Obesity and over-nutrition together constitute an ever-increasing world health problem( Reference Zambrano and Nathanielsz 1 ). Further maternal over-nutrition has been demonstrated to result in offspring metabolic programming( Reference Waterland and Garza 2 , Reference Vega, Reyes-Castro and Bautista 3 ), in multiple organ systems, for example, pancreas and liver( Reference Waterland and Garza 2 ), as well as in central and peripheral nervous systems involved in energy homoeostasis( Reference Ke 4 – Reference Gocheva, Zeng and Ke 7 ). Human epidemiological( Reference Waterland and Garza 2 , Reference Hall 8 ) and experimental animal studies( Reference Grove, Allen and Grayson 9 – Reference Keen, Lonnerdal and Clegg 12 ) have shown a correlation between maternal diet and milk composition. Few studies address the mechanisms by which maternal obesity induced by a high-fat diet regulates liver and mammary gland (MG) differentiation and function during lactation and the implications for the observed changes in milk synthesis and composition( Reference Rodriguez-Cruz, Tovar and Palacios-Gonzalez 13 ). Long-chain PUFA (LC-PUFA) are essential for normal neonatal mammalian development. Arachidonic acid (AA, 20 : 4n-6) and DHA (C22 : 6n-3) are essential for neuronal developmental and cognitive function( Reference Heird and Lapillonne 14 – Reference Diau, Hsieh and Sarkadi-Nagy 16 ). EPA (20 : 5n-3) is the precursor of several eicosanoid products that are necessary for normal function of the immunological system( Reference Vernon 17 , Reference Ganapathy 18 ). The concentration of these lipids, AA and EPA=2 % and DHA=0·17 %, in rat milk fat is minimal( Reference Vernon 17 , Reference Bell 19 ). Milk is by far the greatest source of these fatty acids for the neonate, as enzymatic activity of desaturases Δ6D and Δ5D and elongases ELOVL 5 and 2 is low in early life and the fetus and neonate cannot produce the required amounts of LC-PUFA( Reference Koletzko, Agostoni and Carlson 20 , Reference Rodriguez-Cruz, Sanchez and Sanchez 21 ).
We hypothesised that a maternal obesity (MO) phenotype resulting from consumption of a high-fat diet during growth, gestation and lactation induces (1) alterations in maternal liver metabolism; (2) variations in MG differentiation and function; (3) adverse changes in maternal milk nutrient concentration; and (4) programmes offspring liver, fat and brain development in a sex-dependent manner.
Methods
To ensure homogeneity of mothers studied in the different experimental groups, female albino Wistar rats were obtained exclusively from Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán (INCMNSZ), Mexico. All procedures were approved by INCMNSZ Animal Experimentation Ethics Committee. Animals were held in American Association for Accreditation of Laboratory Animal Care. We studied female rats F0 fed control (C, control n 5) (laboratory chow Zeigler Rodent RQ22–5) or obesogenic diet (MO, n 5) (Table 1) during growth (21–120 d of life) and pregnancy and lactation, as previously described in detail( Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 22 ). Female rats were mated at 120 d of age. Mothers delivering litters over fourteen or under ten were excluded from the experiment. To ensure F1 offspring homogeneity, on postnatal day (PND) 2, all F1 litters studied were adjusted to ten pups with equal numbers of male and female pups whenever possible. A fixed amount of fresh food was provided daily. During lactation, the food remaining after 24 h was weighed daily( Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 22 ). Offspring were weaned at PND 21, housed five per cage and fed the control diet.
Table 1 Maternal diet composition (Percentages)
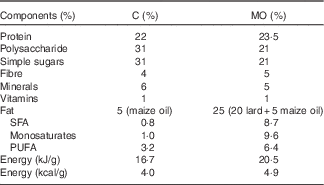
C, control group; MO, maternal obesity group.
Measurement of milk composition
Milk was obtained at PND 20. Food and pups were removed from mothers at 06.00 hours. After 4 h, 0·8 U oxytocin (ip) was administered to the mother and milk expressed 15 min later and quantified as total milk yield (ml). Milk samples were vortexed, divided into aliquots and frozen at −20°C until analysis. Milk samples were thawed at 37°C and vortexed before pipetting to ensure sample uniformity. Milk water was studied by gravimetric analysis. Milk carbohydrate concentration was determined by spectrophotometry (absorbance 492–550 nm) using the enzymatic glucose oxidase method (AccuTrack). Protein concentration was determined by the Bradford assay (Bio-Rad). Milk leptin concentration was determined by RIA, as previously described( Reference Bautista, Boeck and Larrea 11 ), using a commercial rat kit (Linco Research Inc.) with a detection limit of 0·5 ng/ml. Milk samples were assayed in duplicate.
