Introduction
Hereditary hemorrhagic telangiectasia (HHT) is an autosomal dominant genetic disorder with an estimated prevalence of 1 in 5000 people in North America,Reference Govani and Shovlin1 the majority of whom have not been screened at an HHT screening and treatment center. This disorder is associated with abnormal vascular development and organ-specific over-activity of vascular endothelial growth factor, the mechanisms of which have not been fully elucidated. The most common clinical manifestations include spontaneous recurrent epistaxis, mucocutaneous telangiectasia, and most importantly, visceral arteriovenous malformations (AVMs) particularly prevalent in the brain, liver, and pulmonary circulation.Reference Dupuis-Girod, Bailly and Plauchu2 It is estimated that 35%-40% of individuals with HHT have pulmonary AVMs (PAVMs) and 10% have cerebral AVMs (CAVMs).Reference Easey, Wallace, Hughes, Jackson, Taylor and Shovlin3, Reference Faughnan, Palda and Garcia-Tsao4
There is limited data on the risk for both hemorrhagic stroke and ischemic stroke in HHT in comparison to the general population. A study in a large HHT clinic, comprising approximately 10% of all HHT cases in the United Kingdom, found a 16-fold increase in hemorrhagic stroke risk and a seven-fold increase in ischemic stroke risk compared to the general population.Reference Easey, Wallace, Hughes, Jackson, Taylor and Shovlin3 Presence of AVMs increases the likelihood of a stroke occurrence in an individual; studies have shown that there is a higher correlation of PAVMs with ischemic stroke and CAVMs with hemorrhagic events.Reference Blauwblomme, Bourgeois and Meyer5-Reference Woodall, McGettigan, Figueroa, Gossage and Alleyne8
Hereditary hemorrhagic telangiectasia is unusual in that the unique vascular pathophysiology makes it one of the few known genetic conditions that can lead to hemorrhagic, thrombotic, and embolic strokes.Reference Akers, Ball and Clancy9-Reference Caplan and Bogousslavsky11 Precise estimates of risk are impeded by the rarity of the condition and the lack of population-based data on risk assessment. Using a province-wide, population-based, administrative health data set, we examined the stroke risk in patients with HHT compared to the general population.
Methods
We conducted a retrospective population-based study using information from the Alberta Health (AH) databases. As Alberta maintains a publicly funded, universally available, healthcare system, residents of the province must register with the Alberta Health Care Insurance Plan (AHCIP). Each registrant with the AHCIP is issued a person health number that acts as a unique lifetime identifier, and is captured in all interactions with the healthcare system. Alberta has a population-based, province-wide, discharge abstracts database which includes all inpatient hospitalizations, ambulatory hospital visits, and emergency room visits for patients of all ages. The province also maintains a comprehensive physician claims database which captures physician visits for inpatient and outpatient settings and associated International Classification Disease (ICD)-10 diagnostic codes. Alberta Ministry of Health staff extracted the data and provided statistical analysis.
Inclusion criteria for this project were all strokes (both in pediatric and adult patients) in Alberta as captured through the Alberta Health databases who presented to an emergency department and/or were hospitalized in the province of Alberta between 1997 and 2012. We excluded any individuals who did not meet either of the above criteria.
To have an HHT diagnosis for the purposes of this study, all individuals captured required two different physician clinic visits within one year with a diagnosis of HHT (ICD-09: 448.0) or two outpatient hospital visits or emergency room visits, each with a diagnosis of HHT (ICD-09: 448.0), or one inpatient visit with a diagnosis of HHT (ICD-09: 448.0). The more stringent definition of requiring two physician visits to confirm the HHT diagnosis (although not requiring the physicians to be HHT specialists) ensures the dataset errs on the side of high specificity for HHT.
The AH databases were reviewed from 1997 to 2012 for four major forms of stroke-related codes presenting to the emergency department and/or requiring hospital admissions: acute ischemic stroke (AIS), intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), and transient ischemic attack (TIA). The HHT diagnoses were correlated through this same registry. We retrieved patient age and gender demographics with all stroke admissions. Patients were sub-divided by gender into five age groups: Birth, 0-17 years, 18-30 years, 31-60 years, and over 60 years of age.
