The efficacy of new antidepressants introduced in recent years generally appears to be equal to that of the established classes of drugs, particularly the tricyclics (TCAs) and the selective serotonin reuptake inhibitors (SSRIs) (Reference Anderson, Nutt and DeakinAnderson et al, 2000). However, some new compounds claim advantages over other agents, especially the SSRIs. These advantages, which differ from drug to drug, either refer to specific side-effects, such as sexual dysfunction, or to symptoms of the depressive illness that are not adequately controlled by the SSRIs, such as sleep. Nevertheless, head-to-head comparisons between the new agents and the SSRIs, designed specifically to test these claims, are still scarce.
Antidepressants and sleep
One of the new compounds, nefazodone, is said to confer an advantage over the SSRIs in improving sleep, even before the onset of antidepressant action. This clinically useful characteristic is shared with the TCAs but the latter group, unlike nefazodone, is handicapped by danger in overdose and troublesome anticholinergic side-effects. By contrast, the SSRIs are not considered sleep-promoting. Disturbed sleep is one of the most frequent and distressing symptoms in moderate and severe depression. Objective sleep changes in depression include shortened rapid eye movement (REM) latency, disruption of sleep continuity, early morning waking and reduction of slow wave sleep, particularly in the first sleep cycle (Reference Benca, Obermeyer and ThistedBenca et al, 1992). Antidepressants such as the TCAs and SSRIs produce marked suppression of REM sleep. The TCAs tend to improve sleep fragmentation acutely whereas SSRIs decrease sleep continuity until there is resolution because of improvement of the depressive illness (Reference Wilson, Bell and CouplandWilson et al, 2000).
Nefazodone weakly antagonises the reuptake of serotonin (5-HT) and noradrenaline (Reference Tatsumi, Groshan and BlakelyTatsumi et al, 1997); it also blocks the post-synaptic 5-HT2 receptor and the α1-adrenoceptor, but does not block histamine or cholinergic receptors, the supposed mechanism producing the sedative effects of TCAs (Reference Cusack, Nelson and RichelsonCusack et al, 1994). In a multi-centre sleep laboratory study in major depression (Reference Rush, Armitage and GillinRush et al, 1998) nefazodone improved sleep over an 8-week period compared with fluoxetine. No study, however, has looked at the first 2 weeks of treatment or at patients sleeping in their home environment.
METHOD
Protocol and study objectives
This was a single-site, double-blind, randomised, parallel-group, 8-week study of sleep in patients with moderate to severe depression without psychotic features. The objective of this trial was to compare the effects of nefazodone and paroxetine on sleep and mood. The study was approved by the local ethics committee and all patients gave written informed consent. At the end of the acute phase of the treatment (8 weeks) patients who showed at least minimal improvement on the Clinical Global Impression (CGI) Improvement scale (Reference GuyGuy, 1976) continued to be studied clinically, but without sleep measures, for a further 16 weeks.
Patient population
Consecutive referrals to a hospital psychiatric out-patient clinic and direct referrals from two general practices were assessed for eligibility in the study. They were screened with full medical and psychiatric history, mental state examination and physical examination. To be randomised into the study, patients had to fulfil diagnostic criteria for DSM—IV (American Psychiatric Association, 1994) moderate to severe depression, scoring 18 or over on the Hamilton Rating Scale for Depression (HRSD; Reference HamiltonHamilton, 1960). Exclusion criteria included schizophrenia, history of mania, active suicidal ideation, alcohol misuse and illicit drug use. Patients unable to maintain a consistent sleep pattern, such as shift workers or those with a current sleep/wake disorder, were also excluded. Pre-menopausal women were required to have a negative pregnancy test at screening and to take precautions against pregnancy during the trial. Subjects who had previously taken psychoactive medication including benzodiazepines were required to undergo a 2-week (or 5-week in the case of fluoxetine) washout period before entering the trial. In the event, six of the patients had received benzodiazepines, none in the previous year, and four patients had received fluoxetine in the previous year, one stopping for the study.
Patients who had received any other investigational drug up to 30 days before the initiation of therapy, or who were participating in another clinical study at the time of the assessment, were not considered for inclusion in this protocol. Pregnant or nursing females, or women of child-bearing potential who were not using adequate methods of birth control were also excluded.
Determination of sample size
This was calculated to detect the smallest clinically relevant differences in two sleep parameters measured by electroencephalogram (EEG) (total sleep time and number of awakenings) with an 80% power (P=0.05) based on variance data from a previous sleep study conducted in our laboratory (Reference Wilson, Bell and CouplandWilson et al, 2000), and the two-sample method (Reference Gore and AltmanGore & Altman, 1982) was applied. The estimated sample size was 18 patients in each group providing valid data for at least three sleep assessment days (see later). The target sample size was then extended to 22, to allow for dropouts. In the event of the study, fewer dropouts occurred than had been anticipated.
