There has been a rapid development of knowledge of the role of autoimmunity in common medical conditions and also in psychiatric presentations and intellectual disability. Autoimmune disorders form a heterogeneous group of conditions mediated by a variety of mechanisms, which in turn are modulated by gene and environment interactions that are not yet fully understood. Various tried and tested medical therapeutic approaches are widely used in a range of clinical settings, but they are rarely considered in relation to psychiatric presentations. Although autoimmune conditions were previously considered to be entirely discrete disorders, it is now recognised that there is a significant rate of co-occurrence of multiple different autoimmune disorders at both the individual and family level. For example, in Down syndrome an increased risk of a number of autoimmune disorders reflects a range of defects in the immune system associated with the genotype (Box 1) (Reference ChistiakovChistiakov 2007).
BOX 1 Autoimmune disease in Down syndrome

A triad of autoimmune disorders is commonly found in association with Down syndrome:
-
type 1 diabetes
-
Hashimoto’s disease
-
coeliac disease
Suggested monitoring of children with Down syndrome to be reviewed in any presentation to child and adolescent mental health services:
-
monitor growth
-
screen for signs and symptoms of diabetes and thyroid disease
-
monitor thyroid function tests (note: annual thyroid function tests are advised in all individuals with Down syndrome), screen for anti-thyroid and anti-islet cell antibodies, measure serum levels of antigliadin and anti-endomysium autoantibodies
It has been estimated that it takes an average of 17 years for new scientific discoveries to be translated into routine clinical practice (Reference Morris, Wooding and GrantMorris 2011). The realisation that the blood–brain barrier (made particularly vulnerable by stress, infections and inflammation) is variably permeable to autoimmune complexes has been a significant advance in understanding the brain effects of autoimmune disease. Postulated mechanisms include autoimmune effects on neurotransmitter production, ion channels and the integrity and functioning of neurons and microglia, mediated by autoantibodies (many not yet identified) and the complement system. The role of immunology in normal brain development is also a rapidly emerging area of research, for example microglia have a function in relation to synaptic connection that has an impact on neural circuitry (Reference Leckman and VaccarinoLeckman 2015).
The potential impact of improved diagnosis and treatment of autoimmune disorders on psychiatric practice makes this an exciting area for psychiatrists and clinical researchers to keep abreast of and they have a responsibility to ensure that patients benefit as soon as possible. A recent editorial in the journal Brain Research concludes cautiously that ‘while work in developmental psychoneuroimmunology engenders a good deal of excitement, the “promise” of the field clearly remains greater than the “deliverables”, in terms of any direct benefits on patient care’ (Reference Leckman and VaccarinoLeckman 2015). Considering the need to find a balance between the future possibilities implied by current research and the realities of clinical practice, this article aims to give a practical summary of autoimmune disorders in child psychiatry, concentrating on the ‘deliverables’ within current practice – recognition and a multidisciplinary liaison approach to assessment and therapy.
Presentation to psychiatric services
Children with autoimmune disorders presenting to psychiatry can be thought of in three main ways:
-
those with neuropsychiatric complications of a known autoimmune disorder (paediatricians may ask for psychiatric input in case management)
-
those with psychiatric disorder and a co-occurring autoimmune disorder (collaboration of paediatric and mental health teams could be helpful)
-
those with what appears to be a primarily psychiatric illness but with indicators suggesting autoimmune aetiology (where the child and adolescent psychiatrist may need to seek the help of a paediatrician).
This categorisation, although helpful, is an oversimplification as disease processes can occur separately or simultaneously and it may not be possible in current clinical practice accurately to apportion presenting psychiatric symptoms to a single particular aetiology or mechanism. As in all medical specialties, any presentation is in the context of individual and family biological, psychological and social characteristics. Given the range of autoimmune disorders and the variation in the current levels of scientific understanding of the interaction of psychiatric and medical conditions, this article will illustrate key points that may apply across various disorders, by discussion of a selection of specific clinical presentations, some common and some extremely rare.
Common autoimmune medical conditions
Psoriasis
Psoriasis is an autoimmune skin condition with, as yet, no known direct cerebral autoimmune involvement. However, it is important to note that recently, psoriasis has been recognised as being more of a systemic illness than traditionally thought (Reference Laws, Young, Warren, Weinberg and LebwohlLaws 2014). Psoriasis has a significant negative impact on quality of life, well-being and social functioning. Living with a chronic condition that causes itchy, flaking skin and can also affect nails and joints can be difficult. The visibility of the illness can result in lowered self-esteem, social stigma and bullying, and in some circumstances a vicious cycle of stress leading to worsening of the condition can be identified. The treatment of psoriasis may have an effect on mental health, for example large areas treated with potent corticosteroids can result in systemic effects, and mood changes are a recognised side-effect of methotrexate therapy. In some cases, of course, the presenting psychiatric illness is entirely unrelated to the diagnosis of psoriasis, and autoimmune disorder is a coincidental finding in the patient’s history.