Maternal liver and mammary gland collection
At PND 21, after a 4-h fast, mothers were rapidly euthanised by decapitation by experienced personnel trained in the use of the rodent guillotine (Thomas Scientific), and trunk blood was collected into polyethylene tubes, allowed to clot at 4°C for 1 h, centrifuged at 1500 g for 15 min at 4°C and serum was stored at −20°C until assayed. Maternal liver( Reference Torres, Bautista and Tovar 23 ) and MG chain were excised and weighed( Reference Bautista, Rodriguez-Gonzalez and Torres 24 ). The right inferior liver lobule was fixed for morphometric analysis and immunohistochemistry (IHC), whereas the left inferior liver lobule was immediately frozen at −75°C for protein analysis by Western blotting (WB). The remainder of the liver was collected at −20°C for Folch analysis and fatty acid profile. The MG beneath the 6th right nipple (counted from the cephalad end) was sectioned longitudinally into two halves and immediately immersion-fixed in 4 % paraformaldehyde in neutral PBS. After 24 h of fixation, tissue sections were dehydrated with ethanol at increasing concentrations from 75 to 95 % and embedded in paraffin. Sections (5 µm) were stained with haematoxylin–eosin. The MG beneath the 4th and 5th right nipples was frozen at −20°C for Folch analysis and fatty acid profile. The MG below the 4th, 5th and 6th left nipples was immediately frozen at −75°C for WB analysis( Reference Bautista, Rodriguez-Gonzalez and Torres 24 ).
Morphometric analysis
The percentage area stained for liver fat was evaluated at 100× magnification.
For each animal, ten MG pictures were analysed containing at least 150 lobules/rat at 10×. Area was expressed as the percentage of adipose and parenchymal tissues (acinar and ductal epithelium). In all, fifty acini per animal were measured at higher magnification (100×), and results were expressed as acini area; nucleus and cytoplasm area for cells in each acini (approximately 7–15 cells/acinus) were measured and results were expressed as cytoplasm and nuclei size. All procedures were evaluated using the AxioVision software( Reference Bautista, Rodriguez-Gonzalez and Torres 24 ). All histological measurements were performed by two independent observers without knowledge of the source of the tissues, and the results were averaged.
Western blot analysis of protein concentration
A section of liver tissue and MG were homogenised in RIPA lysis buffer (PBS 1 %, NP-40 1 %, sodium deoxicolate 0·5 %, SDS 0·1 % and sodium azide 0·006 % (w/v)). The total lysate protein concentration was determined by the Bradford method. Protein samples were separated on 10 % SDS-PAGE gels and transferred onto a polyvinylidene fluoride membrane (0·45 μm Millipore). Each membrane was probed with antibodies from Santa Cruz biotechnology either to Δ6D (sc-98480), Δ5D (sc-101953), ELOVL 5 (sc-374138) or ELOVL 2 (sc-54874) at a 1:500 dilution in Tris-buffered saline Tween (20 mm TrisHCl, 500 mm-NaCl, pH 7·4, 0·01 % Tween-20) with 5 % non-fat dried milk, and incubated for 60 min. Δ6D and Δ5D were incubated with goat anti-rabbit IgG-horseradish peroxidase (sc-2004) secondary antibodies and ELOVL 5 and 2 with donkey anti-goat IgG-HRP (sc-2020). Proteins were detected by chemiluminescence (Millipore). Images were captured by an E3 biochemical imaging system (UVP) and spot densitometry analysis was performed using the vision work system software (UVP). The first four spots of each gel contained samples from the C group and the next four spots contained samples from the MO group. A total of eight samples were included per gel. All results were normalised to β-actin as the loading control( Reference Bautista, Rodriguez-Gonzalez and Torres 24 ).