Study design and medication
Forty patients were randomly assigned on day 1 of the study, after the baseline period, to receive either nefazodone or paroxetine. Randomisation was carried out by Bristol-Myers Squibb, in blocks of four, so that in any one block there were two patients taking nefazodone and two taking paroxetine. All study medication was re-encapsulated. Capsules and container bottles were identical in shape, but of two different colours. Medication was provided for an extra 3 days a week, to allow for late follow-up visits. Treatment was taken in a twice daily regimen by mouth, with patients taking one or two capsules from the nefazodone/placebo bottle twice a day and a capsule from the paroxetine/placebo bottle in the morning. Patients commenced on day 1 with either morning nefazodone (100 mg twice daily), evening nefazodone (100 mg+placebo) or morning paroxetine (20 mg+placebo), evening placebo. After 1 week, the dose of nefazodone was increased to 200 mg twice daily and that of paroxetine remained at 20 mg once a day. Further titration was according to clinical response and side-effects, and occurred during scheduled visits or urgent visits if there were any problems. Adverse events were monitored specifically from a checklist of 95 symptoms and recorded during each visit. Patients also had 24-h telephone access to an investigator if anything untoward occurred. The patients were instructed to return all unused medication in the original package at each visit. No additional psychoactive medication was allowed during the washout and treatment phase of the study. Wherever possible, all other non-psychotropic concomitant medications and non-pharmacological therapies were recorded and kept constant for the duration of the study.
Assessments
At baseline, a physical examination was performed; vital signs (pulse, blood pressure) were recorded, and demographic data were collected. Information about previous episodes of depression was obtained from case notes and patients' and relatives' recollection. A pregnancy test was performed as indicated above. A baseline EEG was performed using the home ambulatory monitoring system (Medilog, Oxford Instruments Medical, Old Woking). Subjects were visited in their homes during the evening and the recording equipment for electro-encephalography, electro-oculography and electromyography was attached, according to the standard sleep montage (Reference Rechtschaffen and KalesRechtschaffen & Kales, 1968). The subjects were then left to sleep normally at home. They were asked not to bathe or shower with the equipment on but told that otherwise they could carry out their normal domestic routine; they were asked not to drink alcohol for the 48 h before the recording but were allowed their normal caffeine intake. They were instructed to keep to their normal bedtime routine and to press the event marker on the recorder when they turned out the lights and tried to sleep, and on waking finally the following morning. Subjective measures of sleep using the St Mary's Hospital Sleep Questionnaire (SMHSQ; Reference Leigh, Bird and HindmarchLeigh et al, 1988) were obtained on the morning after the recording. Thereafter sleep recordings and subjective sleep questionnaires (SMHSQ and Leeds Sleep Evaluation Questionnaire (LSEQ; Reference Parrott and HindmarchParrott & Hindmarch, 1978)) were performed at days 3 and 10 and week 8 of treatment. Patients kept a diary of sleep quality and number of awakenings for the first 21 days of treatment.
Sleep analysis
Sleep was scored automatically by the Medilog 9002 with visual correction by an experienced sleep scorer (J. A. H.) according to the Rechtshaffen & Kales (1968) criteria. The following parameters were derived from the sleep recordings. (a) Staging time — the interval between the patient pressing the event markers (when these were omitted, the sleep scorer judged these times from the EEG recording when the patient closed their eyes at night and when they opened them and started to move around in the morning). (b) Total sleep time (TST) — time in all stages of sleep. (c) Sleep efficiency — %TST/staging time. (d) Number of awakenings — these had to be greater than 16 s in duration. (e) Sleep onset latency — time from the patient pushing the button to start their night's sleep to the first 2 min of stage 2 sleep. (f) Duration of stage 1-4, and REM sleep and REM onset latency — the time to the first continuous 2 min of REM from the onset of stage 2 sleep. (g) Wakefulness after sleep onset — total time spent awake after sleep onset.
Efficacy of the antidepressant treatment was measured with the HRSD (Reference HamiltonHamilton, 1960), Montgomery—Åsberg Depression Rating Scale (MADRS; Reference Montgomery and ÅsbergMontgomery & Åsberg, 1979) and CGI Severity and Improvement scales (Reference GuyGuy, 1976). These were completed by the clinician at baseline, days 3 and 10, and at the end of weeks 3, 4, 6, 8, 16 and 24 (end of study) or at any point that a patient was prematurely withdrawn from the study.
Statistical analysis
Statistical analysis of objective sleep measures was carried out using STATA version 5.0 for Windows (STATA Corporation). Descriptive statistics were derived for each of the variables. Tests for normality showed that the variables stage 1, stage 3, sleep onset latency, REM onset latency and wakefulness after sleep onset were not normally distributed. The values for stage 1 sleep and sleep onset latency and wakefulness after sleep onset were normalised by logarithmic transformation, and those for stage 3 sleep, REM onset latency and wakefulness after sleep onset, by square root transformation. Complete data-sets (four sleep assessments) were available for 29 individuals and this resulted in an unbalanced design. Consequently, a sequential fitting of the different sum of squares was used. Split plot analysis of variance (split plot ANOVA) was used to investigate the effects of the two drugs on all the sleep variables. This analysis separates the variance ascribable to pre-treatment differences between the two treatment groups, resulting from the effect of time, the interaction between time and treatment and the effect of the treatments themselves.
Data from HRSD, MADRS and CGI severity and improvement scales were tabulated using both the observed values and with last observation carried forward (LOCF) in the whole (intent to treat; ITT) group. Number of responders (50% or more reduction on baseline HRSD) and remitters (HRSD ≤8) were tabulated for each treatment group. Total scores for the rating scales were analysed using ANOVA, first at baseline and on the change from baseline at the end of the specific weeks and end-point (subject's last available observation).