The incidence of stroke was captured using diagnostic codes: ischemic stroke (ICD-10: I63.9), non-traumatic subarachnoid hemorrhage (ICD-10: I60.0), non-traumatic (ICD: I61.9), and TIAs (ICD-10: G45.9).27 All strokes among the HHT patient group were captured in a similar manner and incidence rates per 100,000 person-years calculated for each age group.
Statistical Analysis
Descriptive statistics were used to calculate the stroke incidence rate in both HHT and non-HHT groups. The direct method was used for age-standardization, and standardized rate ratio (SRR) was used to compare the stroke incidence rates between HHT and non-HHT population groups. Excel for Mac (version 15.26 [2016], Microsoft Corporation, Redmond, WA, USA) was used to calculate the SRR. Incidence rate ratios of each stroke subtype in both the HHT and general population were calculated with confidence intervals using the same software.
Results
From the years 1997 to 2012, we found a significantly higher incidence of strokes in patients with HHT compared to the baseline population. The stroke incidence rate in HHT was about 450 per 100,000 people as compared to 260 per 100,000 people in the general population (Figure 1). The proportions of each stroke subtype were the same in both groups. The majority of strokes were AIS, which accounted for about 52% of all strokes in the diagnosed HHT group and about 50% of all strokes in the non-HHT group (Table 1). Transient ischemic attacks were the second most frequent type in both populations (41% versus 40%). Notably, rates of each stroke subtype were higher in the HHT population than in the general population when ratios of incidence to person-years are compared.
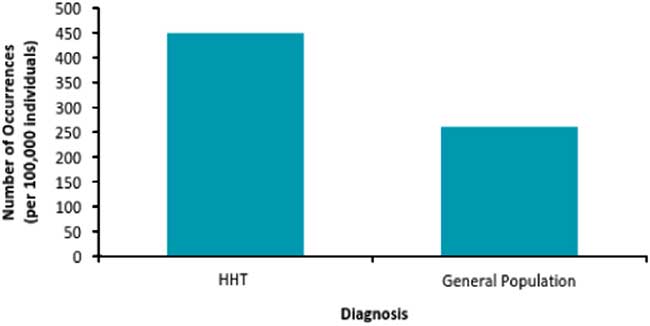
Figure 1 Age-standardized stroke incidence rate in HHT and non-HHT population in Alberta. Stroke incidence rate in Albertans with HHT—450 per 100,000 person-years (95% CI [226-673]) compared with 260 per 100,000 person-years (95% CI [259-262]) in the general population.
Table 1 Stroke subtypes in HHT and non-HHT groups
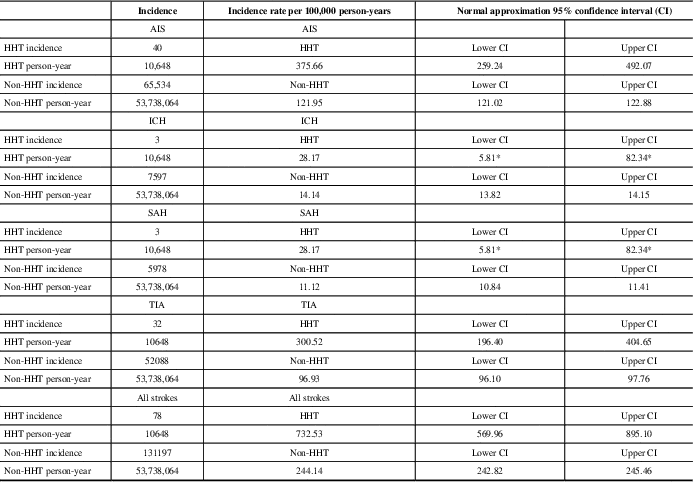
AIS=acute ischemic stroke, ICH=intracranial hemorrhage, SAH=subarachnoid hemorrhage, TIA=transient ischemic attack.
The cumulative incidence of different types of stroke in patients with and without HHT over 16 years. The adjacent column shows the ratio of this incidence by 100,000 person-years for comparison between the HHT and non-HHT groups with CI.
