of antimicrobial susceptibility of Salmonella isolated from diarrhoeal infection indicate that all isolates were resistant to at least one of the 12 antimicrobials, with exception to chloramphenicol (Table 4). Resistance was most commonly detected to tetracycline (78·8%), followed by azithromycin (66·7%), ciprofloxacin (57·6%), trimethoprim/sulfamethoxazole (51·5%), streptomycin (48·4%), and nalidixic acid (45·5%). In addition, we identified a high level of resistance to third-generation cephalosporins including cefotaxime (42·4%) and ceftriaxone (36·4%). A lower resistance rate was observed against gentamicin (27·3%), ampicillin (12·1%), and amoxicillin/clavulanate (6·1%) (Table 4). Among the 33 non-typhoidal Salmonella isolates, multidrug resistance to three or more antimicrobials was recorded in 28 (84·9%) of the isolates, and resistance to five or more antimicrobials was detected in 19 (57·6%) of the total isolates. MDR to seven antimicrobials were observed in seven (21·2%) of the isolates. In the present study isolates, S. Typhimurium were more frequently encountered with resistance to clinically relevant antibiotics compared with isolates from other serotypes (Table 5).
Table 4. Antimicrobial susceptibility patterns of non-typhoidal Salmonella isolates (n = 33) from children <5 years old admitted in two hospitals in Thi-Qar Governorate, Iraq
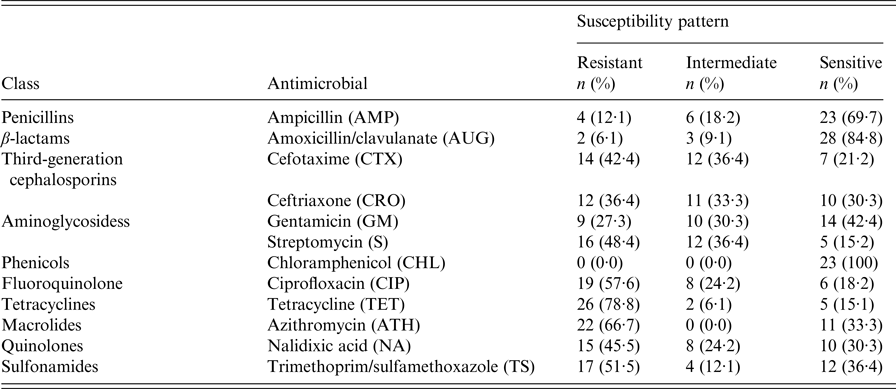
Table 5. Antimicrobial multi-resistance profiles of Salmonella serotypes isolated from children <5 years old admitted in two hospitals in Thi-Qar Governorate, Iraq
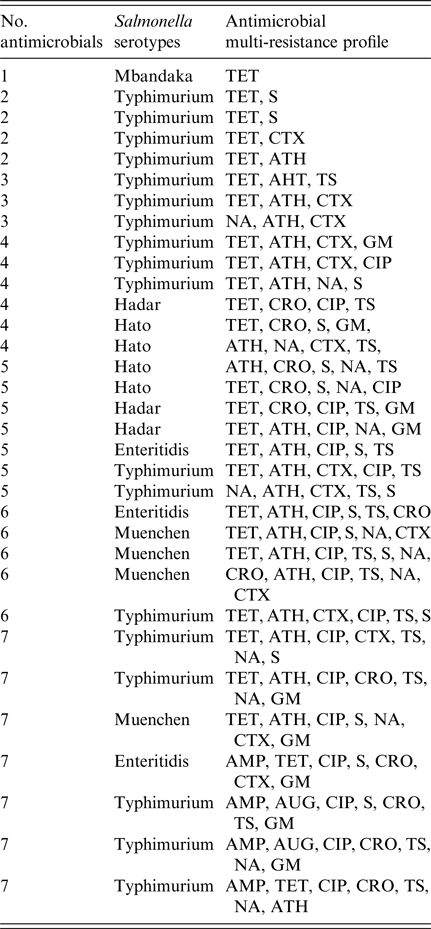
AMP, ampicillin; AUG, amoxicillin/clavulanate; CTX, cefotaxime; CRO, ceftriaxone; GM, gentamicin; S, streptomycin; CHL, chloramphenicol; CIP, ciprofloxacin; TET, tetracycline; ATH, azithromycin; NA, nalidixic acid; TS, trimethoprim/sulfamethoxazole.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the role of non-typhoidal Salmonella in the epidemiology of diarrhoea in children <5 years old in south of Iraq. The key findings arising from this study provides better insight on prevalence and key risk factors associated with non-typhoidal Salmonella infection in children in Thi-Qar, the poorest governorate in Iraq.
