Introduction
Invasive alien species have long been recognized as a major cause of extinctions on islands, with rodents being among the most destructive animals in this regard (Jones et al., Reference Jones, Tershy, Zavaleta, Croll, Keitt, Finkelstein and Howald2008). Over recent decades efforts to eradicate rodents from islands have increased in number, scale and rate of success. Pioneered largely in the New Zealand archipelago, where the need was urgent and extreme (Towns & Broome, Reference Towns and Broome2003), but then implemented more widely, rodent eradications increased in scale from 0.01 km2 (Maria Island) in the early 1960s (Moors, Reference Moors1985) through 113 km2 (Campbell Island) in 2001 (McClelland & Tyree, Reference McClelland and Tyree2002) to 128 km2 (Macquarie Island) in 2011 (Springer, Reference Springer2016). In each case, operations were informed by lessons learned and experience gained from earlier eradications.
The island of South Georgia, an Overseas Territory of the UK, has long been known to host damaging alien species introduced by successive waves of human visitors over more than 2 centuries. Formally discovered and named by Captain Cook in 1775, the wealth of wildlife on land and offshore, sustained by the highly biologically productive waters on and beyond its shelf, attracted sealers, then whalers, then fishers. Visiting ships often carried stowaway rats and mice, which were accidentally introduced to many sites along South Georgia's long and indented coastline. Brown rats Rattus norvegicus and house mice Mus musculus persisted into the 21st century. It is likely that black rats Rattus rattus were also introduced but were unable to survive the hostile, cold climate.
The impact of rodents, especially rats, on South Georgia's native wildlife was obvious to anyone visiting both the main island and any of the vegetated rodent-free offshore islands in recent decades. The endemic South Georgia pipit Anthus antarcticus and many burrow/cavity-nesting seabirds were unable to breed successfully in the presence of rats, and nested only on offshore islands. Some prions Pachyptila spp. and diving petrels Pelecanoides spp. persisted at high elevations on the main island, although even there they were not safe from predation. Rats crossed large areas of barren scree, nesting at elevations of up to 500 m (S. Poncet, Island LandCare, Falkland Islands, pers. comm.).
Despite the damage caused by rodents to South Georgia's native wildlife having been recognized for decades, until the end of the 20th century there seemed to be no realistic prospect of controlling their numbers, let alone eradicating them entirely. Although the number and size of islands successfully cleared of rodents globally was increasing, by 1990 the largest attempted eradication was in an area of only 170 ha (Cromarty et al., Reference Cromarty, Broome, Cox, Empson, Hutchinson, McFadden, Veitch and Clout2002), < 0.2% of the land area requiring treatment on South Georgia. However, a breakthrough was made in 2001, when the 11,300 ha Campbell Island was successfully cleared of brown rats (McClelland & Tyree, Reference McClelland and Tyree2002). Not only was this eradication larger than any of its predecessors, but the island was, like South Georgia, remote and in the Subantarctic.
A small-scale but important rodent eradication operation was carried out on 30 ha Grass Island, in South Georgia's Stromness Bay, in 2000 (Poncet et al., Reference Poncet, Poncet, Poncet, Christie, Dockrill, Brown, Veitch, Clout and Towns2011). This project demonstrated that the second-generation rodenticide Brodifacoum could be deployed successfully to destroy R. norvegicus in summer, and indicated that an operation across all parts of South Georgia harbouring rodents could be feasible.
The largest rodent eradication operations, including that on Campbell Island, had hitherto been funded and carried out by governments or large NGOs. The Government of South Georgia and the South Sandwich Islands identified the eradication of rats as an objective (McIntosh & Walton, Reference McIntosh and Walton2000) but did not have the capacity in 2007 to take on such a huge challenge (Christie & Brown, Reference Christie and Brown2007). Around that time the newly formed South Georgia Heritage Trust, a small, independent UK charity, decided to raise funds for a rodent eradication campaign and subsequently assumed responsibility for managing the operation.
