If our batteries do not behave the way we expect, we have a problem. Such unpredictability can arise from many sources, including difficult-to-detect nanoscale defects in the materials of the batteries. In the August 11 issue of Chemistry of Materials (DOI: 10.1021/acs.chemmater.5b01234; p. 5212), Michaël Deschamps of the University of Orléans and the National Centre for Scientific Research, along with a team of researchers from France, detail their work to locate and characterize these defect structures in the electrodes of lithium-ion batteries.
The researchers’ lithium-ion battery uses a LiVPO4F electrode in which the material is intercalative, meaning that a mobile atomic species moves into the spaces between the LiVPO4F layers. In general, atoms in intercalation materials can have any of several oxidation or spin states. The standard method of measuring these states leaves their local nature unresolved—thus, sufficiently small atomic features go undetected.
Deschamps and co-workers used nuclear magnetic resonance (NMR) to characterize the local atomic structures at the nanoscale in this material. Unexpectedly, they found paramagnetic lithium environments associated with crystallographic Li sites, and calculated that these environments accounted for as much as 20% of the Li content in the electrode. Furthermore, they applied a radio frequency (RF) dipole–dipole-recoupling technique on a time scale roughly equal to the very short lifetimes of the atomic spin states, and registered enough of a signal for meaningful analysis. Combined with NMR spectra, the recoupling data allowed the researchers to determine unambiguously that these environments were associated with systemic structural defects in the crystal lattice, rather than representing a simple impurity phase. “These defects were completely undetected by other techniques, and we were really happy that NMR could show that there was more than meets the eye—or, in this case, the transmission electron microscope,” Deschamps says.
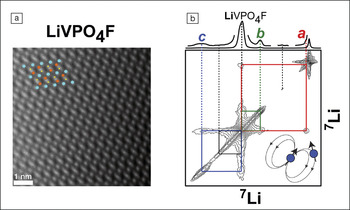
(a) Scanning transmission electron microscope image of phase-pure LiVPO4F along the [010] crystal orientation. A LiVPO4F unit cell is superimposed over the image, where vanadium, lithium, and phosphorus atoms are represented by blue, orange, and yellow spheres, respectively. (b) Solid lines represent correlated two-dimensional signals between crystallographic LiVPO4F and the other paramagnetic 7Li environments, establishing their respective 7Li dipole−dipole interactions and hence subnanometer-scale proximities. Credit: Chemistry of Materials.
In addition to the discovery of unexpected defects in a well-crystallized LiVPO4F electrode, Deschamps and his colleagues demonstrated a new way to characterize certain kinds of paramagnetic materials: “One could imagine NMR spectroscopy being used to monitor the quality of materials, ensuring the batteries made with them will function as expected,” Deschamps says. By characterizing the lithium-defect environments down to the atomic scale with remarkable precision, this work now provides researchers with a new, powerful tool to continue improving battery technology.