Descriptive statistics, comparison tests at days 3, 10 and week 8 and ANOVA were performed on the scores of LSEQ, the items 5, 6, 9-11 and 13 of SMHSQ and the sleep items of HRSD. Values from the daily diary of sleep quality were analysed with descriptive statistics and scrutinised for possible trends in the data. An average score for each week of the study was compared for the two groups.
Adverse events were cross-tabulated by treatment, severity and clinical estimate of relation to study medication, to detect any evidence of drug-related trends or increased incidence.
All statistical tests and analysis of subjective and mood measures were performed using the standard SPSS package (version 10.0 for Windows).
RESULTS
Participant recruitment and follow-up
Forty patients (23 females, 17 males) were randomised to nefazodone (n=20) or paroxetine (n=20). Demographic data and past psychiatric history data, including comorbidity, are presented in Table 1. There were no significant differences between the groups in these variables. The number of patients that completed the study and the reasons for early discontinuation are presented in Table 2.
Table 1 Baseline demographic and clinical data
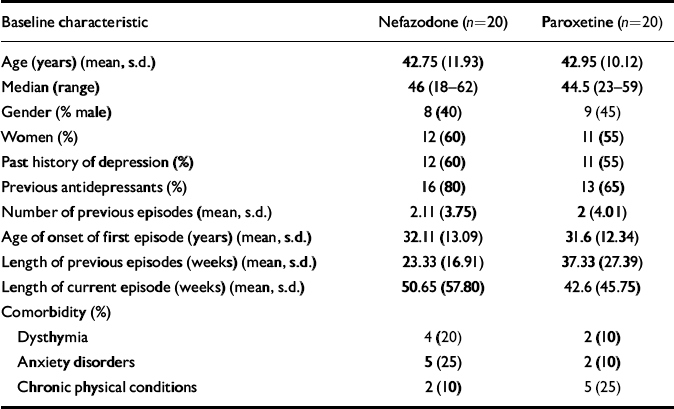
| Baseline characteristic | Nefazodone (n=20) | Paroxetine (n=20) |
|---|---|---|
| Age (years) (mean, s.d.) | 42.75 (11.93) | 42.95 (10.12) |
| Median (range) | 46 (18-62) | 44.5 (23-59) |
| Gender (% male) | 8 (40) | 9 (45) |
| Women (%) | 12 (60) | 11 (55) |
| Past history of depression (%) | 12 (60) | 11 (55) |
| Previous antidepressants (%) | 16 (80) | 13 (65) |
| Number of previous episodes (mean, s.d.) | 2.11 (3.75) | 2 (4.01) |
| Age of onset of first episode (years) (mean, s.d.) | 32.11 (13.09) | 31.6 (12.34) |
| Length of previous episodes (weeks) (mean, s.d.) | 23.33 (16.91) | 37.33 (27.39) |
| Length of current episode (weeks) (mean, s.d.) | 50.65 (57.80) | 42.6 (45.75) |
| Comorbidity (%) | ||
| Dysthymia | 4 (20) | 2 (10) |
| Anxiety disorders | 5 (25) | 2 (10) |
| Chronic physical conditions | 2 (10) | 5 (25) |
Table 2 Primary reasons for discontinuation
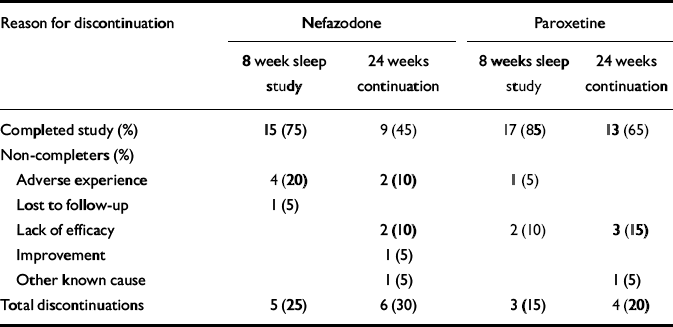
| Reason for discontinuation | Nefazodone | Paroxetine | ||
|---|---|---|---|---|
| 8 week sleep | 24 weeks | 8 weeks sleep | 24 weeks | |
| study | continuation | study | continuation | |
| Completed study (%) | 15 (75) | 9 (45) | 17 (85) | 13 (65) |
| Non-completers (%) | ||||
| Adverse experience | 4 (20) | 2 (10) | 1 (5) | |
| Lost to follow-up | 1 (5) | |||
| Lack of efficacy | 2 (10) | 2 (10) | 3 (15) | |
| Improvement | 1 (5) | |||
| Other known cause | 1 (5) | 1 (5) | ||
| Total discontinuations | 5 (25) | 6 (30) | 3 (15) | 4 (20) |
Patients excluded from the sleep analysis were: one patient who completed baseline only; one patient who completed baseline and day 3, but day 10 recording was technically unsatisfactory; and one patient whose baseline recording was technically unsatisfactory. One other patient's day 3 recording was technically unsatisfactory but baseline, day 10 and week 8 were included, and six other patients had no week 8 recording as they left the study.
The mean nefazodone dose (and standard deviation (s.d.)) used in the study was 495 mg/day (82.6) and for paroxetine it was 29.5 mg/day (8.9), well within the therapeutic range advised for these drugs in the treatment of depression.
Analysis of sleep
In the paroxetine group, four patients at day 3 and one patient at day 10 had no REM sleep at all. In these patients the REM latency was taken to be the staging time minus sleep onset latency.
Table 3 shows polysomnographic data of sleep parameters with results of ANOVA statistical analysis.