The only methodology offering any chance of success on an island with the land area and topography of South Georgia was aerial (helicopter) spreading of bait that contained a rodenticide with a proven capacity to eradicate rodents. This technique had been successful on Campbell Island and was the standard methodology for islands unsuitable for ground baiting methods (Broome et al., Reference Broome, Cox, Golding, Cromarty, Bell and McClelland2014). Although it provided a plausible means of clearing rodents from South Georgia, aerial bait spreading held risks for non-target wildlife and perhaps even for the people living on the island. Firstly, the spreading of a toxin could potentially poison native wildlife and, if it got into potable water supplies, people too. Secondly, aircraft flying over bird concentrations have the potential to cause substantial damage, including the loss of eggs or young, and even mass mortality (Rounsevell & Binns, Reference Rounsevell and Binns1991; Cooper et al., Reference Cooper, Avenant and Lafite1994; Harris, Reference Harris2005; Hughes et al., Reference Hughes, Waluda, Stone, Ridout and Shears2008). South Georgia has hundreds of colonies of albatrosses, giant petrels and penguins, the majority of which would have to be over-flown by bait-spreading helicopters because they are in areas that contained rats. Operational success would therefore be dependent on three factors: the killing of every target animal, the well-being of people living on the island, and wildlife mortality being kept sufficiently low to ensure recovery was possible at the population level.
Rats were far more widespread and probably more destructive than mice on South Georgia, but mice are known to cause substantial damage to seabird populations elsewhere (Wanless et al., Reference Wanless, Angel, Cuthbert, Hilton and Ryan2007), and may also have been doing so on South Georgia. Therefore, the decision was made to attempt to eradicate mice while the infrastructure was in place to eradicate the higher priority rats.
Operational area
South Georgia is a crescent-shaped island, 170 km long and typically 25–36 km wide, with a planar land area of 3,528 km2. The terrain is mountainous, with 11 peaks > 2,000 m (highest 2,934 m). At latitude 54°S, South Georgia is south of the Antarctic Convergence and has a Subantarctic climate. Snow is possible on any day of the year, and storms are frequent. Over half of the island is covered by permanent ice and snow. Many glaciers flow from the ice-cap to the north and south coasts, although most are receding rapidly as a result of climate change (Cook et al., Reference Cook, Poncet, Cooper, Herbert and Christie2010). Glaciers that end in the sea form total barriers to rodents. They effectively divide the island into many independent land areas, most with their own population of rats or, in two cases amounting to 49 km2, mice. No areas of land were known to host both rats and mice in recent times.
Almost all terrain not under permanent ice and snow was occupied by rodents at the beginning of the 21st century, amounting to c. 1,080 km2. Only a narrow ice-free strip of land along the southern end of the south coast was free of rodents (Fig. 1), probably because conditions are too inhospitable for them to survive there. Little wildlife occurs in this area; even low-lying land is snow-covered until well into summer, leaving insufficient time for burrow-nesting birds to breed before the onset of winter. Overall, rodents occupied c. 30% of the land area of the main island of South Georgia, and all of the terrain favoured by native wildlife.
