Introduction
Eighty-five per cent of cases of SAH are due to aneurysm rupture. Reference Macdonald and Schweizer1 The morbidity, mortality and economic burden of aneurysmal subarachnoid haemorrhage (aSAH) continues to be considerable, with an estimated mean cost for hospitalisation of 82,514 USD per patient. Reference Modi, Shah, Schultz, Tahir, Affan and Varelas2 Half of the aSAH patients die within 1 month of the haemorrhage, and 40% of those who survive the first month remain dependent. Reference Natarajan, Sekhar, Ghodke, Britz, Bhagawati and Temkin3 Additionally, early survivors are at elevated risk for a rebleed and secondary complications such as delayed cerebral ischaemia and hydrocephalus. Reference Fraser, Riina, Mitra, Gobin, Simon and Stieg4 Timely treatment of ruptured intracranial aneurysms reduces the risk of rebleeding and long-term sequelae.
The two primary techniques of aneurysm repair include surgical clipping and endovascular coiling. The International Subarachnoid Aneurysm Trial (ISAT) concluded that for patients with ruptured aneurysms suitable for either treatment modality, endovascular coiling led to improved clinical outcomes at 1-year follow-up, as assessed by the modified Rankin scale (mRS). Reference Molyneux and Kerr5 As a result of ISAT and improvements in both coil technique and technology, coil embolisation has since become the preferred first-line treatment for most ruptured aneurysms in many centres, Reference Molyneux and Kerr5–Reference Bederson, Connolly and Batjer7 with post-ISAT studies showing greater usage of endovascular coiling regardless of rupture status. Reference Alshekhlee, Mehta and Edgell8,Reference Qureshi, Vazquez, Tariq, Suri, Lakshminarayan and Lanzino9
However, many critiques have been raised regarding the ISAT study and its narrow choice of participants, study protocol, lack of data on excluded patients and lower durability as well as higher retreatment rates for coiled versus clipped patients in longer term follow-up. Reference Molyneux, Birks, Clarke, Sneade and Kerr10–Reference Zhao, Rabinstein, Murad, Lanzino, Panni and Brinjikji12 Although there are studies that echo the results of ISAT, Reference Zhao, Tan and Yang13 more recent prospective data show no significant difference in clinical outcomes between the two techniques, Reference Spetzler, McDougall and Zabramski11 or an even greater mortality rates in coiled versus clipped poor-grade aSAH patients. Reference Zhao, Rabinstein, Murad, Lanzino, Panni and Brinjikji12 Although these smaller studies do not possess the degree of stringency of ISAT, they offer important data that potentially reflect the outcomes achievable in actual clinical practice as compared to the artificial environment of a randomised controlled trial (RCT).
We report the first cohort study in Canada using prospectively collected data to examine the treatment of ruptured intracranial aneurysms using surgical clipping and endovascular coiling techniques and associated patient outcomes over the past 15 years since the publication of ISAT at a quaternary care centre. We hypothesised that patients treated with endovascular coiling would have better clinical outcomes than those treated with craniotomy and clipping.
Methods
Study Population
Research ethics approval was received from the local health authority (REB:1014100). A review of prospectively collected data was performed on the cerebrovascular database consisting of records of all patients with spontaneous aSAH managed at a Canadian quaternary care centre from January 2002 to December 2017.
Exclusion Criteria
Patients with non-aneurysmal SAH, those who died before receiving treatment, or who received surgical clipping and endovascular coiling simultaneously were excluded from this study.
Treatment Decision and Protocols
Decision on the aneurysm treatment modality was made on a case-by-case basis based on clinical status, imaging, aneurysm dome/neck ratio, morphology and location. Cross-sectional imaging (CT angiography) was performed in all cases, with preoperative digital subtraction angiography obtained in cases where it was felt useful or necessary to inform treatment decisions and operative technique. Each case was reviewed with the cerebrovascular team consisting of a neurosurgeon and neurointerventionalist. Pre and post-operative management was performed according to the institutional standard of care for aSAH.
Aneurysm treatment was performed by or under the direct supervision of a specialist with extensive experience. Patients treated with surgical clipping underwent frontal, pterional or skull base approaches depending on the aneurysm location. Patients treated with endovascular coiling received optional balloon or stent assistance as required. Aneurysmal obliteration was confirmed using intra- and post-operative angiography.
All patients were admitted to the intensive care unit or neurosurgical intermediate care unit post-operatively for management of vasospasm, hydrocephalus or other complications under the supervision of a neurosurgeon. Follow-up was performed in an outpatient setting at 6 months and 12 months after the discharge date.