* Exact CI used for small case counts (under 20).
In both groups <3% of strokes occurred under 30 years of age. In the diagnosed HHT group, 21% of strokes occurred in the middle-age group (31-60 years), and 76% in the elderly group (>60 years). These percentage breakdowns for stroke by age groups are nearly the same for the non-HHT population as well (0-17 years and 18-30 years: 2%, 31-60 years: 23%, and over 60 years: 75%). Figure 2 illustrates the rising incidence of HHT over 16 years in the Alberta population.
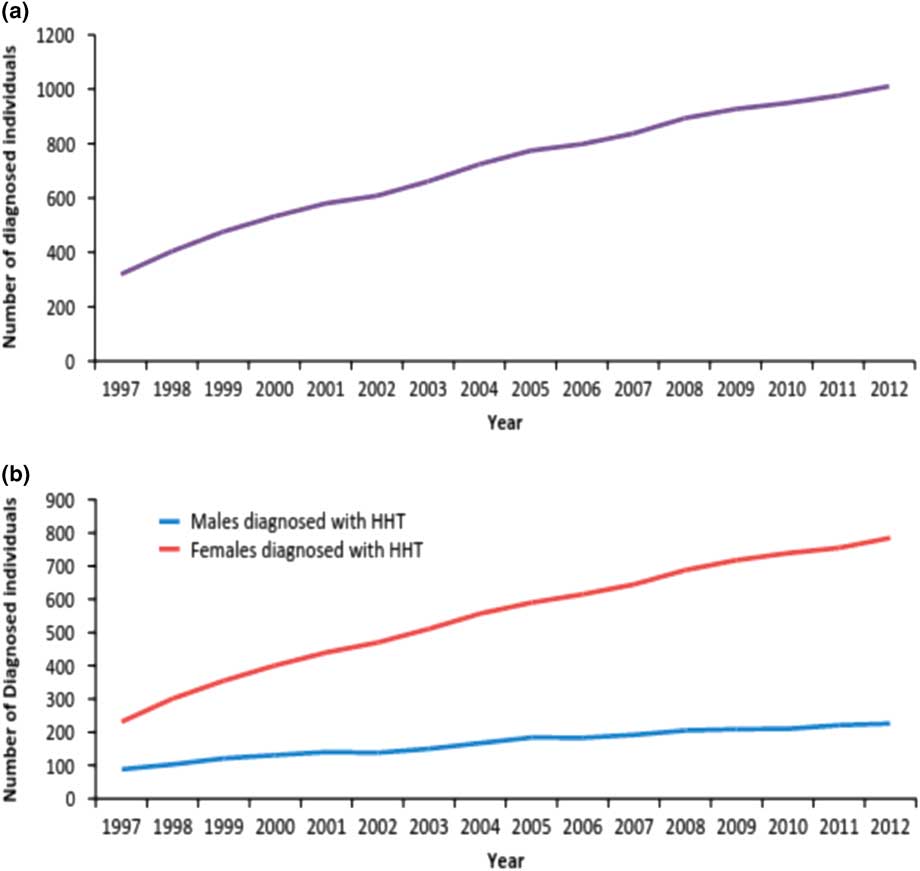
Figure 2 Trends in the total number of patients diagnosed with HHT per year in Alberta between 1997 and 2012. a) Total number of Albertans diagnosed with HHT per year. The overall HHT prevalence in Alberta is about 1 in 3800, compared with the North American estimate of 1 in 5000. b) Albertans diagnosed with HHT classified by gender. Though the total number of individuals diagnosed with HHT is steadily increasing, a closer analysis by gender reveals the male incidence is not growing at the same rate as female incidence per year.
Three key trends emerged from our data on increased burden of disease in Alberta: a definite female predilection for HHT diagnosis, a high incidence of stroke in females with HHT compared to men with HHT, and a higher overall prevalence for HHT in Alberta compared to the worldwide prevalence. Interestingly, the female to male ratio of HHT incidence averaged over 16 years is 3.25:1 (95% CI [3.24-3.25]). Moreover, women with HHT were also more likely than men to have strokes at a 1.88:1 female to male ratio. There is an overall HHT prevalence of 1 in 3800 in Alberta, which is higher than the estimated 1:5000 worldwide prevalence. These specific gender trends are broken down by stroke subtype by four-year clusters in Table 2.