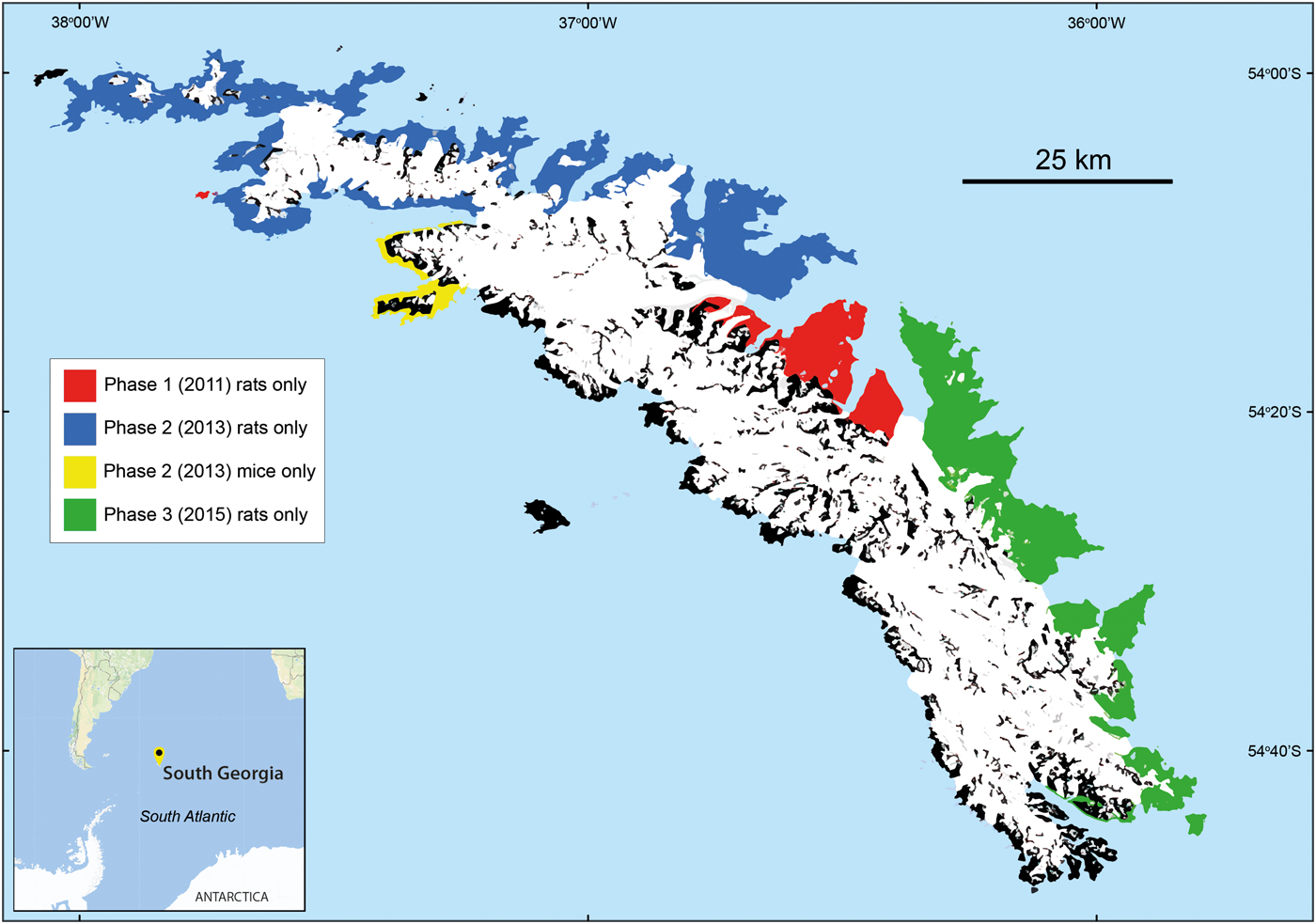
Fig. 1 The location of baiting zones treated during the 3-season operation to eradicate rodents from the island of South Georgia. The Phase 1 area (128 km2) was baited in early 2011, Phase 2 (580 km2) in 2013 and Phase 3 (360 km2) in early 2015. Mice Mus musculus occurred only in two adjacent zones, where there were no rats Rattus norvegicus. All other zones were occupied by rats, with no mice. The black-shaded area was not occupied by rodents. The interior is mostly covered with permanent snow and ice (white), with no rodents present.
Methods
Operational strategy
The scale of the task on South Georgia, requiring bait to be spread over an area greater by an order of magnitude than that of the largest island tackled to date, was such that it could not be completed in a single season, as had all previous rodent eradications. Glaciers on South Georgia provided the opportunity for the eradication to be spread over several seasons. If the entire land area between two glaciers could be cleared of rodents in a season, those glaciers would prevent rats or mice reinvading. The ability to spread the work across years also provided the crucial opportunity to test whether the methodology was successful before continuing. The work was carried out over three seasons, each separated by 2 years. A trial (Phase 1) took place in 2011 and covered 128 km2 of the central north coast, centred on the island's main base, at King Edward Point. Phase 2 (2013) treated the north and west, an area of 580 km2, including the two adjacent baiting zones that were inhabited by mice. Phase 3 (2015) covered the south-eastern part of South Georgia, sowing bait over 360 km2 (Fig. 1). Spreading the work over three seasons had the advantage of not putting all non-target fauna (birds) at risk in any one season, and allowing time for fund raising to continue ahead of successive phases.
The aim was to eradicate rodent populations, not control them, and this ruled out some methodologies from the outset. Two operational objectives had to be met: (1) a high probability of killing every rodent on the island, from coast to mountaintop, in caves and man-made structures, no matter the weather or the degree of snow cover, and (2) no unsustainable damage to non-target species.
Rodent eradications are normally carried out in winter, when population size is low and natural food resources are minimal, rendering bait more attractive (Broome et al., Reference Broome, Cox, Golding, Cromarty, Bell and McClelland2014). Winters on South Georgia, however, carry a high risk of deep snow, which would increase the probability of operational failure because of rodents being prevented from encountering bait. Baiting in summer was not favoured, for two reasons. Firstly, the number of birds at risk of poisoning would be high because of the influx of breeding season migrants. Secondly, rodents are both more abundant and in the process of breeding, the latter possibly leading to some offspring surviving. Those near weaning when bait is spread could start foraging independently after all pellets in the local area had been consumed. A compromise between these opposing pressures was adopted, whereby baiting commenced in late summer and was planned to continue into autumn, although it finished in winter in Phase 2 because of prolonged bad weather. Bait-laying dates were 1 March–26 March 2011 (Phase 1), 4 March–18 May 2013 (Phase 2) and 13 February–23 March 2015 (Phase 3).