Data Definition and Collection
Baseline patient characteristics included age at admission, gender, current smoking status, hypertension, diabetes mellitus, previous aneurysm repair, prior stroke, time to treatment, number of aneurysms, aneurysm location, maximal diameter of culprit aneurysm (in mm), hospital stay (in days), admission scores (World Federation of Neurological Society [WFNS] score, Fisher scale Reference Fisher, Kistler and Davis14 ) and length of hospital stay (in days).
Age at admission was dichotomised into <50 years or ≥50 years. Admission scores were dichotomised into the following groups: WFNS score (grades 1–3 for good, 4–5 for bad) and Fisher score (grades 1–2 for good, 3–4 for bad). Aneurysm location was dichotomised into anterior or posterior circulation (Table 1), and size into <10 mm or ≥10 mm.
Table 1: Baseline patient characteristics
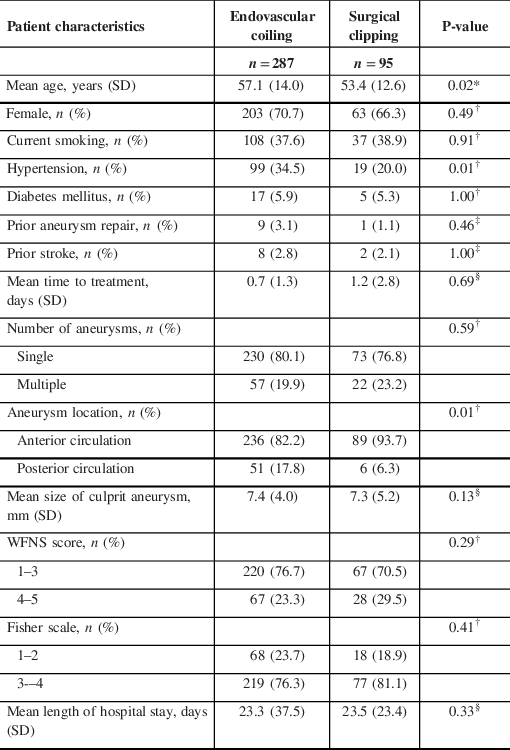
SD = standard deviation; WFNS = World Federation of Neurological Society.
* Welch’s t-test is used to compare the variables.
† Chi-squared test is used to compare the variables.
‡ Fisher’s exact test is used to compare the variables.
§ Wilcoxon rank-sum test is used to compare the variables.
The primary outcome was the Glasgow Outcome Scale (GOS) at discharge, 6 and 12 months’ follow-up after the discharge date, which was categorised into dead (GOS 1), dependent (GOS 2–3) or independent (GOS 4–5). Secondary outcomes included post-operative complications such as rebleed, vasospasm, infarction, hydrocephalus, seizure, infection and pneumonia.
Statistical Analysis
Baseline patient characteristics were reported as means with standard deviations or as counts with percentages, and then subsequently compared between the two treatment groups using Welch’s t-tests, Wilcoxon Rank sum, chi-squared and Fisher’s exact tests with a significance level of α = 0.05.
Univariate analyses of outcome variables were performed using chi-squared and Fisher’s exact tests. Crude and adjusted odds ratios with 95% confidence intervals were calculated using an ordinal logistic regression model for GOS at discharge, 6 months and 12 months’ follow-up. Models were adjusted for age at admission, hypertension and aneurysm location using multivariable ordinal logistic regression. Interaction between age at admission and hypertension was also assessed in the models using an interaction term. The proportional odds assumption of the final adjusted model was assessed using the Brant test, model fit was assessed with Hosmer and Lemeshow, Lipsitz goodness of fit and Pulkstenis–Robinson chi-squared and deviance tests and model performance was assessed using the misclassification error. Crude and adjusted odds ratios with 95% confidence intervals were calculated using binary logistic regression models for secondary outcomes. Model fit and predictive ability were assessed with the Hosmer–Lemeshow test and receiver operating characteristic curves, respectively. To evaluate for potential differences in trends in patient demographics, clipping or coiling treatment and treatment outcomes over time, additional subgroup analysis was also performed by grouping patients into three time periods by admission date: 2002–2007, 2008–2012 and 2013–2017. All statistical analyses were performed with R version 3.6.1.