Immunohistochemistry analysis
Liver and MG paraffin sections (5 µm) were immunostained with Santa Cruz Biotechnology; rabbit polyclonal Δ6D (sc-98480) and Δ5D (sc-101953), and goat polyclonal ELOVL 5 (sc-374138) and ELOVL 2 (sc-54874) 1:200 dilution, ABC Elite kit, Vector Laboratories and visualised using 2·5 % nickel sulphate with 0·02 % DAB (3,3′-diaminobenzidine tetrahydrochloride) chromogen in 0·175 m-sodium acetate. Cell counts were performed on an Olympus BX51 light microscope using image analysis software (Image-Pro Plus, version 3.1; Media Cybernetics Inc.). For the data analyses of protein in the liver by IHC, we used the imageJ software as previously described( Reference Vega, Reyes-Castro and Bautista 3 ). For MG, the IHC was used only for immunolocalisation, as the distribution of lobules and fat was very different between control and MO mothers.
Fatty acid analysis
Maternal liver, MG, milk and offspring liver lipids were extracted by a modified Folch technique. Samples were homogenised with 2 ml of 0·9 % NaCl and 5 ml of chloroform–methanol (2:1), as previously described( Reference Torres, Bautista and Tovar 23 , Reference Bautista, Rodriguez-Gonzalez and Torres 24 ). Fatty acid extraction was performed by the addition of chloroform (3×2 ml). The organic phase was pooled and 120–150 µl of methanol was added until the organic phase turned transparent, and then 1 g of Na2SO4 was added and vortexed to provide the residue for analysis. The organic phase was evaporated under a stream of N2.
Preparation of fatty acid methyl esters
Samples for fatty acid methyl esters (FAME) were prepared as previously described( Reference Bautista, Rodriguez-Gonzalez and Torres 24 ). Briefly, 2 ml of methanol, 100 µl of toluene and 40 µl of 2 % methanolic sulphuric acid were added to the above residue and heated at 90°C for 2 h. Tubes were then placed on ice, and 1 ml of 5 % NaCl was added. FAME were extracted with hexane (3×2 ml), and the mixture was centrifuged at 1500 g for 1 min. The organic phase was pooled and evaporated under a stream of N2. Hexane (200 µl) was added to the residue, which was then centrifuged at 1500 g for 5 min. The clear solution was injected in an Agilent model 6850 GC equipped with a flame ionisation detector. Automatic split injection was carried out using an Agilent 6850 auto sampler. The chromatographic column was an HP-INNOWax capillary column (30 m, 0·25 mm, 0·25 m) (J & W Scientific). One hundred twenty-five micrograms of heptadecanoic acid was added to 100 mg of tissue as an internal standard. A 1-µl sample was injected in split mode (50:1) at 250°C. The carrier gas was He2 with a constant linear velocity of 24 cm/s, and the interface temperature was maintained at 280°C. The oven temperature was raised from 50 to 230°C. Identification of the FAME was based upon retention times obtained for methyl ester standards from PolyScience, and each one was expressed as a percentage of total fatty acid in the sample.
Quantification of TAG
Liver TAG were extracted using the Folch method and quantified with a colorimetric (absorbance 546 nm) commercial kit from RANDOX CE® (RX MONZA Method GPO-PAP). Briefly, 10 µl of lipids was diluted in 1000 µl of enzyme reagent and incubated for 10 min at 20–25°C. All samples were assayed in duplicate.
Offspring parameters at postnatal day 21 and 36
After weaning, all offspring were fed chow diet. Offspring body weight was determined at random in two male and two female F1 pups/litter per age (n 5 litters). At PND 21 and 36, pups were fasted for 4 h and euthanised by decapitation. Offspring adipose tissue from visceral and retroperitoneal areas was collected and weighed together with the brain and liver.
Statistical analysis
Statistical analysis was performed using unpaired Student’s t test with P set at <0·05. Results from WB were normalised with β-actin and compared between groups. Offspring data from the same litter were averaged for analysis to provide 5 litters per group. Preliminary analysis for differences according to the sex of the pup at PND 21 revealed no difference, and thus all data at this age were pooled. At PND 36, the results were expressed by sex. All data are presented as mean values with their standard errors.