Table 3 Objective (EEG) sleep
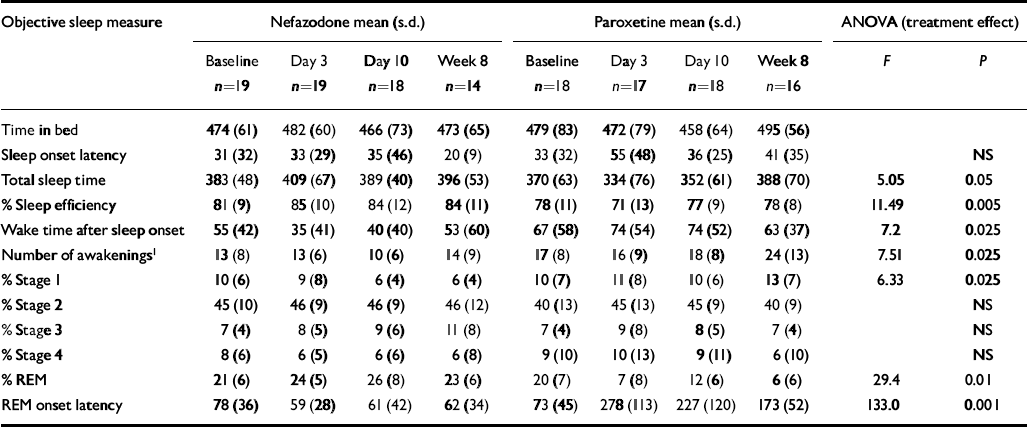
| Objective sleep measure | Nefazodone mean (s.d.) | Paroxetine mean (s.d.) | ANOVA (treatment effect) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Day 3 | Day 10 | Week 8 | Baseline | Day 3 | Day 10 | Week 8 | F | P | |
| n=19 | n=19 | n=18 | n=14 | n=18 | n=17 | n=18 | n=16 | |||
| Time in bed | 474 (61) | 482 (60) | 466 (73) | 473 (65) | 479 (83) | 472 (79) | 458 (64) | 495 (56) | ||
| Sleep onset latency | 31 (32) | 33 (29) | 35 (46) | 20 (9) | 33 (32) | 55 (48) | 36 (25) | 41 (35) | NS | |
| Total sleep time | 383 (48) | 409 (67) | 389 (40) | 396 (53) | 370 (63) | 334 (76) | 352 (61) | 388 (70) | 5.05 | 0.05 |
| % Sleep efficiency | 81 (9) | 85 (10) | 84 (12) | 84 (11) | 78 (11) | 71 (13) | 77 (9) | 78 (8) | 11.49 | 0.005 |
| Wake time after sleep onset | 55 (42) | 35 (41) | 40 (40) | 53 (60) | 67 (58) | 74 (54) | 74 (52) | 63 (37) | 7.2 | 0.025 |
| Number of awakenings1 | 13 (8) | 13 (6) | 10 (6) | 14 (9) | 17 (8) | 16 (9) | 18 (8) | 24 (13) | 7.51 | 0.025 |
| % Stage 1 | 10 (6) | 9 (8) | 6 (4) | 6 (4) | 10 (7) | 11 (8) | 10 (6) | 13 (7) | 6.33 | 0.025 |
| % Stage 2 | 45 (10) | 46 (9) | 46 (9) | 46 (12) | 40 (13) | 45 (13) | 45 (9) | 40 (9) | NS | |
| % Stage 3 | 7 (4) | 8 (5) | 9 (6) | 11 (8) | 7 (4) | 9 (8) | 8 (5) | 7 (4) | NS | |
| % Stage 4 | 8 (6) | 6 (5) | 6 (6) | 6 (8) | 9 (10) | 10 (13) | 9 (11) | 6 (10) | NS | |
| % REM | 21 (6) | 24 (5) | 26 (8) | 23 (6) | 20 (7) | 7 (8) | 12 (6) | 6 (6) | 29.4 | 0.01 |
| REM onset latency | 78 (36) | 59 (28) | 61 (42) | 62 (34) | 73 (45) | 278 (113) | 227 (120) | 173 (52) | 133.0 | 0.001 |
There were significant pre-treatment differences between the two treatment groups on nearly all the sleep measures, with sleep in the paroxetine group being generally worse. This occurred entirely by chance, as allocation was random. As described in the Method section, we separated this baseline difference from the treatment effects.
There were significant drug effects on total sleep time, sleep efficiency (see Fig. 1) and wakefulness after sleep onset, with nefazodone improving these and paroxetine worsening them early in treatment but both drug groups returning towards baseline by 8 weeks. Stage 1 sleep and number of awakenings also showed significant treatment effects and these were more obvious at 8 weeks, with both measures being increased in the paroxetine group (see Fig. 2). Number of awakenings showed a significant time × treatment effect, with the nefazodone group showing an early decrease and returning towards baseline at 8 weeks and the paroxetine group continuing to increase during treatment.