In the present study, the prevalence of non-typhoidal Salmonella in children <5 years in Thi-Qar was slightly lower compared with findings from studies in north of Iraq (15%) [Reference Alrajab, Abdullah and Shareef18] and in the neighbouring Kuwait (18%) [Reference Sethi and Khuffash24]. Nevertheless, the prevalence concluded from the current study was shown to be higher than what was concluded from studies in Baghdad, the capital of Iraq (4%) [Reference Al-Kubaisy17], and from many other Middle Eastern countries, such as, Turkey (5·2%) [Reference Küçük25] and Iran (7·6%) [Reference Jafari26], all in paediatric patients. It is important to note that direct comparison of our results with findings from other regional studies should be treated with caution, given differences in sample size, study period, and laboratory testing methods in each study. The sampling period in our study was mainly focusing on the summer months, which might also affect the prevalence results. Nevertheless, this is the first study on prevalence and antimicrobial resistance of Salmonella infection among children in this setting. In the present study, the proportion of cases cultured positive for Salmonella was in line with data from neighbouring countries including Jordan [Reference Battikhi27] and Saudi Arabia [Reference Al-Jurayyan28], with Salmonella isolation rates in children as 10·7% and 10·5%, respectively. These former studies also concluded that in children <5 years and the highest isolation rates of Salmonella were reported during summer months. In concordance with our findings regarding the dominance of S. Typhimurium, similar results could be revealed from studies in Iraq [Reference Alrajab, Abdullah and Shareef18] and Bangladesh [Reference Rahman12], underlying S. Typhimurium as an important serotype associated with diarrhoeal illnesses in children <5 years old in Iraq, as well as other developing countries.
In the present study setting, age and gender of children were highlighted by the final multivariable model as important risk factors associated with non-typhoidal Salmonella diarrhoeal illness in children <5 years old in Thi-Qar. Despite being intriguing findings, future case–control studies might be needed to confirm the validity of this finding. Previous studies from neighbouring countries such as Qatar [Reference Ehlayel, Bener and Abu Laban29] and Turkey [Reference Küçük25] indicated no statistically significant difference between boys and girls. However, other investigations from Taiwan [Reference Lee30] found a resembling finding to ours, with statistically significant higher Salmonella infection in boys compared with girls. With regard to age, our results show that the highest numbers of cases among the study subjects were among children aged between 1 and 2 years. This is consistent with previous studies done in Iraq [Reference Alrajab, Abdullah and Shareef18], and also in neighbouring countries such as Jordan [Reference Youssef31] and Iran [Reference Jafari26], where also the highest frequency of Salmonella-positive stool cultures were reported in the age group between 1 and 2 years. This could be due to an immaturity of the immune system and the slow development of immune competence in the post-natal and childhood years [Reference Upham32]. Furthermore, the risk of putting contaminated fingers and fomites in the mouth increased susceptibility to infectious diseases due to physiological behaviour such as teething and crawling which begins at this age [Reference Shah33].
Our findings indicate that in the current study setting, children belonging to the mothers with lower education level (illiterate and primary) were more likely to have Salmonella infection compared with the mothers with a higher education level. On the contrary to our findings, a study in the USA (between 1997 and 2007) found that the highest rates of Salmonella infections are in families with a high educational attainment [Reference Younus34]. The reasons for this contradiction are not clear. However, it could be hypothesized that, in the USA where the study was done, the more educated the mothers are, the more they could be capable of searching and receiving health care.
In this study, the pipe source of water (municipality supplied) was found to be a significant risk factor associated with Salmonella infection in children, and boiling water had a protective effect against Salmonella infection. These findings might indicate that contamination of drinking water by Salmonella could occur at some stages from the source to the point of use. Added to that, Salmonella has a well-documented capacity for colonizing surfaces and replicating in biofilms of distribution system pipes [Reference Jones and Bradshaw35]. Studies in some South Asian countries corroborated that the use of municipal water was a major risk factor for typhoid and non-typhoidal Salmonella outbreaks as reported in Pakistan and Nepal, respectively [Reference Farooqui, Khan and Kazmi36, Reference Bhatta37]. In Iraq, it is a common practice to store water in tanks and barrels at home for days before consumption [Reference Latif and Rossle38]. According to the United Nations data in 2013, the availability of drinking water in Thi-Qar province was reported as ‘bad or very bad’ by 69% of the population [19]. Collectively, our finding adds to the body of evidence that unsafe water consumption should be regarded among the significant factors associated with children infection with non-typhoidal Salmonella in Iraq.
In the present study, exposure to domestic animals in the household was a significant risk factor for Salmonella infection. This finding is not surprising as the correlation between Salmonella infection in children and contact with animals have been well documented in several studies [Reference Sato39–Reference Williams41]. Our study supports the hypothesis that animals living in proximity to the household are potential sources of human salmonellosis.
