In the UK, 23 antipsychotic drugs are available on prescription, many by more than one route. Most side-effects of these drugs are dose related, cause substantial morbidity and may contribute to poor treatment adherence (American Psychiatric Association, 1997). It remains unclear whether the risk of sudden death, acknowledged to occur with antipsychotic drugs, is dose related (Royal College of Psychiatrists, 1997). Existing research offers limited guidance on optimal prescribing in individual circumstances. However, reviews have concluded that, in general, the use of high doses or of polypharmacy (simultaneous use of more than one antipsychotic drug) offers little, if any, benefit over moderate doses of a single drug, in relation to the disadvantages (Royal College of Psychiatrists, 1993). This evidence has influenced the development of national guidelines and consensus statements.
Method
Development of the audit standards
Five English-speaking countries have published national guidelines or consensus statements that refer to the prescribing of antipsychotic drugs (American Psychiatric Association, 1997; EPPIC Statewide Services, 1999; New Zealand Ministry of Health, 1996; Royal College of Psychiatrists, 1993, 1997; Working Group for the Canadian Psychiatric Association and the Canadian Alliance for Research on Schizophrenia, 1998). All advise against the use of high doses other than in exceptional circumstances. Four make a similar, explicit recommendation in respect of polypharmacy. Audit standards were derived from these documents and were presented to, and agreed by, a separate ‘expert panel’ of psychiatric pharmacists and psychopharmacologists. The standards audited, and the measures used to audit them, are shown in Table 1.
Table 1. Audited standards and the findings
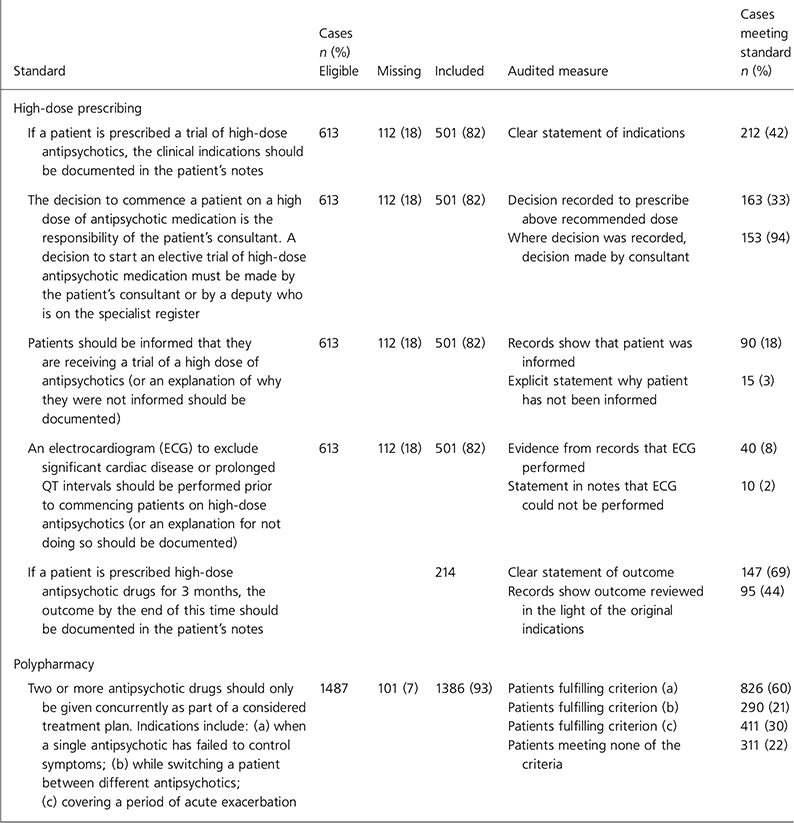
| Standard | Cases n (%) Eligible | Missing | Included | Audited measure | Cases meeting standard n (%) |
|---|---|---|---|---|---|
| High-dose prescribing | |||||
| If a patient is prescribed a trial of high-dose antipsychotics, the clinical indications should be documented in the patient's notes | 613 | 112 (18) | 501 (82) | Clear statement of indications | 212 (42) |
| The decision to commence a patient on a high dose of antipsychotic medication is the responsibility of the patient's consultant. A decision to start an elective trial of high-dose antipsychotic medication must be made by the patient's consultant or by a deputy who is on the specialist register | 613 | 112 (18) | 501 (82) | Decision recorded to prescribe above recommended dose | 163 (33) |
| Where decision was recorded, decision made by consultant | 153 (94) | ||||
| Patients should be informed that they are receiving a trial of a high dose of antipsychotics (or an explanation of why they were not informed should be documented) | 613 | 112 (18) | 501 (82) | Records show that patient was informed | 90 (18) |
| Explicit statement why patient has not been informed | 15 (3) | ||||
| An electrocardiogram (ECG) to exclude significant cardiac disease or prolonged QT intervals should be performed prior to commencing patients on high-dose antipsychotics (or an explanation for not doing so should be documented) | 613 | 112 (18) | 501 (82) | Evidence from records that ECG performed | 40 (8) |