Bait and baiting
The multi-year nature of the operation rendered purchase of the baiting helicopters more economical than leasing. Two aircraft were purchased initially, and a third added prior to Phase 2. All were of the same model, the Bölkow 105, a light, twin-turbine helicopter capable of operating in extreme gusty conditions and lifting an underslung load of 500 kg. Bait pellets in loads of 295 kg (Phase 1) and 363 kg (Phases 2 and 3) were delivered from a stainless steel bucket (HeliOtago Ltd, New Zealand) using a mechanical spinner. This provided a baiting swath of 80 m (Phases 1 and 2) or 105 m (Phase 3). Hand baiting was carried out only in areas and spaces inaccessible to aerial baiting, such as the island's abandoned whaling stations.
The bait selected was manufactured by Bell Laboratories Inc., Wisconsin, USA. It comprised hard, extruded cereal pellets containing 25 ppm Brodifacoum (rats) and 50 ppm (mice). Brodifacoum is a second-generation anticoagulant that is normally fatal after one feed (Eason et al., Reference Eason, Murphy, Wright and Spurr2002). A single intact pellet (13 mm diameter, 25 mm long, 3 g) would usually kill a rat. Mouse pellets were smaller (10 mm diameter, 15 mm long, 1 g). Brodifacoum is not water soluble, and consequently poses little risk of harmful contamination of drinking water or of leaching into soils (DOC, 2007). Because of the scale of the task, a repeat treatment some days after the first, as recommended by Broome et al. (Reference Broome, Cox, Golding, Cromarty, Bell and McClelland2014), was not feasible on South Georgia. The single-treatment approach had worked successfully on Campbell Island (McClelland & Tyree, Reference McClelland and Tyree2002).
Rat baiting strategy
Given expected concentrations of rats in vegetated areas at low elevations, where most food and shelter are available, a greater density of bait was spread in this habitat than elsewhere. Bait was spread in three passes. One pass delivered a low density of bait over all ice-free land, with no overlap between adjacent swaths. A second delivered a higher density of bait over all vegetated land, each swath overlapping the adjacent one to reduce the risk of gaps in coverage. A third pass delivered bait to a narrow coastal swath. In this way, the coastal margins received three independent bait sowings, cumulatively providing the target density and ensuring no gaps in coverage.
For the Phase 1 trial operation a bait density of 6.5 kg ha−1 was adopted for vegetated areas, and 2 kg ha−1 for unvegetated areas, informed by the successful operation on Campbell Island (McClelland & Tyree, Reference McClelland and Tyree2002) and local knowledge on South Georgia that rat density in unvegetated (higher elevation) terrain was lower than in vegetated areas. For Phases 2 and 3, in light of Phase 1 experience, bait density was reduced to 5 and 1.5 kg ha−1, respectively, with an increased overlap (25 m) between adjacent swaths over vegetation. The coastal swath added a further 7 kg ha−1 along a 40 m wide strip adjacent to water.
Mouse baiting strategy
A single application of 10 kg ha−1 was made over all areas that were not bare rock or ice, with a 60% overlap between swaths. Steep slopes and cliffs were treated with an additional 5 kg ha−1 (planar area). A coastal swath was also sown at 3 kg ha−1.
Non-target mortality
The poisoning of some non-target fauna is unavoidable in a bait-spreading operation of this scale, especially when predators and scavengers are present and likely to consume dead and dying rodents, thereby themselves becoming vulnerable to the toxin (secondary poisoning). In anticipation of this, South Georgia Heritage Trust established an international panel of experts to assess risks to birds and advise on appropriate mitigation measures (South Georgia Heritage Trust Advisory Panel on Non-target Mortality, 2011). Following Phase 1 baiting, concern focused mainly on brown skuas Stercorarius antarcticus. The Panel proposed a specific mitigation protocol for Phase 2, encouraging the early migration of breeding adults by oiling eggs to prevent them hatching, and this was adopted on Bird Island, at the western end of South Georgia, where 40% of South Georgia's skuas breed. The measure would cause little population damage in this long-lived seabird, yet offered a high probability of protecting the breeding adults from poisoning. Bird Island was rodent free and would not be baited, but skuas from this breeding concentration routinely foraged on the main island (Phillips et al., Reference Phillips, Catry, Silk, Bearhop, McGill, Afanasyev and Strange2007).
The bird with the greatest apparent risk of significant population-level mortality from poisoning was the endemic South Georgia pintail Anas georgica georgica. To estimate the rate of fatal poisoning of this taxon, 20 VHF transmitters with unique frequencies were attached to the legs of adults early in the Phase 1 operation, and another 20 during Phase 2. Transmitter signals were subsequently sought on foot and by helicopter, using a receiver and yagi antenna, to relocate birds, whether dead or alive. This information was required to assess whether baiting could continue in future seasons without jeopardizing the entire island population.