Results
Maternal parameters at postnatal day 21
Maternal body weight in the C and MO groups (C=352 (sem 6) g and MO=356 (sem 14) g) at PND 21 were similar. However, total body adipose tissue weight was higher in MO than in C mothers (C=4 (sem 0·2) g and MO=19 (sem 3) g; P<0·01). Using average data for the lactation period, food intake (C=63 (sem 2·6) g and MO=50 (sem 2·8) g/d) and energy intake (C=1054 (sem 42) kJ/d (C=252 (sem 10) kcal/d) and MO=1025 (sem 59) kJ/d (MO=245 (sem 14) kcal/d)) were similar between groups. Fat intake was higher in MO than in C (C=3·2 (sem 0·2) g/d and MO=12·5 (sem 0·7) g/d; P<0·05).
Maternal liver analysis at postnatal day 21
Liver weight was similar in both groups, whereas the percentage of liver fat was higher in MO than in C ( Table 2). The percentages of AA and EPA were lower in MO than in C, whereas DHA was similar in the two groups (Fig. 1(a), (b) and (c)). Finally, the hepatic fatty acid profile exhibited more SFA and monosaturates and less n-3 and n-6 PUFA in MO than in C (Table 2).

Fig. 1 Maternal liver at postnatal day (PND) 21. (a) Arachidonic acid (AA, %), (b) EPA (%) and (c) DHA (%), and maternal mammary gland (MG) at PND 21, (d) AA (%), (e) EPA (%) and (f) DHA (%). Values are means (n 5), with their standard errors represented by vertical bars. * Mean value was significantly different (P≤0·05). ![]() , Control (C);
, Control (C); ![]() , maternal obesity (MO).
, maternal obesity (MO).
Table 2 Maternal liver and mammary gland (MG) parameters at postnatal day (PND) 21 (Mean values with their standard errors; n 5)
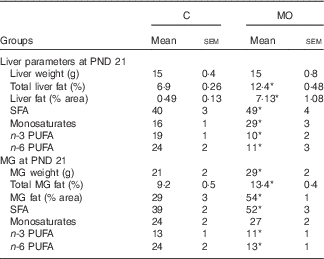
C, control group; MO, maternal obesity group. * Significantly different compared with C (P≤0·05).
Maternal liver Δ6D, Δ5D, ELOVL 5 and 2 protein expression by Western blotting and immunohistochemistry at postnatal day 21
Hepatic Δ6D and Δ5D protein abundance and the percentage of immunostained tissue area were higher in MO than in C mothers (Fig. 2(a) and (b)). ELOVL 5 was similar in both groups (Fig. 2(c)). ELOVL 2 immunostaining was lower in MO than in C, but protein levels by WB were similar in the two groups (Fig. 2(d)).

Fig. 2 Maternal liver Western blot analysis (![]() , control (C), n 4 and
, control (C), n 4 and ![]() , maternal obesity (MO) n 4) and immunohistochemistry (C, n 5) and (MO, n 5) at postnatal day 21. (a) Delta 6 desaturase (Δ6D), (b) delta 5 desaturase (Δ5D), (c) elongase 5 (ELOVL 5) and (d) elongase 2 (ELOVL 2). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different (P≤0·05).
, maternal obesity (MO) n 4) and immunohistochemistry (C, n 5) and (MO, n 5) at postnatal day 21. (a) Delta 6 desaturase (Δ6D), (b) delta 5 desaturase (Δ5D), (c) elongase 5 (ELOVL 5) and (d) elongase 2 (ELOVL 2). Values are means, with their standard errors represented by vertical bars. * Mean value was significantly different (P≤0·05).
Mammary gland weight and morphometric analysis at postnatal day 21
At PND 21 MG weight, total fat by Folch and percentage of fat by histological analysis were higher in MO than in C (Table 2 and Fig. 3(d)). Histological analysis showed smaller acini and cytoplasm area and nuclei size in MO than in C (Fig. 3(a), (b), (c) and (e)). The percentages of AA and DHA were similar between groups (Fig. 1(d) and (f)), whereas EPA was lower in MO than in C (Fig. (1)). Fatty acid analysis showed more SFA and less n-3 and n-6 PUFA in MO than C mothers (Table 2).

Fig. 3 Maternal mammary gland at postnatal day 21. (a) Acini area (μm2), (b) cytoplasm (μm2), (c) nuclei size (μm2) and (d) microphotography at 10× the adipose tissue area in white and parenchymal tissue in black, and (e) microphotography at 100× cytoplasm and nuclei size. Values are means (n 5), with their standard errors represented by vertical bars. * Mean value was significantly different (P≤0·05). ![]() , Control (C);
, Control (C); ![]() , maternal obesity (MO).
