The prevalence of autistic-spectrum disorder in the UK has recently been estimated to be approximately 1%, and is 3–4 times higher in males than in females (Reference Baird, Simonoff and PicklesBaird et al, 2006). However, despite the relatively high prevalence and heritability of this disorder, its pathophysiology remains incompletely defined. Recent studies have helped define the neuroanatomical and functional abnormalities underlying the condition (reviewed by Reference Toal, Murphy and MurphyToal et al, 2005); however, these data have been acquired in male-only (or predominantly male) samples, and at different ages. To date there has been no study of regional differences in grey and white matter in female-only samples, or in adult women (when brain development is complete). Hence the biological associates of autistic-spectrum disorder in adult women are largely unknown. It has been reported that the behavioural phenotype of females with the disorder is different from that of males (Lord et al, Reference Lord, Schopler and Revicki1982; McLennan et al, Reference McLennan, Lord and Schopler1993; Reference Gillberg and ColemanGillberg & Coleman, 2000). Also, there are gender differences in postnatal brain development and ageing (Reference Murphy, DeCarli and McIntoshMurphy et al, 1996; Reference Giedd, Castellanos and RajapakseGiedd et al, 1997; Reference Gur, Gunning-Dixon and TuretskyGur et al, 2002). It is therefore possible that the neuropathology of autistic-spectrum disorder in females is different from that reported in males.
Brain anatomy in vivo can be measured using magnetic resonance imaging (MRI) and a variety of analytical approaches, including hand tracing methods and voxel-based morphometry (VBM). Hand tracing allows measurement of relatively large regional bulk volumes (i.e. with no differentiation of grey and white matter), whereas the latter technique allows analysis of subtle regional differences in grey and white matter. We therefore used MRI and VBM to investigate the brain anatomy of women with autistic-spectrum disorder.
METHOD
The sample consisted of participants in a clinical research programme enabled by the Medical Research Council UK Autism Imaging Multicentre Study (AIMS) network, and the study was jointly conducted by South London and Maudsley National Health Service (NHS) Foundation Trust and the Institute of Psychiatry, London. We included 19 women in a control group (mean age 35.0 years, s.d.=14.0) and 14 women with an autistic-spectrum disorder: 10 with Asperger syndrome and 4 with autism (mean age 37.9 years s.d.=11.4). Participants were diagnosed using the ICD-10 clinical research criteria (World Health Organization, 1992). This was achieved by consensus between two clinicians, experienced in diagnosis of autistic-spectrum disorders, and a nurse, all trained in the use of the autism diagnostic measures used in the study. The diagnosis was based on clinical interviews, collateral information from family members and review of other information available, such as school reports. In addition, we were able to use the Autism Diagnostic Interview – Revised (ADI–R; Reference Lord, Rutter and Le CouteurLord et al, 1994) to assess 7 individuals whose parental informants were willing and available and the Autism Diagnostic Observation Schedule (ADOS; Reference Lord, Rutter and GoodeLord et al, 1989) to assess a further 5 participants who were willing to undertake further interviewing. Thus, we confirmed clinical research criteria in all participants using ICD–10, and in 12 of the 14 individuals with the ADI–R or ADOS. All assessments were masked to MRI data.
All participants underwent a structured clinical examination and routine clinical blood tests to exclude biochemical, haematological or chromosomal abnormalities. Individuals were excluded if they had a history of major psychiatric disorder (e.g. psychosis), head injury, toxic exposure, diabetes, abnormalities in routine blood tests, drug or alcohol misuse, clinical abnormality on routine MRI, or a medical or genetic disorder associated with autistic symptoms (e.g. epilepsy, tuberous sclerosis or fragile X syndrome). All participants gave informed consent and/or assent (as approved by the Institute of Psychiatry and the South London and Maudsley NHS Trust research ethics committee). None was taking medication at the time of testing.
Neuropsychological testing
Overall intellectual ability (IQ) was determined using an abbreviated Wechsler Adult Intelligence Scale (WAIS–R; Reference Canavan and BeckmannCanavan & Beckmann, 1993).
Image acquisition
All MRI data were obtained using a GE Signa 1.5 T neuro-optimised magnetic resonance system (General Electric, Milwaukee, USA). Whole-head coronal three-dimensional spoiled gradient recalled (3D-SPGR) images (repetition time=13.8 ms, echo time=2.8 ms, 256 × 192 acquisition matrix, 124 slices, thickness 1.5 mm) were obtained from all participants.