Results
Patient Characteristics
A total of 577 patients were treated with coiling or clipping during this time period, and 382 had 12-month follow-up data available (Figure 1), of whom 287 patients received coiling and 95 patients received clipping (Table 2). The mean age of clipped patients was significantly younger than coiled patients (53.4 ± 12.6 vs. 57.1 ± 14.0 years, p = 0.02), and hypertension was significantly more common in those who were coiled versus clipped (99/287 [34.5%] vs. 19/95 [20.0%], p = 0.01). A greater proportion of coiled aneurysms were located in the posterior circulation compared to clipped aneurysms (51/287 [17.8%] vs. 6/95 [6.3%], p = 0.01). There were no significant differences in gender, current smoking status, diabetes mellitus, prior aneurysm repair, prior stroke, mean time to treatment, number of aneurysms, mean size of culprit aneurysm, WFNS score, Fisher score or mean length of hospital stay between the coiling and clipping groups. No patients experienced a rebleed in the time period between admission and treatment. Subgroup analysis of mean age by time period showed that in 2008–2012, clipped patients were significantly younger than coiled patients (52.1 ± 10.7 vs. 56.7 ± 15.0 years, p = 0.04). There was no significant difference in age between the two treatment groups in 2002–2007 and 2013–2017.

Figure 1: Patient flow diagram.
Table 2: Distribution of culprit aneurysms by location

* These aneurysms are classified here as anterior circulation due to their anatomical location on the ICA, despite their embryological origin.
Univariate Analysis of GOS and Secondary Outcomes
There were no significant differences in GOS between coiling and clipping patients at discharge, 6 months or 12 months’ follow-up (Table 3). There was a moderate improvement in GOS from dependent to independent between discharge and 6 months in both groups, but virtually no change in GOS from 6 months to 12 months. Additionally, no significant differences in post-surgical complications were seen between the two groups in terms of rebleed, vasospasm, infarct, hydrocephalus, seizure, infection and pneumonia.
Table 3: Univariate analysis of Glasgow Outcome Scale and secondary outcomes associated with surgical clipping compared to endovascular coiling
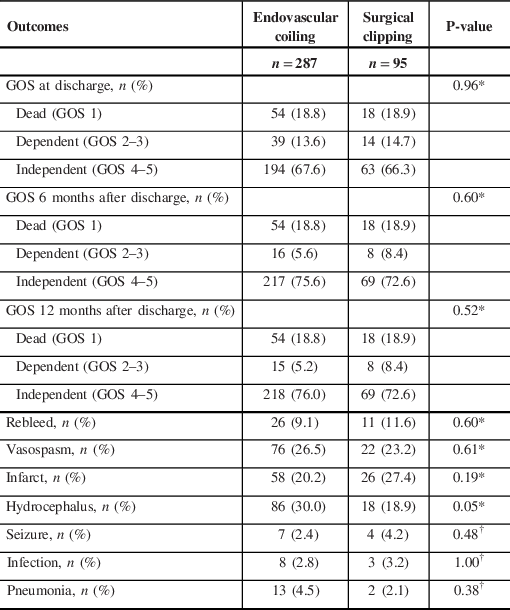
GOS = Glasgow Outcome Scale.
* Chi-squared test is used to compare the variables.
† Fisher’s exact test is used to compare the variables.
Multivariable Analyses of GOS and Secondary Outcomes
In the overall multivariable analysis and analysis by time period, patients who were treated with coiling showed no significant difference in odds of having a favourable GOS compared to patients who were clipped at any of the time points: discharge, 6 months or 12 months’ follow-up (Table 4). Patient’s age >50 years had lower odds of having a favourable GOS at all time points. Patients with hypertension had lower odds of having a favourable GOS at discharge, however, this relationship lost significance after adjusting for treatment type, age at admission and aneurysm location (Adjusted OR 0.66 [95% CI 0.42–1.04], p = 0.07). There was no evidence of significant interaction between age and hypertension in the regression model at all time points. Adequate model fitting and performance were demonstrated for GOS at all time points and the proportional odds assumption was satisfied for all three models.
Table 4: Ordinal regression analysis of Glasgow Outcome Scale

CI = confidence interval; OR = odds ratio.
* Models were adjusted for all other covariates in the table within their respective follow-up time points.
Regarding secondary outcomes, patients who were treated with coiling had lower odds of experiencing infarction at discharge compared to patients who were clipped after adjusting for age, hypertension and aneurysm location (Adjusted OR 0.52 [95% CI 0.29–0.92], p = 0.02). Patients who were treated with coiling had greater odds of experiencing hydrocephalus compared to patients who were clipped, but this relationship lost significance after adjustment for age at admission, hypertension and aneurysm location (Adjusted OR 1.62 [95% CI 0.92–2.99], p = 0.11) (Table 5). There were no significant differences in the other secondary outcomes (rebleed, vasospasm, seizure, infection, pneumonia) between clipped versus coiled patients.