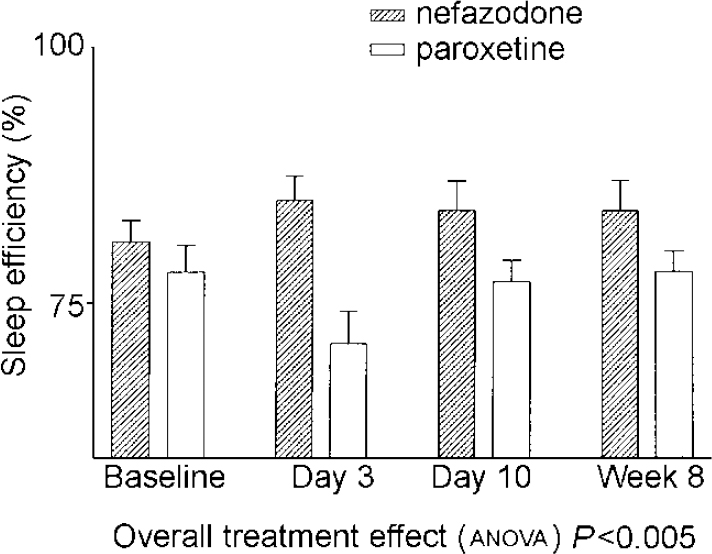
Fig. 1 Mean sleep efficiency. Error bars indicate s.e.m.
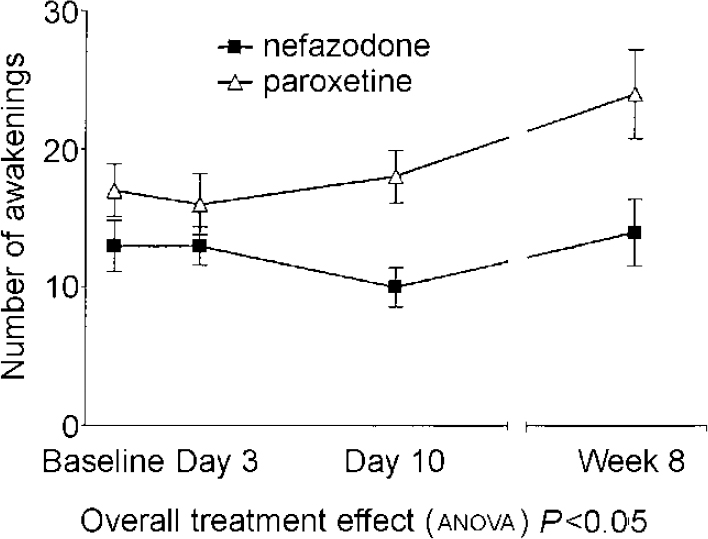
Fig. 2 Mean number of awakenings. Error bars indicate s.e.m.
There were highly significant treatment differences on REM sleep, with paroxetine increasing REM latency (see Fig. 3) and decreasing the amount of REM throughout the 8 weeks' treatment. The nefazodone group showed a slight increase in REM and decrease in REM latency. Neither slow wave sleep nor stage 2 sleep showed significant time or group differences.

Fig. 3 Mean REM latency. Error bars indicate s.e.m.
Subjective data from the SMHSQ were not distributed normally and there was a significant difference between the two treatment groups at baseline.
Changes from baseline scores were derived for all patients and these were found to be distributed normally and therefore used in subsequent testing. A significant difference was found at night 3 for sleep quality (item 5: how well did you sleep?) (T=2.12, d.f.=36, P=0.04) with the nefazodone group showing greater improvement from baseline. On the ANOVA there was a treatment effect for sleep quality (P=0.042) and for sleep depth (P=0.042) with the nefazodone group showing more improved scores on both (Table 4 and Fig. 4).
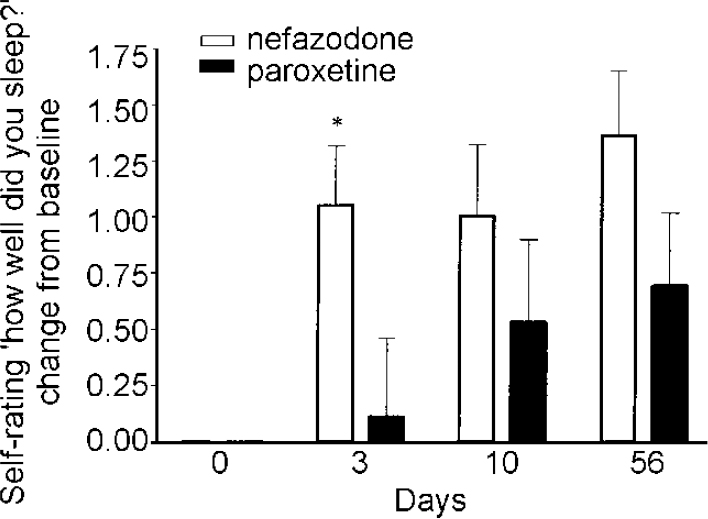
Fig. 4 Subjective sleep measures — change from baseline St Mary's Hospital Sleep Questionnaire Quality of sleep. Error bars indicate s.e.m. * P<0.05.