VBM pre-processing
Voxel-based morphometry pre-processing was performed on the 3D-SPGR data using Statistical Parametric Mapping software (SPM2; Wellcome Department of Imaging Neurosciences, University College London, UK). The image processing steps have been described in detail elsewhere (Reference Abell, Krams and AshburnerAbell et al, 1999; Reference Good, Johnsrude and AshburnerGood et al, 2001).
The segmentation algorithm implemented in SPM2 incorporates a priori knowledge of the likely spatial distribution of tissue types in the brain through use of prior probability tissue maps derived from a large number of individuals. To ensure the most accurate segmentation possible, we created study-specific customised prior probability maps based on all 33 participants. The pre-processing stages were as follows:
-
(a) scans were segmented into probabilistic maps of grey and white matter and cerebrospinal fluid using a modified mixture model clustering algorithm;
-
(b) the segmented grey matter map was mapped to a grey matter template and the derived warping parameters were applied to the original T 1-weighted image in order to map it into standard space (this procedure prevents skull and other non-brain voxels from contributing to the registration, while avoiding the need for explicit skull-stripping);
-
(c) the registered image was then resegmented, which is necessary as the a priori knowledge incorporated into the SPM2 segmentation algorithm means that it works optimally on images in standard space. The segmented maps were then corrected for volume changes introduced during the registration and smoothed using a Gaussian filter of 5 mm full width at half-maximum. Total grey and white matter densities were calculated from the segmented maps in native space.
VBM analysis
For the VBM analyses, between-group differences in grey- and white-matter density were calculated by fitting an analysis of covariance (ANCOVA) model at each intracerebral voxel in standard space, covarying for total grey-matter (or white-matter) density. Structural brain changes are likely to extend over a number of contiguous voxels and therefore test statistics incorporating spatial information, such as three-dimensional cluster mass (the sum of supra-threshold voxel statistics), are generally more powerful than other possible test statistics which are informed only by data at a single voxel. Therefore, our approach was to provisionally set a relatively lenient P value (P⩽0.05) to detect voxels putatively demonstrating differences between groups. We then searched for spatial clusters of such voxels. At the cluster level, rather than set a single a priori P value below which we would regard findings as significant, we calculated for a range of P values the number of clusters that would be expected by chance alone. We then set the statistical threshold for cluster significance by data-driven permeation testing. This was done such that the expected number of false positive clusters is less than 1, and we quoted the P value at which this occurs (Reference Bullmore, Suckling and OvermeyerBullmore et al, 1999; Reference Sigmundsson, Suckling and MaierSigmundsson et al, 2001).
Post hoc analysis of behavioural scores
Finally, we carried out a preliminary (post hoc) analysis to determine if differences in brain density were associated with behavioural abnormality within people with autistic-spectrum disorder. To do this, we related (using Pearson product-moment correlation coefficients) severity of clinical symptoms within people with the disorder as measured by the ADI–R to the density of brain regions, which differed significantly from controls.
RESULTS
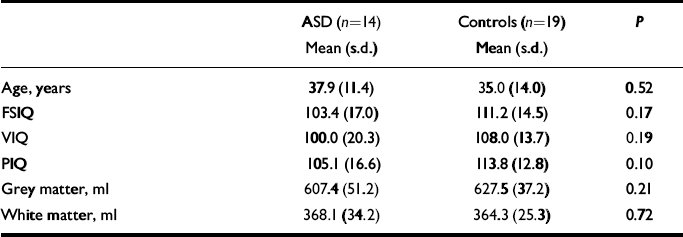
The characteristics of the sample are given in Table 1. There was no significant difference between women with autistic-spectrum disorder and controls in age, IQ or total brain grey- and white-matter density (in native space generated by SPM2).
Table 1 Sample characteristics and volumes of grey and white matter
| ASD (n=14) | Controls (n=19) | P | |
|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | ||
| Age, years | 37.9 (11.4) | 35.0 (14.0) | 0.52 |
| FSIQ | 103.4 (17.0) | 111.2 (14.5) | 0.17 |
| VIQ | 100.0 (20.3) | 108.0 (13.7) | 0.19 |
| PIQ | 105.1 (16.6) | 113.8 (12.8) | 0.10 |
| Grey matter, ml | 607.4 (51.2) | 627.5 (37.2) | 0.21 |
| White matter, ml | 368.1 (34.2) | 364.3 (25.3) | 0.72 |
ASD, autistic-spectrum disorder; FSIQ, full-scale IQ; PIQ, performance IQ; VIQ, verbal IQ
Voxel-based morphometry
The three-dimensional cluster maps of the between-group differences in grey- and white-matter volume were large and extended into several regions.