Table 5: Regression analysis of post-operative secondary outcomes

CI = confidence interval; OR = odds ratio.
* Models were adjusted for age at admission, hypertension and aneurysm location.
Discussion
Our study compared surgical clipping versus endovascular coiling and subsequent patient outcomes at discharge, 6 months and 12 months over a 15-year post-ISAT period at a Canadian quaternary centre. There was no significant difference in GOS at any of the follow-up time points between the two treatment methods. This is in contrast with ISAT, which demonstrated a 22.6% (95% CI 8.9–34.2%) relative risk reduction for being dead or dependent in coiled versus clipped patients at 12 months based on the mRS. Reference Molyneux and Kerr5 Although different measures of patient outcome were used between our study and ISAT, comparison can still be made as both studies grouped outcomes to look at the likelihood of being independent versus dead or dependent. The most recent data from the Barrow Ruptured Aneurysm Trial favoured coiling on short-term follow-up, but showed no significant difference in risk of death or poor outcome (defined as mRS > 2) at 10 years follow-up between patients who were coiled or clipped. Reference Spetzler, McDougall and Zabramski11 Although a comparison cannot be made between our results and this recent trial due to a difference in follow-up time points, this trial along with a number of other trials, Reference Li, Wang, Chen and Quan15,Reference Koivisto, Vanninen, Hurskainen, Saari, Hernesniemi and Vapalahti16 meta-analysis, Reference Xia, Liu and Wang17 single-centred Reference Taha, Nakahara and Higashi18–Reference Steklacova, Bradac, Charvat, De Lacy and Benes25 and multicentred studies Reference Zhao, Tan and Yang13,Reference Wang, Song and Gao26 continue to echo a lack of difference in patient outcomes between the two treatment modalities at various follow-up time points.
Although we cannot definitively conclude that clipping is non-inferior to coiling for patients with aSAH from the results of our study alone, our findings combined with those emerging in literature post-ISAT suggest that surgical clipping remains an important treatment option for patients with aSAH that would not have met the equipoise criteria for ISAT. Further research is required to examine the benefits of clipping versus coiling for patients who do not meet the inclusion criteria for ISAT. The ongoing ISAT-2 study aims to further elucidate this by including the subgroup of aneurysms that were not well represented in the original ISAT study. Reference Darsaut, Roy and Weill27
Our results suggest that clipping remains a potentially effective and important treatment option compared to coiling with respect to patient outcomes at 6–12 months post-treatment in real-world conditions despite a clear decline in clipping for ruptured intracranial aneurysm repair since the ISAT study was published. Reference Grasso, Alafaci and Macdonald28 This may be attributable to improvements in the clipping procedure, post-surgical care and/or improved patient allocation to treatment modality. Reference Brinjikji, Lanzino, Rabinstein, Kallmes and Cloft29,Reference Hwang, Shin, Lee and Koh30 Furthermore, the prognosis of aSAH may be more importantly determined by patient comorbidities and aneurysm characteristics as opposed to treatment modality. Reference Connolly, Rabinstein and Carhuapoma31 Our results suggest that aSAH patients who receive either treatment experience the greatest chance of improving from dependence to independence during the 6 months post-treatment, with recovery to independence beyond this time frame being unlikely, which may have implications for clinical decision-making and understanding of the natural recovery progression of aSAH.
Additionally, our results showed that patients who were 50 years or older were less likely to have favourable outcomes at discharge, 6 months and 12 months independent of the effects of hypertension, which is consistent with post hoc analyses of ISAT and a number of other studies. Reference Mitchell, Kerr, Mendelow and Molyneux32–Reference Lanzino, Kassell and Germanson34 Patients with advanced age tend to present with poorer status and more comorbidities, contributing to less favourable outcomes. Reference van Donkelaar, Bakker and Birks35 Our study also demonstrated a statistically significant reduction in odds of experiencing an ischaemic infarct for coiled versus clipped patients independent of age at admission, hypertension and aneurysm location. Literature shows mixed conclusions, with some showing no association Reference Dumont, Crowley and Monteith36,Reference Li, Pan and Wang37 and others echoing our results. Reference Li, Wang, Chen and Quan15 Finally, we observed significantly greater odds for coiled patients to develop hydrocephalus, which lost significance after adjustment. This observation was likely confounded, as the coiled group had older mean age and greater proportion of posterior circulation aneurysms. These are both poor prognostic factors for clipping and confer greater risk for developing hydrocephalus, hence the loss of significance after statistical adjustment.