Type 1 diabetes
In child and adolescent type 1 diabetes, mental health problems occur with greater frequency than microvascular and other comorbid autoimmune conditions (Reference Cameron and NorthamCameron 2012). Possibly most well recognised are the psychological difficulties of diabetes as a chronic disease occurring alongside childhood and adolescent psychological and social development, including the transition to self-management of parenteral insulin. The magnification of self-harm risks associated with insulin use increases the challenge in managing comorbid psychiatric disorder. The metabolic effects of hypoglycaemia and hyperglycaemia can also affect the brain. For example, children who have an initial presentation of diabetic ketoacidosis are more likely to have morphological brain changes and difficulties with memory and concentration at least in the medium term (Reference Cameron, Scratch and NadebaumCameron 2014). There are also potentially direct brain effects of the disease process. Type 1 diabetes is associated with autoantibodies against pancreatic glutamic acid decarboxylase (GAD), which may also show affinity for brain-expressed GAD, a key enzyme in the synthesis of the neurotransmitter gamma-aminobutyric acid (GABA) from glutamate (Reference Benros, Eaton and MortensenBenros 2014). Type 1 diabetes has increased prevalence in Down syndrome and its diagnosis may be delayed if this risk is not borne in mind.
Hashimoto’s disease
Hashimoto’s disease is characterised by an autoimmune thyroiditis that is the most common cause of thyroid disease in children, with prevalence rates of up to 3% in some populations (Reference Sari, Karaoglu, Yesilkaya and HuangSari 2011). This disorder has a genetic basis and may be triggered by infection. Damage to thyroid tissue is mediated by T-lymphocyte production of thyroid-specific autoantibodies. The insidious onset of hypothyroidism, with symptoms that may be suggestive of hyperthyroidism (such as excessive sweating, nervousness, irritability and over-activity) can lead to delayed diagnosis, particularly as goitre is not necessarily present. More rarely, psychotic symptoms associated with gradual cognitive decline can point to the development of Hashimoto’s encephalopathy, which is a rare condition with a diagnosis confirmed by the presence of elevated thyroid autoantibodies (Reference Sari, Karaoglu, Yesilkaya and HuangSari 2011). In Down syndrome a clinical diagnosis of thyroid disorder may be difficult, but there is a high prevalence of Hashimoto’s disease, with a risk that reversible cognitive decline and behavioural disturbance may be missed or even misdiagnosed as an Alzheimer-type presentation (which is recognised as a risk in young adults with Down syndrome).
Coeliac disease
An association between coeliac disease and psychiatric illness has been hypothesised for many years – observational studies in the 1950s and 1960s described a higher than usual number of patients with both schizophrenia and coeliac disease. More recent population studies have been inconclusive (Reference Benros, Eaton and MortensenBenros 2014). Similarly, there has been controversy about a possible link between coeliac disease and autism spectrum disorder (ASD); this raised the possibility of a false association, as children with ASD might be more likely to be screened for coeliac disease as a result of the debate. A recent Swedish nationwide study failed to confirm this link, but did provide evidence suggesting increased ‘non-coeliac gluten intolerance’ in individuals with ASD (Reference Ludvigsson, Reichenberg and HultmanLudvigsson 2013). The classification of coeliac disease and gluten sensitivity is an evolving area. The extra-intestinal manifestations of coeliac disease (previously called atypical symptoms) are now recognised as being more common than was thought. As with other gastrointestinal disorders, abdominal symptoms are not always prominent and children with coeliac disease may present with symptoms including irritability, chronic fatigue, anorexia and weight loss or failure to thrive (Box 2) (Reference Tonutti and BizzaroTonutti 2014). Coeliac disease is the third of a triad of conditions with increased prevalence in Down syndrome (Box 1). This is important as there may be a reversible impact of malabsorption on growth and development that might be misattributed to the underlying chromosomal abnormality.
BOX 2 Case vignette: anorexia and coeliac disease
An 11-year-old girl presented to child and adolescent mental health services with dramatic weight loss associated with dietary restriction, low mood, fatigue and a morbid fear of fatness. Her extreme presentation led to an early psychiatric admission. Serological screening followed by an intestinal biopsy showing gluten enteropathy led to a trial of a gluten-free diet. Rapid weight gain on a suitable diet was accompanied by an improvement in mental state to the point that community management of her eating disorder was achieved swiftly. Understanding the role of her physical condition in the formulation was central to management of her illness.