Grey matter
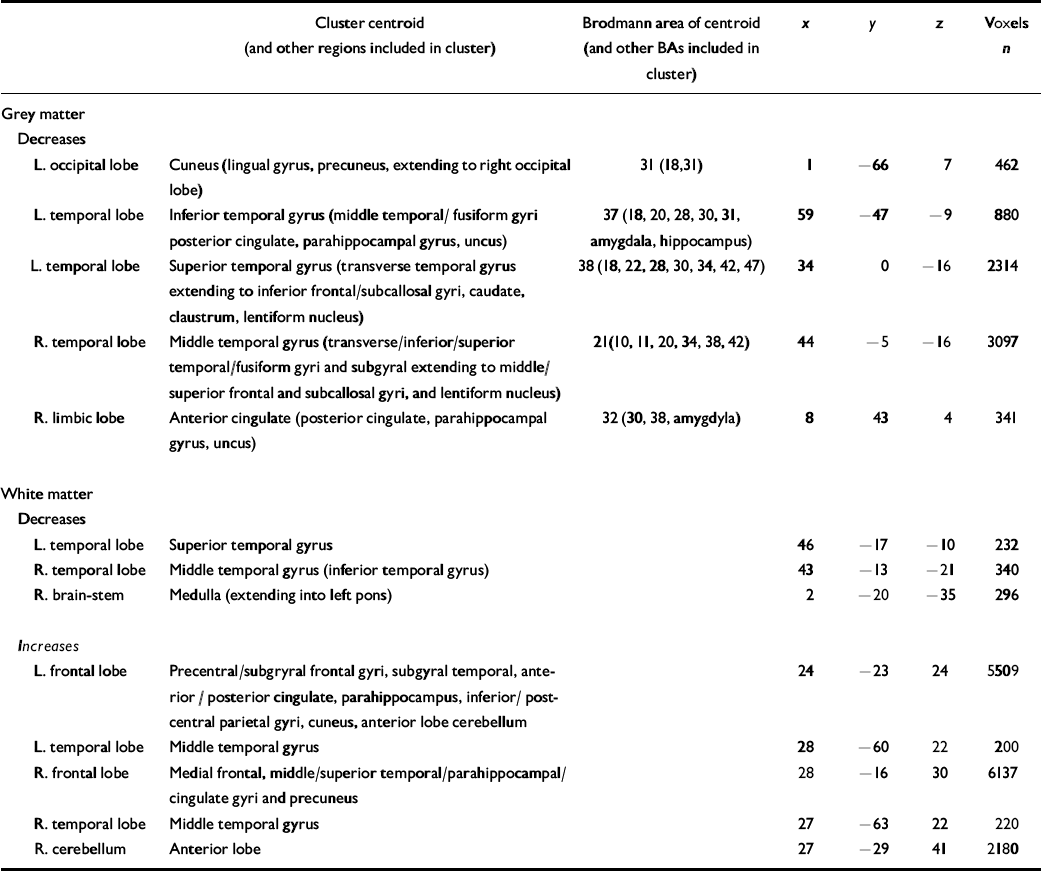
All grey-matter differences between the autistic-spectrum disorder group and the control group were significant at P⩽0.002, the value at which less than 1 false positive cluster was expected by chance alone (Table 2). Women with the disorder had a significantly smaller grey-matter density than controls bilaterally in the temporal lobes (including parahippocampal gyrus), orbito-frontal cortex (medial and lateral) and the basal ganglia (lentiform nucleus and caudate nucleus), in the right medial occipital (left cuneus) lobe, and in the left frontal (right anterior cingulate) lobe (Fig. DS1 in the data suplement to the online version of this paper).