Finally, there was a significant allocation of both anterior and posterior circulation aneurysms to the coiling group in our study, reflective of the post-ISAT era and in contrast to ISAT. We adjusted for the potential effects of aneurysm location in our analysis and found there continued to be no significant difference in patient outcomes between the two treatment modalities, independent of aneurysm location, at any of the follow-up time points.
This study represents the first prospective cohort study to examine the effect of clipping versus coiling on patient outcomes over a 15-year post-ISAT period at a Canadian quaternary centre. It has the advantage of prospective, rigorous data collection and reflects Canadian practice patterns.
However, it was subject to several limitations. In the earlier years of the study period, a significant proportion of patients were referred from other provinces due to the scarcity of interventional resources. This led to a sizeable loss to follow-up from 577 to 382 patients as they were sent back to their home province upon recovery. Our sample size may have limited statistical power and the ability to potentially reach statistical significance for the observed trends.
With 40–50 cases of SAH per year, this represents the experience of a medium–small volume centre. Over the 15-year period, care was delivered by more than one provider in each treatment arm, further reducing the number of cases per provider. This may limit the applicability of our results to current procedural experiences at other institutions. However, while a full discussion of case volume and procedural competence is outside the scope of this paper, we believe our results may be relevant to the many centres in the small-to-medium volume range. Treating physicians were fellowship-trained in large centres with significant case volumes, and annual case volumes were within ranges described for maintenance of competence and reduction of complications. Reference Rush, Romano, Ashkanani, McDermid and Celi38
mRs is thought to be a more sensitive measure of patient outcome than GOS, Reference Tong, Eyngorn, Mlynash, Albers and Hirsch39 and was not incorporated in the data collection protocol until recent years. The mRS requires a structured interview and completion of training by the assessor in order to ensure inter-rater reliability. It is frequently used in clinical trials, but is rarely assessed for all patients in most practicing clinical groups. Given all this, it precluded us from using the scale in the current 15-year study.
We included both anteriorly and posteriorly located aneurysms to ensure the reported outcomes most accurately represented a real-world clinical setting. Although RCTs offer much more robust control for potential confounders, there is still great value derived from this cohort as it presents real-world results for both treatment modalities that were achievable by a multidisciplinary group working in a post-ISAT period.
Despite efforts to adjust for baseline characteristic differences, variables not collected in our study may have influenced the results. The lack of randomisation and locations of aneurysms present in this cohort naturally promoted mutual exclusivity of patients in the treatment modality decision-making process, which may have limited the comparability of the two treatment groups due to the absence of intervention-equipoise. Generalisability of the results may be limited as this was a single-centred study, and a much greater proportion of our cohort was treated through coiling versus clipping. Finally, it represents real-world clinical practice, in which there was no randomisation, and so there is evidently selection bias. However, it does demonstrate that such selection, when done by subspecialty experts, does not lead to inferior patient outcomes in patients treated with surgical clipping.
Conclusion
Our analysis showed no significant difference in clinical outcomes between aSAH patients who were clipped versus coiled at discharge, 6 months and 12 months’ follow-up. Overall in both clipping and coiling groups, patient recovery to independence was observed from discharge to 6 months, with no further recovery seen from 6 to 12 months’ follow-up. In both groups, patient’s age over 50 years decreased the likelihood of recovery to independence. These findings suggest that clipping remains an effective and important treatment option for aSAH compared to coiling when decisions are made by an expert multidisciplinary cerebrovascular team, and can help inform the shared decision-making process between physicians, patients and families. Further research is required to examine the benefits of clipping versus coiling for patients who do not meet the inclusion criteria for ISAT.
Acknowledgements
We acknowledge the imaging technologists and operating room/interventional nurses, without whom this work would not be possible.
Funding
Mr. Alubankudi was supported with a summer research studentship from the Imhotep Legacy Academy (Dalhousie University, Halifax, Canada).
Conflict of Interest
There are no conflicts of interest from any authors of this manuscript, financial or otherwise.
Institutional Review Board Approval
Research ethics approval was granted by the Nova Scotia Health Authority (REB:1014100).
Statement of Authorship
Study design: GEP, JJ, RA, ADW, TJH. Data collection: RA, ADW, JJ, GEP. Data analysis and interpretation: ADW, GEP, AAD, TJH. Drafting the article: ADW, RA, GEP. Critical revision of the article: AAD, GEP, TJH.