Rheumatoid arthritis
The changing understanding of rheumatoid arthritis illustrates the advances in medical knowledge over the past few years. It is not long ago that rheumatoid arthritis was considered to be a classic psychosomatic illness, with a poor maternal relationship in childhood a hypothesised risk factor (Reference Baker and BrewertonBaker 1981). The possible impact of early life stress on the functioning of the hypothalamic–pituitary–adrenal (HPA) axis in an inflammatory disorder is now better understood (Reference Essex, Shirtcliff and BurkEssex 2011), but the confident connection of stress with the progression of rheumatoid arthritis is no longer made. The psychosocial burden on families associated with a child’s diagnosis of rheumatoid arthritis remains an important consideration for paediatric services. In addition, as with other severe autoimmune conditions, consideration must be given to the neuropsychiatric side-effects of medication and also to the effects on the brain of systemic inflammation, including altered brain metabolism, recognised as occurring even in the absence of direct autoimmune attack on the brain (Reference Emmer, van der Bijl and HuizingaEmmer 2009).
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) can present with a variety of symptoms affecting almost any physiological system, for example the characteristic butterfly rash, gastrointestinal disturbance, pulmonary disorders and renal disease, in addition to signs of systemic illness such as fatigue or unexplained fever. The American College of Rheumatology (1999) has described 19 neuropsychiatric syndromes of SLE, including: acute confusional state, anxiety disorder, cognitive dysfunction, mood disorder and psychosis, as well as chorea and seizure disorders. Paediatric SLE is associated with a high prevalence of neuropsychiatric complications, with approximately half of children with SLE experiencing at least one neuropsychiatric symptom (Reference ChanChan 2000). SLE should be held in mind as an important differential diagnosis in young people presenting with an acute onset of psychiatric symptoms, particularly if the presentation is atypical, if there are associated physical symptoms or signs or a strong family history of autoimmune disorder. When psychiatric disorder arises in association with an established diagnosis of SLE, consideration should be given in the formulation to the possible impact of neuropsychological changes, but care should be taken that the potential neuropsychiatric impact of SLE is not overemphasised, leading to neglect of other factors that may be independent of the disease process and open to change.
Neuropsychiatric disorders
In susceptible individuals, certain infections can trigger the onset of autoimmune disorders, resulting in further inflammation. Neuropsychiatric complications may arise from the inflammation associated with the infection and from autoimmune disease, which can affect the brain both independently and synergistically (Reference Benros, Waltoft and NordentoftBenros 2013). Reference Swedo, Leckman and RoseSwedo et al (2012) have developed the concept of paediatric acute-onset neuropsychiatric syndrome (PANS) as a way of describing a range of presentations.
Post-streptococcal disorders
Sydenham’s chorea
Sydenham’s chorea is the major neurological presentation of rheumatic fever, an inflammatory process following infection with group A streptococci (GAS) such as throat infection or scarlet fever. There is evidence that family genetic predisposition may be important in some cases. Sydenham’s chorea is characterised by uncoordinated jerking movements affecting the face and limbs and hypotonia. Some children also experience vocal and motor tics that may persist (Box 3). Behavioural symptoms and psychiatric comorbidities are also common and can have an abrupt onset which can precede the onset of the chorea. Emotional lability, anxiety and symptoms of obsessive–compulsive disorder (OCD) are the most well recognised and there have also been associations with attention-deficit hyperactivity disorder (ADHD), mood disorders and even symptoms of psychosis. All of these conditions can have an impact on learning and development, particularly if they are not identified. In addition, it is possible that impairments in executive functioning may be associated with Sydenham’s chorea in the absence of a psychiatric diagnosis. Sydenham’s chorea usually resolves spontaneously within a few months, although it can occasionally lead to persistent symptoms of chorea and/or fatigue. It may become a recurring illness, with or without further infection; pregnancy and the oral contraceptive pill may precipitate relapses of chorea. The recognised complications of Sydenham’s chorea include joint pains and damage to heart valves, as well as psychiatric symptoms. Basal ganglia damage and impaired executive functioning have been shown to persist into adulthood in some cases (Reference Williams and SwedoWilliams 2015).
BOX 3 Case vignette: tic disorder after Sydenham’s chorea
A boy of 8 presented with an acute-onset movement disorder. As the presentation was suggestive of Sydenham’s chorea, he was referred to the paediatric neurosciences service. At the specialist clinic several weeks later, the child had no chorea but had an obvious motor and vocal tic disorder. He was able to say that ‘his movements’ had changed. Review of a family video made earlier in the illness confirmed a diagnosis of Sydenham’s chorea, leading to discovery of cardiac involvement and a prescription for long-term penicillin. The child continued to tic and fulfilled criteria for a diagnosis of Tourette syndrome, with symptoms worsening during or after childhood illnesses. Understanding the impact of immune factors helped to guide treatment, clonidine was helpful and a tonsillectomy reduced the frequency of relapses.

Paediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS)
Paediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS) describes a disorder within the PANS classification characterised by sudden and dramatic onset of OCD, tic symptoms or severely restricted food intake and neuropsychiatric comorbidity, also of sudden and severe onset, following GAS infection. The timescale for onset of symptoms after infection is variable. Acute rheumatic fever, Sydenham’s chorea and other medical disorders should be excluded. Categories of comorbidity are similar to those of Sydenham’s chorea and include: anxiety (including separation anxiety); emotional lability and/or depression; irritability, aggression and/or severely oppositional behaviours; behavioural (developmental) regression; deterioration in school performance (ADHD-like symptoms, memory deficits, cognitive changes); sensory or motor abnormalities; somatic signs and symptoms, including sleep disturbances, enuresis or increased urinary frequency (Reference Swedo, Leckman and RoseSwedo 2012). These symptoms can have a significant impact on quality of life. A recent study (Reference Murphy, Patel and McGuireMurphy 2015) found that nearly a third of children assessed endorsed items related to suicide on the Child Behavior Checklist.
Encephalitides
Children with encephalitis may present to psychiatry with a variety of symptoms dependent on the subtype. Although many of these presentations are thought to have a post-infection origin some may be paraneoplastic (i.e. associated with underlying cancerous conditions).
Limbic encephalitis
Limbic encephalitis is characterised by an acute onset of memory loss, confusion and seizures and has traditionally been considered either paraneoplastic or viral in origin. However, it has been increasingly recognised that autoimmune forms of limbic encephalitis can occur in the absence of infection or malignancy. The term limbic encephalitis relates to inflammation of the limbic system, including the medial temporal lobes, amygdala and cingulate gyrus, but the label has historically often been applied erroneously to any encephalopathy with seizures, and memory and behavioural difficulties (Reference Armangue, Petit-Pedrol and DalmauArmangue 2012). Limbic encephalitis is rare in children and is associated with significant pathology.
The most frequent paediatric autoimmune encephalitis diagnosis is anti-N-methyl-d-aspartate (anti-NMDA) receptor encephalitis with potassium channel dysfunction mediated by antibodies targeting the NR1 receptor subunit (Reference Dalmau, Gleichman and HughesDalmau 2008) (Box 4). In adults and teenagers, the illness usually follows a staged course (Fig. 1), beginning with a prodromal stage of headache, fever or other viral symptoms and followed by psychiatric and behavioural problems (including anxiety, unusual behaviour, delusions and insomnia) then progressing to impairment of consciousness, seizures, dyskinesias, choreoathetoid movements, autonomic instability and breathing problems (Reference Armangue, Petit-Pedrol and DalmauArmangue 2012). Presenting symptoms in younger children reflect disruption in the limbic system, with behavioural changes (new-onset temper tantrums, agitation, aggression and changes in mood or personality), changes in speech (reduced, mutism, echolalia, perseveration), seizures and movement disorders.
BOX 4 Case vignette: anti-NMDA receptor encephalitis
A 13-year-old boy attended the emergency department, hallucinating and disoriented after an epileptic seizure. There was a history of several weeks of increasing disciplinary problems and declining school performance. Drug screen was negative. Following admission, he had poor sleep and episodes of aggression and confusion, his temperature, pulse and blood pressure were fluctuating and he developed orofacial dyskinesia. Combined assessment by a child neurologist and child psychiatrist achieved early diagnosis of anti-NMDA receptor encephalitis with raised antibodies, and initiation of immunotherapy. Physical recovery was good, but persistent memory impairment complicated his return to school. He required long-term psychological support to re-engage with education.