Table 2 Total patient numbers for all stroke types for HHT and non-HHT groups per 4-year cluster
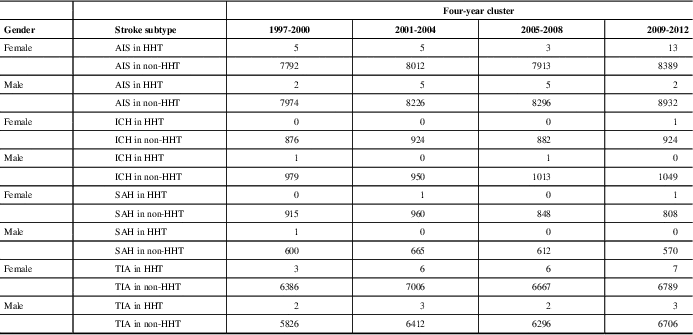
AIS=acute ischemic stroke, ICH=intracranial hemorrhage, SAH=subarachnoid hemorrhage, TIA=transient ischemic attack.
Incidences of each stroke subtype for males and females with and without HHT in Alberta over four-year clusters spanning the period of this study.
Discussion
Stroke occurrence in Alberta is on the rise, likely due to a growing population (from 2.8 million in 1997 to 3.9 million in 2012).Reference Jeerakathil, Burridge, Thompson, Fang and Hill12 There is a single HHT center for the province. Given Alberta’s widely dispersed population, screening individuals for HHT even after a stroke can be difficult. Our study contributed a better understanding of both age and sex-related trends regarding HHT and stroke in Alberta, namely that the age-standardized rate of stroke was significantly higher for persons with HHT compared with the baseline population (Figure 1) and that, despite a wide confidence interval and small population size, there is a higher rate of stroke in HHT.
Regardless of age, an individual diagnosed with HHT is 1.73 times more likely to suffer morbidity and mortality from a stroke than if he or she were not diagnosed with HHT. As HHT predisposes patients to strokes via multiple mechanisms, we attribute this trend to the likely higher probability of an underlying genetic component in strokes occurring before age 60. Screening for HHT through an HHT center should be considered in this group in high risk individuals using historical information (epistaxis, telangiectasia, absence of traditional stroke risk factors). This approach may prompt clinicians to identify patients at risk of having HHT before patients present with sentinel diseases such as stroke at a young age. HHT Centers of Excellence are multidisciplinary care centers, specifically accredited by the Cure HHT Foundation of North America.
Another objective of this study was to understand if any particular age groups were more likely to have strokes with concurrent HHT diagnosis. The database yielded limited data on pediatric strokes as only one documented stroke occurred in the diagnosed HHT group. These findings were expected since pediatric strokes are also rare in the general population, thus the data were not adequately powered to elucidate correlations between HHT and strokes in pediatric patients.
The majority of strokes in both HHT and non-HHT populations occur in those over 60 years of age; a considerable portion of these cases were susceptible to cardiovascular risk factors. The lower risk middle-age strata is an important population because strokes occurring in patients between 31 and 60 years of age are more likely to have atypical causes for their stroke including genetic conditions such as HHT.Reference Kjeldsen, Oxhøj, Andersen, Green and Vase13, Reference Liebeskind23
We also found that stroke occurrence is steadily increasing throughout the 16-year span of this study in the non-HHT group, which concurs with findings from other studies that show a growing proportion of strokes occurs in middle-aged patients.Reference Kissela, Khoury and Alwell14 Individuals with strokes occurring in this age range, in the absence of a substantial risk factor burden, may benefit from an assessment for HHT. It is suspected that out of the general population, some individuals who have experienced a stroke within this age group may have HHT, but may have never been screened for the condition.