, maternal obesity (MO).
Maternal mammary gland Δ6D, Δ5D, ELOVL 5 and 2 protein expression by Western blotting and immunohistochemistry localisation at postnatal day 21
Δ6D, ELOVL 5 and ELOVL 2 protein expression by WB were similar in C and MO (Fig. 4). However, Δ5D was lower in MO than in C (Fig. 4(b)). Δ6D, Δ5D, ELOVL 5 and ELOVL 2 were immunolocalised in parenchymal cells of both groups (Fig. 4).

Fig. 4 Maternal mammary gland Western blot analysis (![]() , control (C) and
, control (C) and ![]() , maternal obesity (MO)) and immunohistochemistry immunolocalisation in parenchymal cells at postnatal day 21. (a) Delta 6 desaturase (Δ6D), (b) delta 5 desaturase (Δ5D), (c) elongase 5 (ELOVL 5) and (d) elongase 2 (ELOVL 2). Values are means (n 4), with their standard errors represented by vertical bars. * Mean value was significantly different (P≤0·05).
, maternal obesity (MO)) and immunohistochemistry immunolocalisation in parenchymal cells at postnatal day 21. (a) Delta 6 desaturase (Δ6D), (b) delta 5 desaturase (Δ5D), (c) elongase 5 (ELOVL 5) and (d) elongase 2 (ELOVL 2). Values are means (n 4), with their standard errors represented by vertical bars. * Mean value was significantly different (P≤0·05).
Milk composition at postnatal day 20
Milk production and milk percentage water, carbohydrate content, EPA, DHA, n-3 PUFA and SFA were lower (Fig. 5(a), (b), (c), (h), (i) and (j)), whereas milk leptin, total fat, AA, monosaturates and fatty acids were higher in MO than in C (Fig. 5(e), (f), (g) and (j)). The percentages of protein and n-6 PUFA were similar in the two groups (Fig. 5(d) and (j)).

Fig. 5 Maternal milk components at postnatal day 20. (a) Total yield (ml), (b) water (%), (c) carbohydrates (%), (d) protein (%), (e) leptin (ng/ml), (f) fat (%), (g) arachidonic acid (AA) (%), (h) EPA (%), (i) DHA (%) and (j) percentage of fatty acid in milk. Values are means (n 5), with their standard errors represented by vertical bars. * Mean value was significantly different (P≤0·05). ![]() , Control (C);
, Control (C); ![]() , maternal obesity (MO).
, maternal obesity (MO).
Pup development at postnatal day 21 and 36
Pup body weights were similar in the two groups at birth as were pup body and liver weights at PND 21 (Table 3). However, pup weight gain during lactation was greater in MO. Body weight at PND 36 was similar between groups in both sexes, but both C and MO male pups weighed more than female pups. Absolute adipose tissue weight and adipose tissue weight relative to body weight was greater in MO pups than in C at PND 21 and 36. Adipose tissue weight in male pups was greater than in female pups at PND 36 in both groups. Brain weight at PND 21 was less in MO compared with C, but when expressed as relative brain weight to body weight they were similar. At PND 36, brain weights were similar in C and MO female pups, whereas in male pups brain weight was lighter in MO than in C (Table 3). Brain weight relative to body weight was lower in male MO than in female MO. Female brain weight was lighter in comparison with male brain weight in the C offspring at PND 36. There was no difference in liver weight at PND 21 and 36 between groups. However, liver weight in MO male pups was heavier than in MO female pups. Liver weight relative to body weight was lower in MO female pups compared with C and in C male pups compared with C female pups. Total liver fat and TAG were similar among groups and sexes (Table 3).
Table 3 Pup parameters at birth and at postnatal day (PND) 21 and 36 (Mean values with their standard errors; n 5 litters)

C, control group; MO, maternal obesity group; ♀, female; ♂, male. * Significantly different compared with C (P≤0·05). † Significantly different compared with male (P≤0·05).