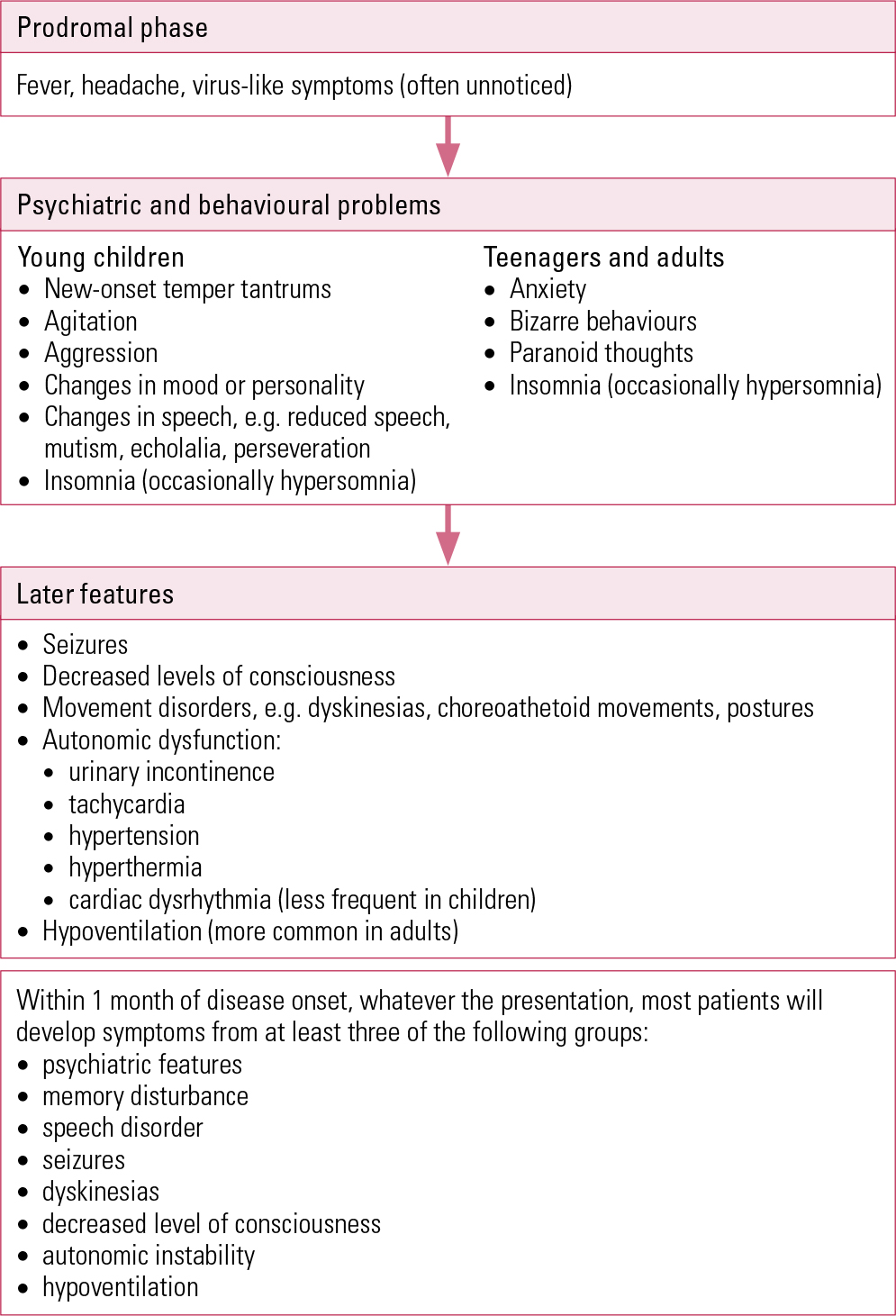
FIG 1 Stages of presentation of anti-N-methyl-d-aspartate (anti-NMDA) receptor encephalitis (after Reference Armangue, Petit-Pedrol and DalmauArmangue 2012).
In adults, 56% of women with anti-NMDA receptor encephalitis have an underlying ovarian teratoma. The incidence of teratoma in females with anti-NMDA receptor encephalitis decreases to 31% in those younger than 18 years and 9% in children under 14 years of age (Reference Florance, Davis and LamFlorance 2009). The treatment of anti-NMDA receptor encephalitis with immunotherapy (first line: corticosteroids, intravenous immunoglobulins and, possibly, plasmapheresis; second line: rituximab, cyclophosphamide), together with prompt removal if a tumour is found, can result in good neurological outcomes at 24 months. Less severe symptoms, prompt treatment and use of second-line immunotherapies if first-line therapies failed were predictors of better outcome in an observational cohort study (Reference Titulaer, McCracken and GabilondoTitulaer 2013).
Rasmussen encephalitis
Rasmussen encephalitis is a rare progressive disease affecting one cerebral hemisphere. Study of the disease’s aetiology has been difficult because of diagnostic delay – current hypotheses include an inflammatory immune response to an antigen, either foreign (e.g. an infectious agent) or autoimmune. The clinical course of the illness includes a prodromal stage with infrequent seizures, then presents with frequent seizures of differing types in the same patient, including unilateral epilepsia partialis continua (continuous focal jerking of a body part), cognitive decline and emergence of a hemiplegia that fluctuates initially before becoming permanent, accompanied by a progressive loss of brain volume in one hemisphere (Reference Varadkar, Bien and KruseVaradkar 2014). Rasmussen encephalitis may be treated by hemispherectomy.