Detecting any surviving rodents
A monitoring plan was developed for each phase of baiting. Part of the Phase 1 area is occupied by the island's main base and administrative centre, and staff from the base travel to other parts of the area regularly, year-round. Rodent traps were set permanently at sites within and around the base. Waxtags (devices designed to attract rodents and retain evidence of gnawing; Pest Control Research, New Zealand) were widely deployed in the months following baiting.
A year after baiting, 815 inert detection devices (waxtags, chew sticks impregnated with vegetable oil, chew boards impregnated with peanut butter, tracking tunnels and trail cameras) were deployed at 13 coastal sites throughout the Phase 2 rat-occupied areas and revisited 13–25 days later. Many were also checked 2 years after baiting.
One year after baiting the mouse zones in Phase 2, 146 inert detection devices were deployed in two localities and checked 13 days later.
Results
Bait uptake and mortality of target species
Phase 1 (rats only)
During the night following the first (low density) sowing of bait, most pellets disappeared. Nocturnal filming demonstrated that rats collected and cached pellets. Many of the pellets from the purposely delayed second (vegetation only) and third (coastal swath) sowings remained untouched for weeks, however, slowly disappearing or disintegrating. Little rodent activity was apparent more than 3 days after the first sowing of bait, and none was seen more than a week after the first baiting. None of the inert detection devices deployed later in the Phase 1 area showed evidence of rats. Clear evidence of a single rat was seen in fresh snow at King Edward Point on 23 October 2014, some 3½ years after the Phase 1 baiting. No rodent sign had been encountered during this time, despite year-round monitoring. In response, bait was spread rapidly within a 1 km radius of the base, and no further sign of the rat was detected. This event occurred within days of two ships docking at the King Edward Point jetty and offloading cargo. It is likely that the rat came ashore from one of the vessels. Alternative explanations, that it survived the baiting or was part of a population founded by survivors, are less plausible. Brown rats rarely live beyond 3 years of age, even in captivity (Turturro et al., Reference Turturro, Witt, Lewis, Hass, Lipman and Hart1999), and there has been no evidence of any other surviving rats in this area during the 6 years since baiting was concluded, despite continuous monitoring.
Phase 2 (rats and mice)
At lower elevations most pellets in the rat-occupied areas often disappeared during the first or second night after sowing. Within whaling station buildings, this occurred to such an extent that entire buildings later appeared to have been overlooked by the hand-baiters. Subsequently, caches of up to 1 kg of pellets were found in dark spaces such as in drawers and under floors, where they could have been placed only by rats. Numerous piles of exactly 12 pellets were placed in these buildings 10–15 days later, but no pellets were removed. No rat activity was observed more than 4 days after baiting at any site. No rodent sign was detected on any of the inert devices deployed later in either the rat or mouse zones during any visit. A relatively brief inspection of the two mouse zones was made 2 years after baiting, and no sign of mice was encountered. Tourists and/or scientists visit many sites in the Phase 2 areas every year, including the whaling stations, and no convincing evidence of surviving rodents has been reported since baiting was completed.
Phase 3 (rats)
Fresh signs of live rats stopped within a week of baiting. No formal attempt was made to detect rodents in the 28 months after baiting concluded. However, thousands of person hours were spent at many sites in that time and no rodents or recent rodent sign were detected. Meanwhile, pipit nests were discovered at several locations: the first in living memory in these areas.
Non-target mortality
South Georgia has no reptiles, amphibians or native terrestrial mammals. The only other introduced mammal to remain until modern times, the reindeer Rangifer tarandus, had been cleared from the rat-infested areas before rodent eradication was attempted. Birds were therefore the only vertebrates at risk from the baiting work. Table 1 outlines the estimated impact of baiting on South Georgia's birds. The majority of species (i.e. those that foraged exclusively at sea) did not recognize bait pellets as being edible and were unaffected by the operation. Carcasses of seven species of birds were discovered with evidence that they had died as a result of consuming bait pellets (Black, Reference Black2011; Lee et al., Reference Lee, Black, Parker and Rexer-Huber2013; South Georgia Heritage Trust, unpubl. data): brown skua, kelp gull Larus dominicanus, two giant petrels Macronectes spp., snowy sheathbill Chionis albus and two ducks (the South Georgia pintail and the yellow-billed teal Anas flavirostris).