| Statement in notes that ECG could not be performed | 10 (2) | ||||
| If a patient is prescribed high-dose antipsychotic drugs for 3 months, the outcome by the end of this time should be documented in the patient's notes | 214 | Clear statement of outcome | 147 (69) | ||
| Records show outcome reviewed in the light of the original indications | 95 (44) | ||||
| Polypharmacy | |||||
| Two or more antipsychotic drugs should only be given concurrently as part of a considered treatment plan. Indications include: (a) when a single antipsychotic has failed to control symptoms; (b) while switching a patient between different antipsychotics; (c) covering a period of acute exacerbation | 1487 | 101 (7) | 1386 (93) | Patients fulfilling criterion (a) | 826 (60) |
| Patients fulfilling criterion (b) | 290 (21) | ||||
| Patients fulfilling criterion (c) | 411 (30) | ||||
| Patients meeting none of the criteria | 311 (22) |
Dose
The British National Formulary (BNF; British Medical Association & Royal Pharmaceutical Society of Great Britain, 1999) states a maximum recommended dose, or a dose range for all antipsychotic drugs except trifluoperazine. The Royal College of Psychiatrists' consensus statement (Royal College of Psychiatrists, 1993) recommends that, when an antipsychotic is given at a dose above the BNF limit the decision should be made by a fully trained psychiatrist; the patient should be informed and his/her consent obtained; an electrocardiogram (ECG) should be performed; regular pulse, blood pressure and temperature checks should be made; and fluid intake should be monitored. A trial of a high-dose prescription should not last longer than 3 months. If there has been no improvement, the dose should be reduced back to within the standard range.
Polypharmacy
The BNF advises against polypharmacy. However, the College consensus statement suggests that there are some occasions when it is appropriate to give more than one antipsychotic drug concurrently for short periods. These include when changing gradually from one drug to another and when giving a more sedative and/or injectable antipsychotic drug to someone who is very agitated and who is already prescribed another antipsychotic drug on a regular basis.
High dose caused by polypharmacy
The College consensus statement clearly states that high-dose prescribing may occur because of the additive effects of two antipsychotic drugs that are prescribed concurrently. There are two methods of calculating whether the ‘total dose of antipsychotic drug’ exceeds the recommended level. One is to convert the doses of the drugs into ‘chlorpromazine equivalents’ and add these. The other approach, which was used in this audit, is to convert the prescribed dose to its percentage of the upper recommended dose (or maximum dose) for each drug and then to add the percentages (Reference Yorston and PinneyYorston & Pinney, 2000). When the sum exceeds 100, the patient is considered to be receiving a high dose.
For the purpose of the audit, the maximum daily dose for trifluoperazine was taken to be 50 mg.