Table 2 Clusters of significantly decreased and increased grey-matter (P=0.004) and white-matter (P=0.01) volume in women with autistic-spectrum disorder compared with controls
| Cluster centroid (and other regions included in cluster) | Brodmann area of centroid (and other BAs included in cluster) | x | y | z | Voxels n | |
|---|---|---|---|---|---|---|
| Grey matter | ||||||
| Decreases | ||||||
| L. occipital lobe | Cuneus (lingual gyrus, precuneus, extending to right occipital lobe) | 31 (18,31) | 1 | -66 | 7 | 462 |
| L. temporal lobe | Inferior temporal gyrus (middle temporal/fusiform gyri posterior cingulate, parahippocampal gyrus, uncus) | 37 (18, 20, 28, 30, 31, amygdala, hippocampus) | 59 | -47 | -9 | 880 |
| L. temporal lobe | Superior temporal gyrus (transverse temporal gyrus extending to inferior frontal/subcallosal gyri, caudate, claustrum, lentiform nucleus) | 38 (18, 22, 28, 30, 34, 42, 47) | 34 | 0 | -16 | 2314 |
| R. temporal lobe | Middle temporal gyrus (transverse/inferior/superior temporal/fusiform gyri and subgyral extending to middle/superior frontal and subcallosal gyri, and lentiform nucleus) | 21(10, 11, 20, 34, 38, 42) | 44 | -5 | -16 | 3097 |
| R. limbic lobe | Anterior cingulate (posterior cingulate, parahippocampal gyrus, uncus) | 32 (30, 38, amygdyla) | 8 | 43 | 4 | 341 |
| White matter | ||||||
| Decreases | ||||||
| L. temporal lobe | Superior temporal gyrus | 46 | -17 | -10 | 232 | |
| R. temporal lobe | Middle temporal gyrus (inferior temporal gyrus) | 43 | -13 | -21 | 340 | |
| R. brain-stem | Medulla (extending into left pons) | 2 | -20 | -35 | 296 | |
| Increases | ||||||
| L. frontal lobe | Precentral/subgryral frontal gyri, subgyral temporal, anterior / posterior cingulate, parahippocampus, inferior/ post-central parietal gyri, cuneus, anterior lobe cerebellum | 24 | -23 | 24 | 5509 | |
| L. temporal lobe | Middle temporal gyrus | 28 | -60 | 22 | 200 | |
| R. frontal lobe | Medial frontal, middle/superior temporal/parahippocampal/cingulate gyri and precuneus | 28 | -16 | 30 | 6137 | |
| R. temporal lobe | Middle temporal gyrus | 27 | -63 | 22 | 220 | |
| R. cerebellum | Anterior lobe | 27 | -29 | 41 | 2180 |
BA, Brodmann area; L, left; R, right
White matter
All white-matter differences between the groups were significant at P⩽0.01, the value at which less than 1 false positive cluster was expected by chance alone (see Table 2). Women with the disorder had a significantly smaller white-matter density bilaterally in the anterior temporal lobes and brain-stem (pons). In contrast, they had a significantly increased white-matter density bilaterally in the association and projection fibres of the frontal, parietal, posterior temporal and occipital lobes, in the commissural fibres of the corpus callosum (splenium) and cerebellum (anterior lobe) (Fig. DS2).
VBM analysis of correlations with ADI score
There was a negative correlation (r=–0.767, n=7, P=0.04) between reduced grey matter in the right limbic regions (including anterior and posterior cingulate, parahippocampal gyrus and uncus) and qualitative abnormalities in reciprocal social interaction (Fig. 1).
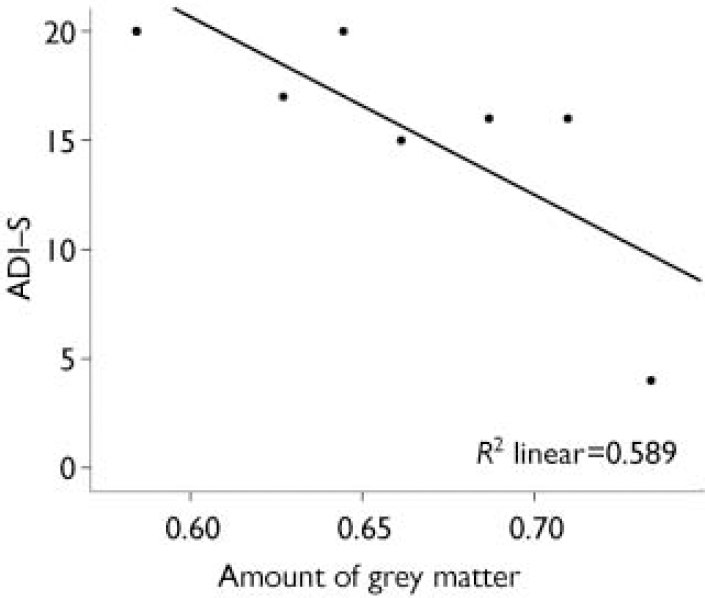
Fig. 1 Negative correlation between the amount of grey matter in the right limbic region, including the right anterior cingulate (centroid) extending into the posterior cingulate, parahippocampal gyrus and uncus, and abnormal reciprocal social interaction measured on the Autism Diagnostic Interview (ADI–S); r=–0.767, n=7, P=0.04.