Table 1 Estimated impact of baiting-induced mortality on non-target (bird) populations, with number of carcasses found, population size, impact on local population within 1 year of baiting, and estimated time to full population recovery.

1The number of carcasses found relates to all three baiting seasons combined, but search effort covered only a limited proportion of the land treated; carcass data are mostly from Lee et al. (Reference Lee, Black, Parker and Rexer-Huber2013) and Government of South Georgia and the South Sandwich Islands (unpubl. data); population size estimates are from Clarke et al. (Reference Clarke, Croxall, Poncet, Martin and Burton2012).
2 Low, < 10%; Medium, 10–50%; High, > 50%
Only a rough estimate of the number of individuals that died was possible because the proportion of carcasses discovered was unknown. Many (probably the majority in Phases 2 and 3) were not recovered because the search effort was limited in geographical extent. Estimation of the proportion of the population affected was compromised further because the number of birds at risk was also poorly known.
Ten VHF transmitters attached to pintails were relocated at least 17 days after Phase 1 bait-spreading, of which six (60%) were on dead birds and four on live birds. Eleven transmitters were relocated at least a month after Phase 2 baiting, of which seven (64%) were on dead birds and four on live birds. Additionally, six ducks were fitted with individually numbered coloured rings prior to Phase 2 baiting, of which three were seen alive at least 8 weeks after baiting, giving an upper limit on mortality of 50%. Combined, these samples indicate a mortality rate of c. 59%, although this figure could be a slight underestimate because some birds fitted with transmitters may have died after the radios ceased to function (cf. discovery curve of fresh carcasses in Lee et al., Reference Lee, Black, Parker and Rexer-Huber2013).
Disturbance
The impact of overflights on bird and seal colonies was closely observed and filmed, especially at the outset of the Phase 1 and Phase 2 operations, to ensure that any problems could be identified before significant damage was caused by the flying. None was discovered.
It was clear that seals of both abundant species, the southern elephant seal Mirounga leonina and the Antarctic fur seal Arctocephalus gazella were broadly tolerant of helicopters flying at the target height of 46 m and that no injuries were likely to be caused by any behavioural response.
The predominant response of adult flighted birds (giant petrels and albatrosses, most importantly) was to take to the air when a helicopter was first in line of sight, at distances of up to 1 km, and to remain airborne until minutes after the aircraft disappeared behind a mountain or headland. All eggs had hatched by the time operations commenced, and chicks of all species were large enough to no longer require brooding. Albatross chicks remained on the nest, as did those of most giant petrels, although the largest of the latter, those close to fledging, sometimes fluttered from the nest in fright, to return when a parent arrived with food.
The two penguin species of most concern, because their colonies were the most abundant, were the king Aptenodytes patagonicus and gentoo penguins Pygoscelis papua. A small colony of king penguins was situated 2 km from the helicopter loading site during Phase 1, was frequently overflown at altitudes of c. 30 m, and suffered no loss of eggs or chicks. A much larger colony (c.7,000 pairs; Poncet & Crosbie, Reference Poncet and Crosbie2005) was observed and filmed in Phase 2, as a helicopter passed overhead at ever decreasing heights from 244 to 153 m. Here, adults brooding eggs or young chicks remained unaffected, whereas non-breeders around the margins of the colony walked away from the disturbance. No loss of eggs or birds was observed or filmed.
Gentoo penguin chicks were large by the time baiting flying commenced. With no brooding parents present, aggregations were free to move and often did so as a helicopter approached, returning long after the source of disturbance had passed. No injuries were observed in any of the colonies overflown.
Recovery of bird populations
Affected by bait
Three bird species suffered significant mortality (Table 1). Brown skuas were heavily impacted at the level of the local population, and most breeding adults may have died during the Phase 1 operation in early 2011. In the following season few breeding territories were unoccupied in this area, and by summer 2012/2013 skua numbers were similar to those before the baiting operation (S. Lurcock, South Georgia Heritage Trust, pers. comm.). Little post-baiting monitoring was carried out in the Phase 2 area, treated in 2013, but the density of breeding pairs 2 years after baiting was not apparently lower than before (ARM, pers. obs.). The number of breeding pairs on Bird Island, where egg oiling had been carried out to induce breeders to migrate away from the island before they were at risk (see Methods), were at pre-baiting levels 2 years after the operation (R.A. Phillips, British Antarctic Survey, pers. comm.). With 40% of South Georgia's skuas nesting there, this was a notable outcome, probably influenced by the fact that baiting was carried out on the adjacent part of mainland South Georgia late in the season. In summary, although a substantial proportion of the local breeding skua population was probably lost to primary or secondary mortality, recruitment from outside the baited area quickly restored losses. Estimating the number of breeding South Georgia pintails is challenging, given the species’ cryptic nature, but many more ducklings than usual were seen in the year immediately after baiting in the Phase 1 area, and the main post-moulting flock 4 years after baiting was considerably larger than before baiting (ARM, pers. obs.; S. Lurcock, pers. comm.). The snowy sheathbill was never abundant in the Phase 1 area, so losses as a result of baiting there could not be a reliable indicator across the island. However, numbers in this area 5 years after baiting were higher than in living memory (S. Lurcock, pers. comm.). During the Phase 2 operation in 2013, losses of adults and chicks were evident, especially in and around penguin colonies; furthermore, a year after baiting observers considered their abundance to be lower than usual. Population recovery has not been quantified in any area but by 2015 sheathbills were plentiful in and near king penguin colonies in the Phase 2 area, and a flock of c. 50 birds was photographed by ARM in April 2015.