The final multivariable model (Table 2) indicates that exclusive breastfeeding and mother's hand washing after cleaning children following defecation are significant protective factors. Despite the significance of these findings, it is important to mind the risk of self-reported bias associated with such outcomes. Previous research showed that exclusive breastfeeding for the first 6 months is protective against development of salmonellosis and other enteric diseases among infants [Reference Williams41]. Added to that, several case–control studies in children with gastroenteritis from different countries [Reference Bassal42–Reference Rowe44] have demonstrated that breastfeeding is a significant factor in the prevention of severe salmonellosis, which might have a biological explanation given the well-documented protective effect of breast milk against infections [Reference France, Marmer and Steele45]. Conversely, bottle feeding was a risk factor associated with Salmonella, which can be explained by inappropriate washing and sterilization conditions of bottles, or unhygienic storage and possible contamination of formula during the preparation of bottles [Reference Williams41]. Another protective explanatory variable was mother's hand washing after cleaning children following defecation. Caregivers should always be encouraged to wash their hands after use of a toilet and before feeding the child in particular, as contamination of hands with faecal matter leads to contamination of surfaces and foods. This indirect contamination pathway has been highlighted previously as important in the transmission of enteric pathogens [Reference Jensen46].
With regard to clinical manifestations, we found that abdominal pain was most commonly occurring in 65·9% of cases (211/320) of salmonellosis among children <5 years in Thi-Qar. This agrees with previous studies in Oman [Reference Patel47] and Turkey [Reference Ince48]. Other features such as fever, vomiting, duration, and consistency of diarrhoea were not as significant in our study, which is similar to a study in Taiwan [Reference Huang49].
Antibiotic treatment is required in severe cases of gastroenteritis and whenever serious complications are caused by Salmonella infection. In our study, Salmonella isolates showed a high frequency of resistance to tetracycline, azithromycin, ciprofloxacin, and trimethoprim/sulfamethoxazole which are currently recommended for empirical treatment by physicians. In a study in Iran, Ranjbar et al. [Reference Ranjbar50] observed a high resistance to nalidixic acid (61·2%) and streptomycin (42·8%) among Salmonella strains isolated from paediatric cases with enteritis during 2007–2008. This is consistent with the present results, which also demonstrate a high percentage of nalidixic acid and streptomycin resistance. Added to that, our study revealed that 42·4% and 36·4% of the isolates were resistant to two common third-generation cephalosporins, cefotaxime, and ceftriaxone, respectively. The rate of resistance to these antibiotics in the current study setting in Thi-Qar, south-eastern of Iraq, was much higher than other findings in Kuwait and United Arab of Emirates [Reference Rotimi51]. This is alarming because third-generation cephalosporin antibiotics are among the first-line drugs for treatment of salmonellosis in children.
We report in this work some low rates of resistance among Salmonella isolated from children in Thi-Qar to gentamicin, amoxicillin/clavulanate, and ampicillin. This is in concordance with a study performed in the neighbouring country Kuwait, where Jamal et al. [Reference Jamal52] observed low levels of resistance to amoxicillin/clavulanate (7%) and ampicillin (17%) among Salmonella spp isolated from children and adults during 1990–1993. We also report an alarming high prevalence of MDR among Salmonella isolated from children in Thi-Qar. This might be explained by the fact that antimicrobial medications in Iraq, as in many other developing countries, are readily available for direct purchase from pharmacies and without the need for medical prescription. Since the Iraq war in 2003, there has been little central governance on antibiotic use in humans or the animal sector, and the widespread potential for misuse and overuse might be a contributing factor to such alarming resistance profiles seen in this study [Reference Jassim53].
Thi-Qar is regarded as the poorest governorate in Iraq. Conducting research in resources-limited settings is very challenging, and in general doing research in Iraq in the shadows of civil unrest, war, and insecurity is even much more confronting. Our study faced with some limitations. First, the study was conducted during the summer, which is the peak season for diarrhoea in Thi-Qar. Such peak might be attributed to increase in outdoor activities, increase in flies and rodents’ density in the summer, and the challenge in maintaining cold chain (e.g. for food commodities) in the hot months, as provoked by frequent electricity outage due to sand storms and heat waves in the study area. This is the first baseline research attempt in this region of Iraq, and it was important to target a sampling time frame that allows us to access a large number of Salmonella cases. Second, this study involved only children <5 years, given the vulnerability of such group to diarrhoea. The findings from this study cannot be generalizable to children older than 5 years; however, it provides valuable baseline information for the public health sector and for combating important enteric illnesses in Thi-Qar.
In conclusion, this study indicates that non-typhoidal Salmonella is an important cause of diarrhoea in children in Thi-Qar, which is the poorest governorate in Iraq. Boiling water, breastfeeding, hand washing practices, and avoiding animal contact in domestic setting could contribute to reducing the risk of transmission of non-typhoidal Salmonella from contaminated environments. A high prevalence of the antimicrobials-resistant isolates is a significant public health concern for treating diseases in children and adults. Therefore, systemic surveillance of antimicrobials and a dedicated centrally run stewardship programme is needed in Iraq in both human and veterinary medicine sectors. This work provides local, specific epidemiological data, which are crucial to understand and combat paediatric diarrhoea in Iraq.
ACKNOWLEDGEMENTS
This study has been supported by the Murdoch University and the Iraqi government. The authors would like to thank the physicians, staffs, and technicians of the children hospitals for their assistance with sampling and processing. The authors would also like to thank the caregivers of children who participated in this study. The authors are grateful to the Department of Biology, College of Science, Thi-Qar University for their technical assistance.