DISCUSSION.
Our main study findings were that women with autistic-spectrum disorder have a significantly reduced density bilaterally of grey matter within the fronto-temporal cortices and limbic system, and of white matter in the anterior temporal lobes. In contrast, they have increased white matter bilaterally in the fronto-parietal, posterior temporal lobes and the cerebellum. Our findings are broadly consistent with prior studies of men of normal IQ with autistic-spectrum disorder reported by our group and others (Reference Abell, Krams and AshburnerAbell et al, 1999; Reference McAlonan, Daly and KumariMcAlonan et al, 2002). For example, we previously reported that men have reduced grey matter in the occipital and temporal cortices and the cingulate region, and white-matter deficits in the brain-stem and temporal lobe. This suggests that similar brain regions are affected in both genders, despite the higher prevalence of autistic-spectrum disorders in males. Furthermore, these brain regions are implicated in some of the higher cognitive functions reported as abnormal in people with this disorder (e.g. social cognition, language, motor control and ‘theory of mind’).
Our preliminary finding was that in women with autistic-spectrum disorder reduced grey-matter density in limbic regions is correlated with abnormal social behaviour. It is possible that this finding is attributable to a type 1 error as we carried out multiple comparisons; however, it is tentatively supported by reports of social and emotional deficits in (macaque) monkeys following lesions of the anterior cingulate (Reference Bachevalier, Merjanian, Bauman and KemperBachevalier & Merjanian, 1994; Reference Rudebeck, Buckley and WaltonRudebeck et al, 2006), and social cognitive deficits in humans following damage to the limbic system (Reference Stone, Cosmides and ToobyStone et al, 2002); further and larger studies are required to examine this issue.
However, there are some differences between our findings and previous neuroanatomical imaging studies of adult males with autistic-spectrum disorder (Reference Abell, Krams and AshburnerAbell et al, 1999; Reference McAlonan, Daly and KumariMcAlonan et al, 2002). For example, in this study we found that women with this disorder have no difference in density of cerebellar grey matter, but they have excess white matter. Prior studies of men have reported both excess (Reference Abell, Krams and AshburnerAbell et al, 1999) and reduced (Reference McAlonan, Daly and KumariMcAlonan et al, 2002) grey matter, but no difference in white matter. Cerebellar pathology has been reported in many post-mortem case studies across a variety of ages and IQ scores (21 out of 29 studies have reported reduced Purkinje cells; Reference Palmen, van Engeland and HofPalmen et al, 2004) and cerebellar hypoplasia has been found by some structural imaging studies (Reference Ciesielski, Harris and HartCiesielski et al, 1997; Reference Levitt, Blanton and Capetillo-CunliffeLevitt et al, 1999; Reference Carper and CourchesneCarper & Courchesne, 2000; Reference Courchesne, Karns and DavisCourchesne et al, 2001). Thus the cerebellum is most probably abnormal in both men and women with autistic-spectrum disorder – but it is unclear if the neuropathology is similar in both genders.