Not affected by bait
Two species of small birds known to be heavily predated by rats were the first to show signs of responding to the eradication operation: the South Georgia pipit and Wilson's storm petrel Oceanites oceanicus. Neither were expected or known to be negatively impacted by baiting. The South Georgia pipit is the only passerine on the island. Its life history and behavioural characteristics (multiple broods of multiple young, post-breeding dispersal, song display, nest above ground; Tyler, Reference Tyler, del Hoyo, Elliott and Christie2004) indicated that it would probably be the first bird to be seen to recolonize formerly rat-infested areas, and this has been the case. Prior to the baiting, pipits rarely if ever nested successfully in rat-infested areas (S. Lurcock, pers. comm.). After baiting, multiple successful nests were reported from areas treated in all three phases of work, with up to four nests in a single bay. One year after the final baiting season was completed, pipits were found routinely throughout the areas once occupied by rats. Previously confined to rat-free offshore islands and the margins of the south coast, the amount of land with suitable nesting habitat now available to pipits is many times larger than before baiting. Wilson's storm petrel is an abundant breeder in scree slopes on rat-free islands but raises a chick very rarely in the presence of rats (S. Lurcock, pers. comm.). With an age at first breeding of several years, and a clutch of one egg (Brooke, Reference Brooke2004), the recovery of this species to levels prior to rodent introduction is likely to take decades, as it is with most of the procellariid seabirds (Kappes & Jones, Reference Kappes and Jones2014). However, the first signs of recovery may already be apparent. Numbers of this species seen from the base in King Edward Cove, the shores of which were cleared of rats in early 2011, have been increasing steadily since that time. Whereas the numbers of birds seen before baiting were in the low tens, 5 years later high hundreds were routinely encountered, many of them flying over suitable breeding habitat that had been infested with rats for over a century.
Discussion
A striking aspect of the South Georgia rodent eradication campaign was that it tackled rats and mice over a land area larger by an order of magnitude than anything previously attempted. There were, however, several other characteristics that differentiated this operation from its predecessors.
The largest rodent eradication campaigns are so expensive and logistically demanding that previously they had been taken on only by governments or large NGOs (e.g. McClelland & Tyree, Reference McClelland and Tyree2002; Springer, Reference Springer2016). The South Georgia project is unique in this context because it was organized and overseen by a small UK charity and funded by donations and grants raised by that same charity and its US counterpart Friends of South Georgia Island. Circa 90% of the project's GBP 7.5 million cost was donated by private individuals and foundations. That a small charity could successfully carry out such a large enterprise may encourage other NGOs with no previous experience in this field of conservation to consider whether they too, with appropriate expert guidance, could restore an island by eradicating destructive invasive species.
The operation was also unique in that it was carried out over multiple seasons. This is not an option for most eradication campaigns because of the inevitability of treated areas being reinvaded from the untreated, and was only possible in this case because of the rodent-proof glaciers. The rapid retreat of those glaciers provided a strong incentive to mount the operation without delay (Gordon et al., Reference Gordon, Haynes and Hubbard2008; Cook et al., Reference Cook, Poncet, Cooper, Herbert and Christie2010).
Another important characteristic of the South Georgia operation was that it utilized low densities of bait: no more than 6.5 kg ha−1, compared to a mean of 17.6 kg ha−1 elsewhere (Donlan & Wilcox, Reference Donlan and Wilcox2007). Bait density is a challenging subject for all those planning an eradication campaign. For reasons of cost and of reducing non-target mortality to a minimum, the aim is always to deploy the minimum amount of bait necessary to achieve 100% mortality of the target species. However, in practice it is not possible to quantify this lower limit reliably before undertaking the work, and cutting bait density even marginally may risk operational failure.