Recruitment of sites
In February 1998, all UK adult mental health services were invited to take part in the audit; 47 did so. Services from every part of the country and from every main socio-demographic group (inner city, urban, mixed and rural) participated. They included one private hospital and one high security hospital. A total of 241 psychiatric wards (154 acute admission, 69 rehabilitation and 18 forensic) were involved. All wards were primarily for the treatment of people aged 18-65.
The audit
Local staff used a pro forma to collect information about all in-patients who were prescribed antipsychotic medication on 5 October 1998. This included details of all prescriptions for antipsychotic medication (drug, daily dose and route of administration). For each prescription, it was noted whether the drug was to be administered routinely or whether it was to be given ‘as required’ at the discretion of nursing staff. Frequency of administration was recorded for antipsychotic drugs prescribed as depot injections and, for zuclopenthixol acetate, the dose prescribed in the previous 72 hours.
Data collectors used a checklist to collate information from case files for comparison of practice with the standards set out in Table 1.
Data collection and management
Staff from each site attended a workshop at which the audit standards and methods for data collection were presented and discussed. Completed forms were returned to the Royal College of Psychiatrists' Research Unit where the data were analysed using SPSS for Windows, version 8.
Results
Returns gave information about prescribing practice relating to 3132 in-patients.
Frequency of prescribing of high doses and polypharmacy
Antipsychotic medication at a total dose above the BNF recommended daily limit was prescribed to 613 patients (20%). For only a small minority of cases (n=34; 5.5% of those prescribed a high dose) was this due to the prescription of a single type of antipsychotic drug at a high dose. For the remainder, high-dose prescribing was due to a combination of two or more types of anti-psychotic drug. If only antipsychotic drugs prescribed for routine administration were considered, 318 patients (10% of the total sample) were prescribed a high dose.
In all, 1487 patients (48%) were prescribed more than one antipsychotic drug on the census day. The results of the audit are summarised in Table 1.
Discussion
As there was no random selection of the participating services, the cohort may not be fully representative. However, units from all parts of the country took part and the sample is large, containing perhaps 15-20% of all people in a psychiatric hospital on the census day. This estimate is extrapolated from the bed numbers for England (Department of Health, 1998).
Why are high-dose prescribing and polypharmacy so common?
The finding that patients in hospital are commonly prescribed high doses is consistent with six smaller scale audits or surveys of psychiatric in-patients carried out in the UK over the past decade (Reference Chaplin and McguiganChaplin & McGuigan, 1996; Reference Krazucki and McfarlaneKrazucki & McFarlane, 1996; Reference Milton, Lawton and SmithMilton et al, 1998; Reference Newton, Murthy and QureshiNewton et al, 1997; Reference Warner, Slade and BarnesWarner et al, 1995; Reference Yorston and PinneyYorston & Pinney, 1997). These studies involved 1084 patients prescribed antipsychotic medication, 32% (n=344) of whom were prescribed a high dose.
A number of factors need to be considered when interpreting the findings from the multi-centre audit, they are as follows.
The sample is cross-sectional
This means that it will contain a higher proportion of patients with longer lengths of stay, and probably higher levels of disturbance and disability, than a cohort of consecutive admissions. Also, the audit gives no indication of longitudinal patterns of prescribing. The potential impact of this is illustrated by the finding that 12% of patients on more than one antipsychotic were in the process of being switched from one antipsychotic to another.
Ward conditions
About two-thirds of the patients were on acute admission wards. The rate of admission to these wards has increased considerably over the past 15 years despite a reduction in bed numbers. This has resulted in people who are severely disabled and highly disturbed being concentrated on these wards. Some of the prescribing might therefore reflect the use of antipsychotics to control behaviour, in the context of a disturbed ward environment, rather than the rational treatment of psychotic symptoms. Furthermore, the need to free up beds for new admissions creates pressure to discharge people quickly. This might encourage rapid escalation of doses or the premature addition of a second type of antipsychotic drug. The commonest reason given for the use of polypharmacy was that a single drug had failed to control symptoms. It is not known how often the trial of a single antipsychotic drug had lasted > 6 weeks, as recommended by a number of national guidelines.