Discussion
Milk protein, carbohydrates and lipids such as LC-PUFA are essential components for optimal offspring growth, brain development and maturation of the immune system. There is a clear association between maternal obesity and failure of milk production in human and animal studies( Reference Turcksin, Bel and Galjaard 25 – Reference Jevitt, Hernandez and Groer 27 ). However, effects of a high-fat diet consumption and maternal obesity on maternal liver and MG synthesis of enzymes related to milk production and composition are not well-documented. Our data show that maternal obesity adversely affects liver and MG function, resulting in decreased milk fatty acid quality, associated with negative effects in offspring development. We previously reported that female rats exposed to a 25 % fat diet from their own weaning to adult life and through gestation and lactation show elevated insulin, glucose, homeostasis model assessment (HOMA), leptin, TAG and cholesterol at the end of their lactation period( Reference Vega, Reyes-Castro and Bautista 3 ). The present study shows that maternal obesity because of a high-fat diet affects maternal hepatic and MG function, alters milk nutrient concentration and negatively programmes offspring metabolism.
The liver has a critical role in fatty acid metabolism, including control of the synthesis and production of LC-PUFA during lactation( Reference Vernon 17 ). The fatty acids are then transported to the MG where they constitute key components of milk. Maternal high-fat intake during lactation alters blood lipid concentrations and liver metabolism with consequences for maternal homoeostasis, oxidative stress, lipogenesis and β-oxidation( Reference Vega, Reyes-Castro and Bautista 3 , Reference Bell 19 , Reference Bertics, Grummer and Cadorniga-Valino 28 – Reference Kuhla, Kucia and Gors 30 ). In the present study, the maternal high-fat diet increased maternal liver fat without any effect on liver weight. Importantly, the percentages of n-3 PUFA and n-6 PUFA and AA and EPA were lower in MO mothers, whereas SFA and monosaturates increased relative to C mothers. Δ6D and Δ5D were increased probably because of negative feedback regulation by decreased ELVOL 2 expression in MO mothers compared with C rats. Availability of milk nutrients is determined by the interaction of dietary intake, intestinal absorption and maternal metabolism in several tissues especially the liver and MG. There is evidence to indicate that desaturases are increased in a fatty liver( Reference Rodriguez-Cruz, Tovar and Palacios-Gonzalez 13 , Reference Niculescu, Lupu and Craciunescu 31 – Reference Nakamura and Nara 34 ), which would explain the increased abundance of desaturases and elongases that we observed in the livers of the obese mothers. However, there is also evidence that low dietary PUFA increase liver desaturases and elongases( Reference Rodriguez-Cruz, Sanchez and Sanchez 21 ) and therefore in our study we might have expected these enzymes to decrease in obese mothers given their increased PUFA intake. A potential explanation of this apparent contradiction would be that it is not the total PUFA in the diet that regulates the hepatic production of these enzymes but the ratio of PUFA:SFA. In agreement with these findings, López-Vicario et al.( Reference López-Vicario, Rius and Morán-Salvador 35 ) produced non-alcoholic steatohepatitis in mice by feeding them with a high-fat diet resulting in an increase of liver Δ5D and n-6 PUFA.
The relative proportions of cellular compartments change markedly during MG development in pregnancy and lactation. Adipose tissue is replaced by parenchymal tissue( Reference Jevitt, Hernandez and Groer 27 ). In our study, MG development in obese mothers did not produce the normal structure with resultant changes in MG function. In our study, MG development in obese mothers did not produce the normal pattern of relative development of parenchymal (acinar and ductal epithelium) and adipose tissues. MO groups showed more adipose and less parenchymal tissue compared with C, likely to result in altered MG function. A high-fat intake produces similar outcomes in obese pregnant mice( Reference Saben, Bales and Jackman 36 ), suggesting that maternal high-fat diet delays lobuloalveolar structure development and differentiation during lactogenesis affecting milk production. Recently, Saben et al.( Reference Saben, Bales and Jackman 36 ) observed that a high-fat diet in lactating mice impairs de novo fatty acid synthesis in the MG through inhibition of acetyl CoA carboxylase mediated by adenosine monophosphate-activated protein kinase; this protein is overexpressed during lactation when lipid production begins (Fig. 6).

Fig. 6 Mechanisms proposed in rats fed an obesogenic diet during growth, pregnancy and lactation and effects on milk composition and offspring outcomes.