The successful eradication of rodents in the Phase 1 area, and perhaps over the entire island, despite the low bait density sown, can be attributed to a number of factors. Firstly, there were insufficient non-target animals to remove substantial quantities of bait. Although skuas, ducks, sheathbills and other birds consumed some pellets, they did not have a significant impact overall. Secondly, the target species occurred at relatively low densities. Even in the most attractive habitats, neither rats nor mice (Cuthbert et al., Reference Cuthbert, Black, Rexer-Huber, Parker and Sommer2012) were ever seen in large numbers. Thirdly, any bait pellets not consumed remained intact and viable for weeks after sowing. Any rodents that may have survived the initial baiting were therefore at risk of consuming the toxin without a repeat sowing of bait, as is often carried out elsewhere (Broome et al., Reference Broome, Cox, Golding, Cromarty, Bell and McClelland2014). Although rodent eradication across the whole island cannot yet be confirmed, success in the Phase 1 area (equal in size to Macquarie Island, the largest island so far successfully cleared of rodents) indicates that the methodology was appropriate and effective. A definitive survey of the Phase 2 and Phase 3 areas, using trained dogs and a range of inert detection devices, is planned for early 2018.
The loss of birds as a result of primary or secondary poisoning was a regrettable but unavoidable consequence of eradicating rodents from South Georgia. In this operation, as is standard practice (Broome et al., Reference Broome, Cox, Golding, Cromarty, Bell and McClelland2014), steps were taken to reduce non-target mortality to a minimum. Nonetheless, all stakeholders recognized that deaths would occur, albeit not at levels where population-level impacts were feasible because of the multi-season approach. The question faced at the outset was not whether the loss of numerous birds was acceptable but whether these deaths (and those of the target rodents) over a limited period of time were preferable to much greater mortality of many more species and individuals in perpetuity. Doing nothing would lead to rodents spreading even further on the main island as a result of glacier recession, and eventually reaching hitherto rodent-free offshore islands. In turn, this range extension of rodents would result in even fewer burrowing petrels on South Georgia, fewer ducks and terns, and the probable extinction of the endemic pipit.
The direct cost of the operation, including the purchase and maintenance of the aircraft, was c. GBP 7.5 million (USD 11 million) at 2015 prices, which amounts to GBP 69.4 (USD 104) per ha. No two invasive eradication operations are identical, and comparisons must take into account such factors as land area (economy of scale) and the number of species being tackled. However, this unit area cost compares favourably with other eradication operations (Martins et al., Reference Martins, de Brooke, Hilton, Farnsworth, Gould and Pain2006; Donlan & Wilcox, Reference Donlan and Wilcox2007) and demonstrates that a project of global significance can be mounted professionally, safely and cost-effectively by a small NGO.
The discovery of the track of a single rat in fresh snow at the island's main base, 3½ years after the last rodent sign and shortly after two ships had tied up at the nearby jetty, was a timely reminder that eradication of damaging invasive species is almost pointless without adequate measures to prevent reinvasions.
Acknowledgements
Funding was generously provided by private donors, trusts and foundations, especially Friends of South Georgia Island (FOSGI), the Department for Environment, Food and Rural Affairs of the UK Government, and the Darwin Initiative. Visitors to South Georgia, and the cruise operators who took them there, were particularly supportive. We thank members of Team Rat, support staff and the many people who contributed in diverse ways to making the operation a success. The Government of South Georgia and the South Sandwich Islands (GSGSSI) provided logistical assistance at cost or gratis, undertook non-target mortality surveys and contributed to post-baiting monitoring. The British Antarctic Survey (BAS) provided crucial expert support on South Georgia and in the UK, and consented to charter its vessel RRS Ernest Shackleton. We gratefully acknowledge vital advice and support from the island eradication community, especially the Island Eradication Advisory Group (Department of Conservation, New Zealand), and Keith Springer, Derek Brown and Andy Cox. Members of the project Steering Committee, including representatives from South Georgia Heritage Trust, FOSGI, BAS and GSGSSI, and its Advisory Committee on Incidental Mortality, guided and supported the operation throughout. This article benefited from the input of two anonymous reviewers, to whom we are most grateful.
Author contributions
ARM was the Project Director and wrote the article. MGR chaired the Project Steering Committee and was closely involved in project strategy, planning and preparations.
Biographical sketches
Tony Martin is a zoologist with special interests in the eradication of invasive species and in the biology and conservation of aquatic mammals. Mike Richardson is now retired. His career was split between nature conservation and involvement with the polar regions, both scientifically and at the political level.