Epilepsy
The psychiatric complications of epilepsy are well recognised, although clinically often underdiagnosed and undertreated (Reference Verrotti, Carrozzino and MilioniVerrotti 2014). It is less well known that some forms of epilepsy have an autoimmune basis. For example, a specific type, faciobrachial dystonia, described in 2008, can precede the onset of limbic encephalitis. Typically, seizures are brief (a few seconds), affect one side of the face and the ipsilateral arm and are frequent (happening as many as 50 times per day). The unusual presentation of the seizures, especially in the context of behavioural and memory disturbance, can result in their mislabelling as being non-organic in origin. Faciobrachial dystonia does not usually respond to anti-epileptic medication. Early recognition and immunotherapy may reduce the degree of post-encephalitic memory and cognitive impairments (Reference Irani, Stagg and SchottIrani 2013a). A large Finnish register study showed that children with an autoimmune disease had a fivefold increase in risk of epilepsy (Reference Ong, Kohane and CaiOng 2014).
Demyelinating disorders
Acute disseminated encephalomyelitis (ADEM) and multiple sclerosis are two autoimmune neurological demyelinating disorders that can occur in childhood. In comparison with multiple sclerosis, a life-long relapsing, degenerative condition with 2–5% of cases beginning in childhood, ADEM is more common in children under 10 years old (Reference Eyre, Absoud and WassmerEyre 2014). Multiple sclerosis is not diagnosed until at least two episodes have occurred; until then, the term clinically isolated syndrome (CIS) is used. Both ADEM and CIS can present with neurological symptoms, but ADEM also includes symptoms of encephalitis. Multiple sclerosis can have a significant impact on the functioning and quality of life of young patients: cognitive dysfunction affects about a third of children and adolescents, and more than half experience a psychiatric disorder (Reference Chou, Whitehouse and WangChou 2014). ADEM has a relatively favourable outcome, with the majority of children having a single-episode monophasic form that resolves within weeks. Follow-up studies of children who have recovered from an episode of ADEM have shown longer-term effects on behaviour and cognition, although a study with longer follow-up has given hope that these effects improve over time (Reference Suppiej, Cainelli and CasaraSuppiej 2014).
Opsoclonus–myoclonus syndrome
Opsoclonus–myoclonus or ‘dancing eye’ syndrome is a rare neurological condition in young children, and an autoimmune pathogenesis is hypothesised. Once thought to be solely a paraneoplastic condition, there is an association with neuroblastoma in about half of childhood cases. In addition to the jerky eye and limb movements, children can experience irritability and sleep disturbance (Reference Sahu and PrasadSahu 2011). The possible impact on behaviour of high-dose steroid treatments in early childhood, together with the potential significance of behavioural symptoms as markers of disease progress, can lead to difficult and complex decision-making in the care of such unusual children.
Aicardi–Goutières syndrome
Aicardi–Goutières syndrome is a rare autosomal autoimmune encephalopathy that most often presents in the first year of life following normal pregnancy and seemingly normal initial development. Symptoms include irritability, sleeping and feeding problems and unexplained fever, followed by psychomotor delay, loss of previously acquired skills, neurological impairment and slower head growth (Reference Orcesi, La Piana and FazziOrcesi 2009). Neonatal presentation can mimic a congenital infection, with neurological impairment, microcephaly, intracranial calcification, hepatosplenomegaly and self-resolving haematological abnormalities. Raised interferon-alpha (IFN-α) levels, triggered by an inappropriate innate immune response, are responsible for the main features of the disease.
Recognition
An important role of the child psychiatrist is to gather a full history from children and their families, including developmental history, family history, psychiatric history and responses to therapies. Given the wide-ranging associated psychopathologies of autoimmune disorder, it is important to consider the full spectrum of psychiatric and behavioural symptoms while taking the history and examining mental state. The fundamental psychiatric skills of taking a careful history and mental state examination can aid diagnosis and prompt timely collaboration with paediatric colleagues. The following particular features in a child’s history should arouse a degree of suspicion for an autoimmune process.
Personal or family risk of autoimmune disorders
It is recognised that there is a high degree of comorbidity among autoimmune disorders. When a child has an autoimmune disorder there is often a family history of either the same or another autoimmune disorder. Although further large population studies are required to investigate the patterns of individual and family co-occurrence, the concept of an autoimmune diathesis is widely accepted (Reference Cooper, Bynum and SomersCooper 2009). A child with Down syndrome should be seen as at high risk by virtue of their genotype. Clinicians should maintain an up-to-date understanding of the developing field of genetic risk in autoimmune conditions.
Recent and current physical illness
A review of systems should be carried out, including constitutional symptoms that might indicate systemic disease, for example rash, fever and weight loss. Enquire about any recent contact with infectious diseases, foreign travel and immunisation history.
Disease features suggestive of an organic aetiology
If a disorder is of sudden, severe onset, if there are impairments of consciousness or memory, or loss of previously acquired developmental milestones, there should be a high degree of suspicion of an organic process.