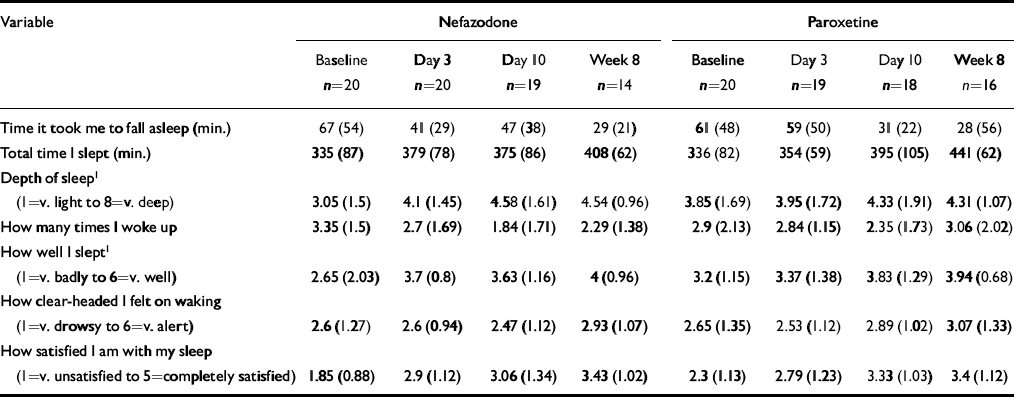
Table 4 Subjective sleep parameters (St Mary's Hospital Sleep Questionnaire). All data presented as mean (s.d.)
| Variable | Nefazodone | Paroxetine | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Day 3 | Day 10 | Week 8 | Baseline | Day 3 | Day 10 | Week 8 | |
| n=20 | n=20 | n=19 | n=14 | n=20 | n=19 | n=18 | n=16 | |
| Time it took me to fall asleep (min.) | 67 (54) | 41 (29) | 47 (38) | 29 (21) | 61 (48) | 59 (50) | 31 (22) | 28 (56) |
| Total time I slept (min.) | 335 (87) | 379 (78) | 375 (86) | 408 (62) | 336 (82) | 354 (59) | 395 (105) | 441 (62) |
| Depth of sleep1(1=v. light to 8=v. deep) | 3.05 (1.5) | 4.1 (1.45) | 4.58 (1.61) | 4.54 (0.96) | 3.85 (1.69) | 3.95 (1.72) | 4.33 (1.91) | 4.31 (1.07) |
| How many times I woke up | 3.35 (1.5) | 2.7 (1.69) | 1.84 (1.71) | 2.29 (1.38) | 2.9 (2.13) | 2.84 (1.15) | 2.35 (1.73) | 3.06 (2.02) |
| How well I slept1(1=v. badly to 6=v. well) | 2.65 (2.03) | 3.7 (0.8) | 3.63 (1.16) | 4 (0.96) | 3.2 (1.15) | 3.37 (1.38) | 3.83 (1.29) | 3.94 (0.68) |
| How clear-headed I felt on waking (1=v. drowsy to 6=v. alert) | 2.6 (1.27) | 2.6 (0.94) | 2.47 (1.12) | 2.93 (1.07) | 2.65 (1.35) | 2.53 (1.12) | 2.89 (1.02) | 3.07 (1.33) |
| How satisfied I am with my sleep (1=v. unsatisfied to 5=completely satisfied) | 1.85 (0.88) | 2.9 (1.12) | 3.06 (1.34) | 3.43 (1.02) | 2.3 (1.13) | 2.79 (1.23) | 3.33 (1.03) | 3.4 (1.12) |
There was no significant treatment effect on the variables of LSEQ but the factor ‘behaviour following wakening’ showed a trend for less clumsiness and tiredness in the morning with paroxetine and more with nefazodone. The differences from baseline, however, were small. The week-by-week analysis of the sleep diary averages for sleep quality (how well I slept) and continuity (how many times did you wake up?) and the HRSD sleep items were not significantly different in the two groups.
Analysis of antidepressant efficacy and safety
Response was defined as a 50% or greater reduction from the initial HRSD score, whereas remission was defined as a final HRSD score of 8 or less. There was no significant difference between the two medications in these variables. At week 8 (end of the sleep study), 11 patients on nefazodone and 16 patients on paroxetine were responding to treatment. At the end of the 24-week study, a total of 12 in the nefazodone group and 14 in the paroxetine group had responded to treatment, while 9 patients on nefazodone and 12 patients on paroxetine wer classified as remitters. Between 8 and 24 weeks, 2 patients in the paroxetine group had experienced a worsening.
There were no significant differences between the two drugs in HRSD, MADRS, CGI Severity and Improvement scales, either on observed data or on LOCF data. The data from HRSD scores (LOCF) are presented in Fig. 5. Data from the MADRS questionnaire, which has only one question about sleep, were as follows (observed cases): nefazodone group baseline 27.5 (s.d.=4.1), 8 weeks 13.0 (s.d.=7.7); paroxetine group baseline 27.1 (s.d.=3.5), 8 weeks 8.4 (s.d.=6.2).
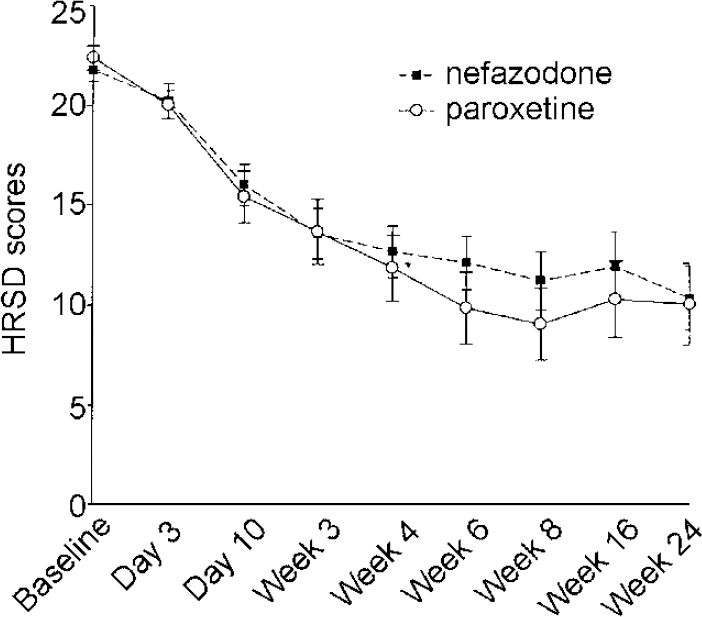
Fig. 5 Hamilton Rating Scale for Depression (HRSD) scores last observation carried forward, intention to treat. Error bars indicate s.e.m.