In the light of our finding that women with autistic-spectrum disorders have abnormalities in brain anatomy that are broadly similar to those previously reported in men, the reasons for the gender difference in the prevalence of this disorder remain unclear. It has been suggested that there is a relative failure to diagnose these disorders in females because of differences in clinical presentation. For example (as noted above), it has been reported that females with the disorder have a different behavioural phenotype to males, with a lower frequency of comorbid challenging behaviours (Reference McLennan, Lord and SchoplerMcLennan et al, 1993) and fewer abnormal special interests (Reference Gillberg and ColemanGillberg & Coleman, 2000); they are less likely to exhibit stereotypic behaviour during play (Reference Lord, Schopler and RevickiLord et al, 1982) and have better superficial social skills and language (Reference Gillberg and ColemanGillberg & Coleman, 2000). Alternatively, it might be that the increased prevalence of autistic-spectrum disorder reported in males (and gender differences in clinical presentation) is due to significant differences in biological vulnerability. Thus the underlying genetic susceptibility for the condition may be similar in both genders, but there may be a lower ‘threshold’ to developing autism in males. If so, the putative increased vulnerability of the male brain is probably due to a number of complex (and interacting) factors, including genomic imprinting (Reference Badcock and CrespiBadcock & Crespi, 2006), hormonal milieu (Reference Baron-Cohen, Knickmeyer and BelmonteBaron-Cohen et al, 2005) and gender differences in the normal maturational trajectory of the brain regions implicated in this disorder (Reference Giedd, Blumenthal and JeffriesGiedd et al, 1999; Reference Gogtay, Giedd and LuskGogtay et al, 2004). For example, the development of frontal and parietal grey matter peaks approximately 1 year earlier in adolescent girls than in boys, and the amygdala increases in density in healthy boys but not in girls.
A further biological explanation for gender differences in the prevalence of autistic-spectrum disorder builds on the concept that autism represents an ‘extreme male brain’ (Reference AspergerAsperger, 1944) by applying empathising–systemising theory (Reference Baron-CohenBaron-Cohen, 2002); this theory suggests that the female brain is predominantly ‘hard-wired’ for empathy, and that the male brain is predominantly ‘hard-wired’ for understanding and building systems (systematising). It is therefore proposed that people with autism may have an ‘extreme male brain’ that is even stronger at systemising and weaker at empathising than the normal male brain, and that this is underpinned by a skew of the normal gender differences in neurodevelopment. This may be due to an ‘extreme’ variation in the typical gender differences observed in brain regions that modulate processes involved in empathy (e.g. the amygdala) and/or systematising. Further, it has been suggested that normal gender differences postulated by empathising–systemising theory might be primarily due to an increase in the ratio of local white-matter tracts (important for systemising) to longer-range, interhemispheric tracts (important for empathising) in males, and that this skewed balance in connectivity is further exaggerated in autism (Reference Baron-Cohen, Knickmeyer and BelmonteBaron-Cohen et al, 2005).
The ‘extreme male brain’ theory implicitly suggests that the skew in normal gender differences (i.e. in the maturation of specific brain regions such as the amygdala and of the ratio of interconnective white-matter tracts) will need to be even more ‘extreme’ in females compared with males with this disorder. The design of the study reported here did not allow us to test this hypothesis directly. Nevertheless, our results do suggest that females with autistic-spectrum disorder have abnormalities in brain regions and systems associated with empathising – such the parietal cortex and limbic regions – which are consistent with the theory. However, further imaging studies are needed to examine directly the differences in the brain anatomy of men and women with this condition.
Our study was limited by a number of factors, including a relatively small sample size, a cross-sectional design and the application of multiple statistical comparisons (i.e. increased risk of type 1 error). However, we believe these limitations are unlikely to explain our results fully. In particular, type 1 errors are unlikely to account for our reported findings with the minimal-assumption, data-driven (permutation) methods we used. Studies of the assumptions of normal theory based methods have often raised issues about the validity of these assumptions (see, for example, Reference Hayasaka and NicholsHayasaka & Nichols, 2003; Reference Thirion, Pinel and MeriauxThirion et al, 2007). However, we used a two-stage inferential procedure in which permutation testing at voxel and cluster levels was used to set the expected type 1 error rate at less than 1 per whole brain with minimal assumptions. In the light of the likely incidence of non-normality in brain imaging data (see Reference Thirion, Pinel and MeriauxThirion et al, 2007), we believe that such a minimal-assumption, data-driven inferential procedure is the best approach to inference in MRI analysis.
In summary, our study suggests that adult women with autistic-spectrum disorder have significant differences from controls in brain anatomy, and these abnormalities are broadly similar to those observed in predominantly male populations with this disorder of similar age and IQ. Larger studies are needed to relate anatomy to behaviour and directly compare females and males with autism across the life span.
Acknowledgements
The authors thank the people with autistic-spectrum disorder who took part in the study, the Medical Research Council UK AIMS programme for infrastructure support, and Professor Nancy Minshew and Dr Marco Catani for their valuable comments during the preparation of this manuscript. This project was assisted by support from the South London and Maudsley NHS Trust.






eLetters
No eLetters have been published for this article.