Our study found HHT to be more common in Alberta than previously reported, at a prevalence of 1 in 3800. There is a wide range for HHT prevalence worldwide. Populations with Dutch genetic backgrounds seemingly have a higher prevalence than others. One such population comes from the Dutch Antilles, where the highest prevalence of HHT exists at 1 in 1331.Reference Westermann, Rosina, de Vries and Coteau15 The Alberta prevalence falls within the available published data. There are many Dutch settlements in AlbertaReference Bletz16 and many rural communities with large family units affected by HHT.
Another significant finding of this study includes the difference between males and females who have been diagnosed with HHT. Interestingly, over the 16 years of our study, females were on average 3.25 times more likely to be diagnosed with HHT. Other studies have shown similar trends, however, not quite as pronounced difference as our data suggests. An HHT population-based study by Donaldson et al found the female to male ratio for HHT diagnosis to be 1.7:1.Reference Donaldson, McKeever, Hall, Hubbard and Fogarty17 Our results do not accurately portray the genetic nature of HHT: it is not sex-linked. This discrepancy, which is unexpected given the autosomal inheritance of HHT, points to two possible reasons that the data show a female gender preponderance and males are being under-diagnosed or females may experience higher penetrance and thus are more symptomatic at the population level.
There are a number of explanations for the discrepancy between our results and the literature with regard to HHT prevalence in females. Hereditary hemorrhagic telangiectasia under-diagnosis in males is a likely contributor as clinical vigilance for rare diseases like HHT is often inadequate. Signs and symptoms of HHT may escape a clinician’s suspicion because they are often obscure or non-specific, such as chronic epistaxis, occult gastrointestinal bleeding, telangiectasias in the oral or nasal mucosa, chronic fatigue, or headaches. Moreover, probably men are less likely to bring such complaints to their primary care provider’s attention as compared with women. These views are supported by the Statistics Canada report “Women and Health” that showed females are more likely than males to have a family physician and are also more likely to seek medical attention.18
Outside of these behavioral considerations, existing literature suggests women are also more likely to have more severe symptoms in HHT. Females have a greater physiological potential to present with bleeding manifestations given that they are prone to all of the bleeding opportunities of males (such as epistaxis and gastrointestinal hemorrhage) as well as uterine bleeding. The combined likelihood of hemorrhage could also increase the chances of presenting to medical care because of iron deficiency anemia. Shovlin et al identified patients to be particularly susceptible to cerebral ischemia if they have iron deficiency anemia in HHT.Reference Shovlin, Chamali and Santhirapala19 Accordingly, we see that women in Alberta with HHT are 1.88 times more likely that males to have a stroke or TIA. Another factor that makes women more vulnerable to severe complications of HHT is pregnancy. This is especially true when obstetricians and patients have no prior knowledge of an HHT diagnosis, so complications such as PAVM hemorrhage, stroke, and maternal death proceed in about 1% of pregnancies in mothers with HHT without the added benefit of clinical vigilance.Reference Shovlin, Sodhi, McCarthy, Lasjaunias, Jackson and Sheppard20
Beyond age and gender trends, our study quantified rates of stroke subtypes in HHT in Alberta. There are multiple etiologies conferring strokes and transient ischemia upon HHT patients. Acute ischemic stroke was the most frequent subtype of stroke in the HHT population at 40 events out of 78. Transient ischemic attacks were also quite frequent at 32 events out of 78. Patients with HHT are at particularly high risk for these two stroke subtypes because of key hallmarks of HHT that predispose to cerebral ischemia, such as PAVMs, CAVMs and brain abscess, or thrombus formation. Abnormal vascular maturation in the pulmonary circulation can result in a right-to-left shunt (RLS) from PAVMs. Velthius et al found a significant correlation between higher-grade pulmonary RLS and cerebral complications (21%).Reference Velthius, Buscarini and van Gent21 Ischemic strokes and TIAs may be seen in individuals with HHT who have PAVMs with larger feeding arteries and not treated with standardized thoracic embolization protocols. Patients who have both CAVMs and PAVMs are also at particularly high risk of cerebral abscess that can lead to ischemia since PAVMS lack the capillary beds that often filter out typical lung pathogens.Reference Akers, Ball and Clancy9 Mathis et al postulate that emboli from PAVMs produce cerebral infarcts where hypoxia and polycythemia produce the necessary conditions for abscess development.Reference Mathis, Dupuis-Girod and Plauchu22 Patients with CAVMs in the context of HHT are at particular risk of thrombus formation. This is a reactive process within the CAVM because of hemodynamic changes that may diminish perfusion, exacerbate venous congestion, and lead to stasis and thrombosis.Reference Liebeskind23, Reference Kristensen, Malm and Calberg24