There were no serious adverse events related to either of the medications. One patient on paroxetine was hospitalised for worsening of her primary diagnosis of depression and emerging suicidal ideation, following the day 3 visit. The randomisation code was broken and the patient was continued on open-label paroxetine and made a good recovery. Table 5 shows the non-serious side-effects attributable to the medications. These were similar to those described in previous reports.
Table 5 Number of non-serious adverse events occurring in more than 5% of patients (% of patients reporting)
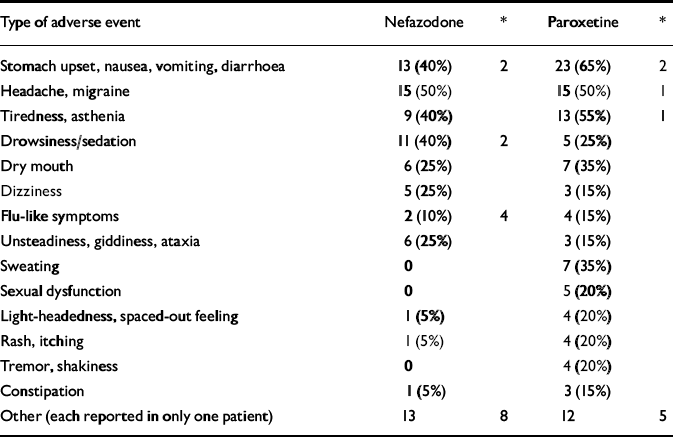
| Type of adverse event | Nefazodone | * | Paroxetine | * |
|---|---|---|---|---|
| Stomach upset, nausea, vomiting, diarrhoea | 13 (40%) | 2 | 23 (65%) | 2 |
| Headache, migraine | 15 (50%) | 15 (50%) | 1 | |
| Tiredness, asthenia | 9 (40%) | 13 (55%) | 1 | |
| Drowsiness/sedation | 11 (40%) | 2 | 5 (25%) | |
| Dry mouth | 6 (25%) | 7 (35%) | ||
| Dizziness | 5 (25%) | 3 (15%) | ||
| Flu-like symptoms | 2 (10%) | 4 | 4 (15%) | |
| Unsteadiness, giddiness, ataxia | 6 (25%) | 3 (15%) | ||
| Sweating | 0 | 7 (35%) | ||
| Sexual dysfunction | 0 | 5 (20%) | ||
| Light-headedness, spaced-out feeling | 1 (5%) | 4 (20%) | ||
| Rash, itching | 1 (5%) | 4 (20%) | ||
| Tremor, shakiness | 0 | 4 (20%) | ||
| Constipation | 1 (5%) | 3 (15%) | ||
| Other (each reported in only one patient) | 13 | 8 | 12 | 5 |
DISCUSSION
At baseline, the depressed patients in this study had disturbed sleep, as in other studies. Total sleep time and sleep efficiency were low, median REM onset latency was slightly short and stage 1 sleep was increased compared with normal sleep. Slow wave sleep, however, was higher in our study than in most sleep laboratory studies in depression, including that of Rush et al (Reference Rush, Armitage and Gillin1998) with nefazodone. This could reflect the use of home recordings for these patients, as we found similar baseline values in a previous study of patients with depression recorded at home. The two groups were different at baseline, with the paroxetine group having generally worse sleep. The key finding in this study was an improvement in sleep maintenance early in treatment in the nefazodone group, with sleep efficiency and total sleep time increasing and wake time decreasing, and the converse effect, i.e increased sleep disturbance, after paroxetine. This is in accordance with the previous US study (Reference Rush, Armitage and GillinRush et al, 1998) which compared nefazodone with fluoxetine. However, in contrast to that study, the differences at 8 weeks were much less marked. Only the number of awakenings and amount of stage 1 sleep were higher at this stage in the paroxetine than the nefazodone group and the sleep onset latency in the nefazodone group was shorter. It seems that paroxetine has a less disturbing effect than fluoxetine on sleep in long-term treatment, although our use of home recordings could contribute to this difference.
REM sleep suppression remains marked throughout treatment with paroxetine but nefazodone has, if anything, a small non-significant promoting effect on REM, as in other studies (Reference Sharpley, Walsh and CowenSharpley et al, 1992). This is different from the TCAs, which produce a similar and sometimes more marked sleep promotion early in treatment but also produce a marked suppression of REM sleep.