Neurological symptoms, abnormal movements and seizures
In a recent study of children with autoimmune encephalopathies (anti-NMDA receptor encephalitis, autoimmune basal ganglia encephalitis or Sydenham’s chorea), three neurologists assessed videos of the children’s movements (Reference Mohammad, Fung and Grattan-SmithMohammad 2014). They found that: chorea and dystonia were experienced by children across all three diagnoses; children with anti-NMDA receptor encephalitis were more likely to have stereotypies and perseveration; children with autoimmune basal ganglia encephalitis were most likely to have akinesia and tremor; and all of the children with Sydenham’s chorea had choreiform movements.
General physical and neurological examination can provide clues to a systemic illness – skin changes, growth disturbance/weight loss and gross neurological abnormalities may be readily observable in young people presenting to child and adolescent mental health services (CAMHS). However, more subtle signs may require examination by a specialist paediatric neurologist. As signs may be transient, video-recording of children undertaken by family or in the clinic can inform discussion with colleagues in neurology.
Investigation
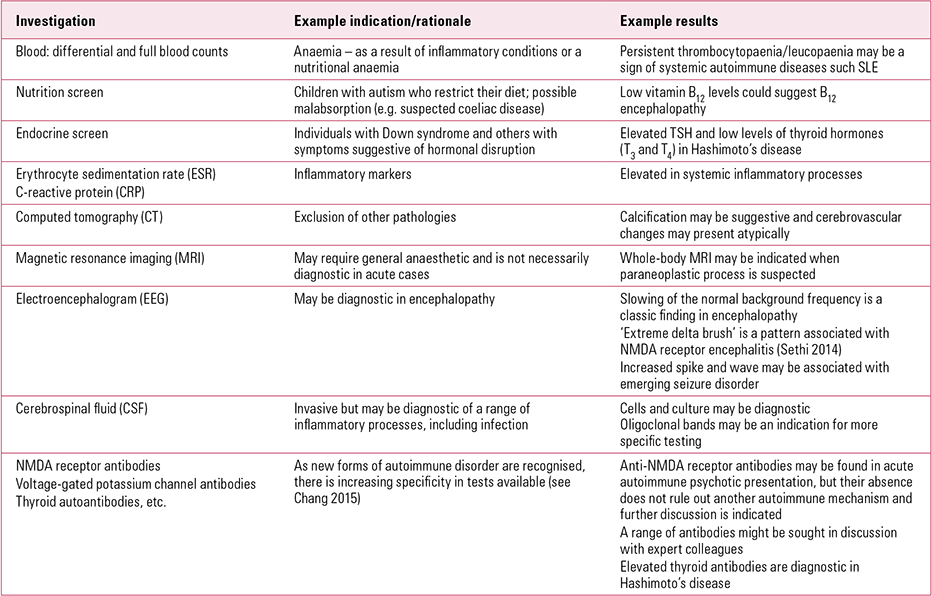
A decision regarding the most appropriate investigations for each child will reflect the differential diagnoses based on findings from the history and clinical examination. The PANS Collaborative Consortium advocates a thoughtful approach to investigation in which a work-up for autoimmune encephalitis, systemic autoimmune disease and other inflammatory diseases should be carried out only in the presence of relevant symptoms (Box 5). This is because of the high frequency of false-positive antibody tests, which cause avoidable distress to families already coping with severely ill children (Reference Chang, Frankovich and CooperstockChang 2015). Early liaison with paediatric and neurology colleagues may help guide investigation, minimising the discomfort of repeated tests for children. Table 1 summarises some possible investigations and their indications, together with points to discuss with paediatricians: the strategy chosen depends on the presentation.
BOX 5 Psychiatric symptoms prompting further investigation

The Pediatric Acute-onset Neuropsychiatric Syndrome (PANS) Consortium Guidance recommends that the following symptoms should prompt consideration of an autoimmune encephalitis work-up:
-
delirium, psychosis and/or diffuse encephalopathy
-
pervasive cognitive decline
-
persistent memory impairment
-
pervasive behavioural deterioration
-
seizure
-
movement abnormality not consistent with tics
TABLE 1 Laboratory investigations and imaging in autoimmune psychiatric presentations
Treatments
Suspicion for and prompt recognition of neuropsychiatric autoimmune disorders in children could offer a broader range of therapeutic options than the traditional psychiatric approaches. A dramatic example is given in the case study of a 29-year-old man with treatable organic suicidal behaviour resulting from NMDA-receptor encephalitis (Reference Irani, Vincent and JacobsonIrani 2013b). Careful psychiatric assessment will contribute to treatment decisions and may help to monitor treatment response. Specific treatments for autoimmune disorders include corticosteroids, plasma exchange, intravenous immunoglobulins or other immunotherapeutic drugs, such as cyclophosphamide and rituximab (Reference Twit and BenselerTwit 2014). These are outside the toolkit of most child and adolescent psychiatrists and both assessment and management should be considered in liaison with specialised paediatric neurologists and/or rheumatologists.