In contrast to ischemic strokes, cerebral hemorrhage was rare in the HHT group, with three cases each of intracranial and SAH, respectively. This finding contrasts the non-population based, but clinically rigorous study by Easey et al found a higher risk of hemorrhagic stroke in a retrospective cohort of HHT probands and their families.Reference Easey, Wallace, Hughes, Jackson, Taylor and Shovlin3 Hereditary hemorrhagic telangiectasia patients are theoretically at risk of hemorrhage as well, since rupture of abnormal intracerebral vasculature from CAVMs can lead to hemorrhagic strokes.Reference Maher, Piepgras, Brown, Friedman and Pollock25, Reference Nataf, Meder and Roux26 These discrepancies in findings might be partially explained by the ease of diagnosis of hemorrhagic stroke and the condition being less subjective to recall bias than ischemic stroke in the retrospective design.Reference Easey, Wallace, Hughes, Jackson, Taylor and Shovlin3 Alternatively, our population-based design would be unlikely to miss cases of ICH that would be very likely to present to emergency departments. However, we may be subjected to misclassification bias and may fail to identify some persons with ICH having probable or possible HHT.
The strength of this study is the population-based design allowing examination of databases of an entire province. Limitations of this study include relatively small number of HHT cases because of the strict method of defining HHT diagnosis. This more stringent definition means we are erring on the side of specificity, biasing toward missing HHT rather than overestimating the rate of HHT. Consequently, our estimates of HHT cases and strokes within the HHT population are conservative. Using a longer period for this study or including more provinces may increase the sample size to show differences between stroke subtypes or age groups more clearly. As HHT is a rare disease, the administrative health data set represents an efficient and cost-effective way to estimate prevalence and impact.
Another shortcoming of this study is the lack of data on AVMs in those patients who experienced strokes. Correlating PAVM and CAVM data with stroke subtypes would offer an added robustness to the study methodology. As similar ratios of different stroke subtypes were seen in the non-HHT population as well, it is difficult to postulate without this information, how much these mechanisms of stroke in HHT factored into the risk of stroke for HHT patients compared with the baseline population. Another limitation of our design is the lack of integration with other provincial data. For example, even if one or two patients with ICH present to neighboring provinces, this skews our data as there were only three ICH events over 16 years in the HHT population.
In conclusion, stroke incidence rate is higher among individuals with HHT. As the age-adjusted stroke rate is higher in the HHT group, there may be an underlying genetic contribution. As such, affected individuals should be screened by history for additional stroke risk factors. This is especially true for male patients, as there seems to be an under-diagnosis of HHT in males in Alberta or under-presentation of males to HHT centers or stroke centers. Given that women have unique risk factors for stroke (oral contraceptive use, pregnancy, migraine with aura, and hormone replacement therapy), special consideration should be given to women with HHT who possess additional risk factors. As HHT is a rare, multi-system, chronic disease, these patients should be referred to an HHT Center of Excellence.
Acknowledgements
The authors thank Ms. Iris de Guzman for her assistance in the preparation of this manuscript.
Financial Support
None.
Disclosures
Farah N. Chowdhury, G. Sanjaya Chandrarathne, Kristopher D. Masilamani, Jennifer T.N. LaBranche, Shaun Malo, Lawrence W. Svenson, and Dilini P. Vethanayagam have nothing to disclose.
Thomas Jeerakathil reports grants and personal fees from CIHR, HSFC, AIHS, CSN, University of Alberta Hospital Foundation, and Bayer, outside the submitted work.