Milk nutrients are derived from dietary intake and liver and MG biosynthesis( Reference Anderson, Rudolph and McManaman 37 – Reference Wattez, Delmont and Bouvet 39 ). Studies in rats indicate that hepatic desaturase and elongase expression is dependent on PUFA availability. High levels of desaturases are present in livers of rats fed a low-PUFA diet( Reference Rodriguez-Cruz, Tovar and Palacios-Gonzalez 13 ). During pregnancy and lactation, some metabolic adaptations occur. LC-PUFA are synthesised in the liver and other extrahepatic tissue such as the MG or they are mobilised from adipose tissue reserves( Reference Rodriguez-Cruz, Sanchez and Bernabe-Garcia 32 ). In the rat, it has been shown that approximately 35 % of dietary linoleic acid is transferred directly to the MG and 12 % to the milk independently of the dietary lipid content. The authors suggest that both the MG and the liver have an important role in the synthesis of LC-PUFA in the milk( Reference Rodriguez-Cruz, Tovar and Palacios-Gonzalez 13 , Reference Rodriguez-Cruz, Sanchez and Bernabe-Garcia 32 ).
Leptin is present in the milk of many different species, including rats( Reference Bautista, Boeck and Larrea 11 ) and humans( Reference Houseknecht, McGuire and Portocarrero 40 ). Nursing rats transfer leptin to the neonates via the milk. This transfer may regulate neonatal food intake( Reference Casabiell, Pineiro and Tome 41 ). Milk leptin concentration is determined by a number of factors. In the present study, milk leptin concentration was higher in MO compared with C, associated with higher fat depots in MO offspring.
Studies in rats programming model of 50 % maternal energy restriction have analysed rat milk composition and found less water in the milk and more fat at days 10 and 21 of lactation, in addition to less carbohydrate at day 10 of lactation( Reference Wattez, Delmont and Bouvet 39 ) results very similar to those we report here. In our study, the milk composition changes were associated with decreased brain weight and more larger fat depots in offspring at PND 21 and 36, supporting our reported findings of negative effects on adult metabolic function( Reference Zambrano, Martinez-Samayoa and Rodriguez-Gonzalez 42 ), as well as cognitive function leading to behaviour impairment( Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 22 ). Absolute weight is the best overall indication of the total number of synapses and neurons, and clearly absolute function throughout life will be affected by having a smaller absolute brain size than controls. In addition, low milk DHA concentrations have been reported( Reference Heird and Lapillonne 14 , Reference Diau, Hsieh and Sarkadi-Nagy 16 ) to affect offspring neural cell membranes and to induce damage in cognitive function during early life.
One very interesting sex-dependent observation in our study was that both absolute and relative brain weight decreased in male pups but not in female pups. These phenotypic changes are in keeping with our demonstration that MO increases offspring hippocampal reactive oxygen stress in adult life( Reference Reyes Castro, Rodrigues Gonzalez and Larrea 43 ). We have reported that MO offspring showed higher TAG, adipose tissue, leptin and insulin resistance at PND 21, 36 and 110( Reference Vega, Reyes-Castro and Bautista 3 , Reference Rodriguez, Rodriguez-Gonzalez and Reyes-Castro 22 ). Other groups have previously demonstrated that adequate lipid concentration in maternal milk is important for offspring organ maturation( Reference Innis 44 , Reference Cetin and Koletzko 45 ).
In conclusion, our results indicate that maternal high-fat diet and obesity impair maternal liver and MG development and function, and modify milk composition, associated with dysregulated offspring brain development and metabolic function in a sex-specific manner.
Acknowledgements
This work was supported by Fundacion Mexicana para la Salud y Fundación Mexicana para la salud Hepatica (C. J. B., FUNSALUD and FUNDHEPA) and Consejo Nacional de Ciencia y Tecnología (CONACyT, E. Z., 155166 and C. J. B., 237643) Mexico. FUNSALUD, FUNDHEPA AND CONACyT had no role in the design, analysis or writing of this article.
C. J. B., researched data, responsible for the study design and manuscript writing; S. M., V. R., researched data; A. M., contributed to the discussion and reviewed the manuscript; P. W. N. and N. A. B., responsible for the study design and preparation of the manuscript; E. Z., responsible for the study design and preparation of the manuscript.
The authors declare that there are no conflicts of interest.