Immunotherapy can have significant psychiatric sequelae. Two cases of psychosis in adolescents with SLE – one as a result of primary neuropsychiatric SLE and the other due to steroid therapy – illustrate the paradox that pharmacotherapy may be a treatment or a cause (Reference Alpert, Marwaha and HuangAlpert 2014). The authors highlighted the role of the clinical history (e.g. exposure to steroids) and biomarkers (e.g. autoantibodies: antiribosomal P, antineuronal or antiphospholipid) in differentiating neuropsychiatric SLE from other presentations (such as thrombotic thrombocytopenic purpura, vasculitis or steroid-induced psychosis) and in guiding the management.
As with all clinical decisions, the risk–benefit ratio should be considered. The familiar evidence-based treatments in child and adolescent psychiatric practice must not be underestimated, particularly for children who are not acutely or life-threateningly unwell. Psychoeducation, cognitive–behavioural therapy, family/systems interventions and psychotropic medication may all play an important part in the holistic approach to treatment. Neuropsychological assessment and support for learning in liaison with school and educational psychology may also be important as there may be long-term impact on cognitive functioning, attention and school performance in a child with no previous difficulties. Psychotropic medication may be considered as indicated, but caution is required when factors such as autonomic dysregulation are complications of an autoimmune presentation. Where a child presents as acutely unwell, monitoring of respiratory or cardiac side-effects of psychotropics may be best managed by paediatric staff in liaison with the psychiatric team.
Conclusions
Recognition of the co-occurrence of autoimmune syndromes has led to greater opportunities for the study of potential mechanisms and treatments than the ‘organ-specific’ single-specialty approach to individual disorders. This highlights the need for cross-specialty working and, where there are psychiatric symptoms, the child psychiatry team is an essential partner. The specific example of Down syndrome exemplifies the growing need for a multidisciplinary approach. The possible role of the paediatric team in screening children with a known autoimmune disease for psychopathology is outwith the scope of this article, but is nonetheless an important consideration in local service design. The liaison psychiatry perspective gives consideration to the interplay between the biological, psychological, social and family factors affecting a young person. Reference Chang, Frankovich and CooperstockChang et al (2015) describe, in the case of PANS, symptoms such as prominent behavioural regression and resultant social disruptions that may give the appearance of a psychosocial rather than biological aetiology, especially in a child with a history of disruptive behaviour. Psychiatric symptoms related to autoimmune conditions may present in any setting and the developing literature should be held in mind in all CAMHS assessments. A careful history and physical and mental state examination are integral to the assessment, treatment planning and follow-up for children and young people with autoimmune disorders and psychiatric symptoms.

MCQs
Select the single best option for each question stem
-
1 Which of the following statements in relation to autoimmune presentations is untrue?
-
a careful exploration of the history can guide management of autoimmune presentations
-
b coeliac disease may present with irritability and fatigue
-
c PANDAS typically presents within 2 weeks of group A haemolytic streptococcus infection
-
d suicidality may be a presenting sign of autoimmune disorder
-
e there are well-recognised mechanisms for neuropsychiatric presentation of diabetes mellitus.
-
-
2 Which of the following examinatio ns and investigations is least likely to be useful when autoimmune disorder is suspected?
-
a careful examination of movement disorder presentations to clarify the diagnosis of autoimmune disease
-
b mental state examination to discriminate likely cases of autoimmune encephalitis in children
-
c an EEG to help in deciding whether there are organic factors contributing to an acute psychiatric presentation
-
d an MRI brain scan in possible Sydenham’s chorea
-
e lumbar puncture in atypical presentation of psychosis.
-
-
3 Which of the following is least likely to be useful in treatment of autoimmune neuropsychiatric disorders?
-
a cognitive–behavioural therapy for OCD in PANDAS
-
b plasmapheresis as a first-line treatment for PANDAS
-
c educational intervention after anti-NMDA receptor psychosis
-
d clonidine in tic disorders following Sydenham’s chorea
-
e high-dose steroids in SLE psychosis.
-
-
4 What is the likely prevalence of neuropsychiatric symptoms in SLE?
-
a 0–5%
-
b 5–15%
-
c 15–25%
-
d 45–55%
-
e 75–80%.
-
-
5 Which of the following autoimmune disorders may be a sign of underlying malignancy?
-
a SLE
-
b anti-NMDA receptor encephalitis
-
c Aicardi–Goutières syndrome
-
d coeliac disease
-
e Hashimoto’s disease.
-
MCQ answers
| 1 | c | 2 | d | 3 | b | 4 | d | 5 | b |






eLetters
No eLetters have been published for this article.