Subjective effects
In our study, both drugs were well tolerated and equally effective in treating depression. Patients taking nefazodone reported increased subjective sleep quality and increased subjective depth of sleep as early as day 3 of treatment. The difference between the two drugs was decreased as the treatment progressed and by the end of the study was not statistically significant. This could be explained by some early sleep-promoting effect of nefazodone, or a decrease of sleep disruption caused by the SSRIs as neuroadaptive changes take place in the brain with prolonged administration and depression improves. Another possible explanation is a change in the perception and/or reporting of sleep difficulties by depressed patients as their clinical status evolves. In a previous study with fluvoxamine (Reference Wilson, Bell and CouplandWilsonet al, 2000), subjective complaints about poor sleep were decreased when patients improved, in spite of lack of significant changes in objective measures of sleep (polysomnography).
Comparison with other studies
The SSRIs have become a first line treatment of depression over the past decade. They offer significant advantages compared with the old compounds (TCAs and monoamine oxidase inhibitors), such as fewer side-effects and non-lethality in overdose. However, some useful properties of the TCAs, such as the promotion of sleep, do not apply to SSRIs. Indeed the SSRIs can increase wakefulness, reduce total sleep time and sleep efficiency. Generally, they have an alerting effect in acute treatment, although sleep disruption can ease with long-term treatment (Reference Wilson, Bell and CouplandWilsonet al, 2000). This alerting effect sometimes results in the use of additional short-term treatment with a benzodiazepine or other hypnotic, with all the well-known problems associated with such a regime. Sleep problems are very prominent in depressive illness, with up to 95% of patients with moderate to severe depression suffering one or more problems with their sleep (Reference ThaseThase, 1999). Therefore, new antidepressants that do not cause further sleep disruption (unlike the SSRIs) and are safe in overdose (unlike the TCAs) could offer an important advantage, especially in patients with pronounced sleep difficulties.
Pharmacological mechanisms
The main action of nefazodone is to block post-synaptic 5-HT2 receptors (Reference Taylor, Carter and EisonTaylor et al, 1995). Evidence for the involvement of 5-HT2 receptors in depression comes from various areas of research. Although this relationship is complex and not yet very well understood, it appears that down-regulation of these receptors could be crucial for the ability of antidepressant drugs to exert their action (Reference Attar-Lévy, Martinot and BlinAttar-Lévyet al, 1999; Reference Yatham, Liddle and ShiaYathamet al, 2000), although this finding has not been replicated in all studies (Reference Massou, Trichard and Attar-LévyMassou et al, 1997). The effectiveness of nefazodone has been established both in the acute and the long-term treatment of depression in a number of randomised controlled trials (Reference Feiger, Bielski and BremnerFeiger et al, 1999; Reference Keller, McCullough and KleinKeller et al, 2000). The relationship of 5-HT neurotransmission and sleep is a complex one (Reference Sharpley, Idzikowski, Idzikowski and CowenSharpley & Idzikowski, 1991). SSRIs are sleep-disturbing early in treatment, presumably as a consequence of increased 5-HT function. Post-synaptic 5-HT2 blockade, by drugs such as ritanserin, has a promoting effect on deep non-REM sleep. Trazodone, an antidepressant which shares the 5-HT2-blocking property of nefazodone, is also sleep-promoting (Reference Mouret, Lemoine and MinuitMouret et al, 1988).
Clinical relevance
We compared the effect of nefazodone and an SSRI on sleep of out-patients with depression, with particular emphasis on the early stages of treatment. We considered that the onset of treatment is a crucial period. The patient's morale is at its lowest and the antidepressant has not yet exerted its effect, therefore the symptoms are at their peak. Further sleep disruption, such as that caused by the SSRIs, can lead either to disaffection with the treatment and early drop-out or poor compliance, negatively affecting the overall outcome, or it could require additional treatment with a hypnotic.
Another difference with the previous study was that patients were studied in their home environment. Patients studied overnight in a sleep laboratory need a period of adjustment to the unfamiliar surroundings and this only adds to the inconveniences already produced by the illness itself. As in our previous study, we found patients more likely to volunteer for the study once they knew that home recordings were involved.
Paroxetine was chosen as a comparator because it is the only SSRI for which sedative properties have been reported (Reference Kerr, Failweather and HindmarchKerr et al, 1997), as opposed to the previously studied fluoxetine. As well as home-based objective sleep assessment, we also used more extensive subjective sleep measures in an attempt to clarify the effect of the two drugs in a variety of sleep parameters. The (unusual for SSRIs) sedative properties of paroxetine in some patients, which could relate to its weak anti-cholinergic effects, could also account for the small differences seen in this study, compared with the clear superiority of nefazodone over fluoxetine in promoting sleep, as reported by Rush et al (Reference Rush, Armitage and Gillin1998).
We conclude that nefazodone is an effective and safe antidepressant that could be a preferable choice over the SSRIs in patients with depression who have prominent sleep problems.
Clinical Implications and Limitations
CLINICAL IMPLICATIONS
-
• Nefazodone significantly improved objective and subjective sleep quality early in treatment compared with paroxetine.
-
• Both drugs were well tolerated and seemed equally effective in treating depression.
-
• By 8 weeks, there was no difference in effects on sleep quality between the two drugs.
LIMITATIONS
-
• There was no placebo group.
-
• The study was powered for sleep measures, so was unable to detect clinically significant differences in antidepressant efficacy for the two antidepressants.
-
• The relationship between subjective and objective sleep is unclear.
Acknowledgements
The authors would like to thank the general practitioners and the staff of West View Road Surgery and Malago Surgery in Bristol for participating in this study and facilitating our work in every possible way.













eLetters
No eLetters have been published for this article.