Medication given at the discretion of nurses
The decision as to whether medication should be given at levels exceeding those recommended by the BNF had, in effect, been left to nursing staff in about half of cases where a doctor had prescribed a high dose. This was through the writing up of ‘as required’ medication. The audit neither determined whether this was intentional (see below) nor whether nursing staff were sufficiently trained in psychopharmacology to make such decisions. The writing of ‘as required’ could also lead to the unintentional and unknowing administration of a high dose (Reference Milton, Lawton and SmithMilton et al, 1998).
Psychiatrists might not be aware that they are prescribing high doses
For two-thirds of patients to whom it applied, the case notes contained no explicit statement that BNF limits had been exceeded. This might reflect sub-optimal record keeping, a lack of awareness or a combination of both. Nearly 95% of high-dose prescribing was due to polypharmacy. It is possible this represents a second cause of ‘covert’ high-dose prescribing wherein clinicians lose sight of total additive dose when they give more than one type of drug at the same time (Reference Tyson, Mortimer and WheelerTyson et al, 1999).
Prescribers might disagree with the guidelines
When an audit highlights significant divergence from recommended practice, the ‘validity’ of the guideline should be reconsidered as well as the behaviour of clinicians. The research base for the guidelines, which informed this audit, is not strong and there might also be questions about the extent to which the research findings can be extrapolated to the real-life clinical situations encountered in modern British psychiatry. Also, the recommendations for maximum doses take no account of patient factors, such as age, gender, ethnicity, body mass, smoking or other medication that might influence response or side-effects. Furthermore, there are apparent inconsistencies between BNF maximum recommended doses and comparisons between drugs based on commonly used tables of equivalence. This is particularly important, given the extent to which highdose prescribing is associated with polypharmacy.
Precautions and consent
Fewer than 10% of patients prescribed a high dose had undergone an ECG. There might be a number of explanations for this:
-
(a) it might reflect the lack of awareness that a high dose has been prescribed
-
(b) psychiatrists might be unaware both of the importance of such a test and of the College guideline (Reference Henderson, Gallagher and StarkHenderson et al, 1997)
-
(c) trainee psychiatrists might not feel proficient in interpreting the results of an ECG (Reference Warner, Gledhill and ConnellWarner et al, 1996).
A lack of awareness of the psychiatrist that a high dose has been prescribed might also partly explain why as many as 80% of patients appear not to have been informed, let alone given their consent. Patients have made allegations of negligence involving doses of antipsychotics outside the BNF recommended range (Reference BradleyBradley, 1997). The lack of objective data to support the efficacy of high doses, in conjunction with the lack of informed consent (Reference Brabbins, Butler and BentallBrabbins et al, 1996) and failure to conduct simple precautionary tests, are likely to make such allegations difficult to defend against.
Prescribing behaviour has probably changed since the 1998 audit owing to the increased use of atypical antipsychotic drugs. This supports the case for further audits of this type.
Declaration of interest
C. P. has very occasionally received speaker fees from Eli Lilly and Pfizer. Over the past year she has been involved with research projects funded by Novartis, Eli Lilly and Janssen—Cilag, but has not received any personal income from those projects. T. S. has been paid honoraria by numerous pharmaceutical companies for contributing to educational events. In 2000 he attended a meeting as a participant in an advisory board for Pfizer. The views expressed do not necessarily reflect those of the Royal College of Psychiatrists.
Acknowledgements
The authors thank the ‘expert panel’ of psychopharmacologists and psychiatric pharmacists who helped draft the standards used in this audit: Professor T. Barnes, Dr J. Cookson, J. Donoghue, Professor M. Lader, Dr L. Pilowsky and D. Taylor. The data were collected by staff from the 47 mental health services that participated in the audit.




eLetters
No eLetters have been published for this article.