Obesity has become increasingly prevalent worldwide as a result of changes in lifestyle, especially eating habits, and it is involved in the aetiology of a number of conditions such as CVD, hypertension, stroke and diabetes(Reference Haffner and Taegtmeyer1, Reference Sharma2). Overconsumption of high-energy food and lower energy expenditure have resulted in an alarming increase in the incidence of obesity(Reference Hardus, van Vuuren and Crawford3). The fact that clinically diagnosed insulin resistance, hypertension, increased fat distribution and high plasma TAG levels have been associated with the development of diseases associated with metabolic syndrome-related parameters has attracted attention from the scientific community. Prevention and improvement of obesity and metabolic syndrome-related diseases are therefore important issues in modern society, and represent an effective strategy for the promotion of better health.
Adenosine is an endogenous purine nucleoside that modulates many physiological processes, with these effects being mediated by the activation of specific subtypes of adenosine receptors, termed A1, A2A, A2B and A3(Reference Jackson and Dubey4, Reference Olah and Stiles5). Adenosine can be extracted from plant and mammalian tissues. Higher adenosine concentrations in the plant and mammalian tissues result from enhanced excretion of adenosine or diminished uptake of adenosine(Reference Zimmermann6).
Previous studies have provided evidence to support the physiological role of adenosine in general health. For example, studies in both rats and human subjects showed that oral administration of adenosine in sucrose solutions decreased blood glucose and insulin concentrations due to its inhibitory effect on α-glucosidase(Reference Fukumori, Maeda and Takeda7, Reference Fukumori, Takeda and Fujisawa8). Continuous intravenous infusion of adenosine in premature infants was also reported to have dramatic beneficial effects on neonatal refractory pulmonary hypertension(Reference Motti, Tissot and Rimensberger9), in addition to attenuating the proliferation of both human and rat glomerular mesangial cells associated with hypertension and diabetes(Reference Dubey, Gillespie and Mi10).
In a previous study in stroke-prone spontaneously hypertensive rats (SHRSP) fed a normal diet, we showed that acute and chronic oral administration of adenosine induced novel effects such as lowering of blood pressure (BP), improvement of hyperlipidaemia and increase in plasma adiponectin levels accompanied by alleviation of hyperinsulinaemia(Reference Ardiansyah, Shirakawa and Shimeno11). Recent studies in mice also demonstrated the role of adenosine and its receptors in the regulation of hepatic fibrosis(Reference Chan, Montesinos and Fernandez12, Reference Peng, Fernandez and Wilder13) and peripheral lipid metabolism(Reference Dhalla, Wong and Voshol14). However, the detailed mechanisms underlying the effects of adenosine on metabolic syndrome-related parameters in rats have not been clarified, especially its effect on adenosine and adiponectin receptors. The purpose of the present study was therefore to extend these previous investigations by determining the effect of adenosine administration on metabolic syndrome-related parameters in SHRSP fed a high-fat diet. SHRSP fed a high-fat diet serve as a suitable animal model of hypertension-related disorders that are similar to human essential hypertension, hyperlipidaemia and insulin resistance.
Material and methods
Animal experiments and diet
Male SHRSP/Izumo strain (Japan SLC, Shizuoka, Japan) were used in the present study. The rats were housed individually in stainless steel cages under a controlled atmosphere (temperature, 23 ± 2°C; humidity, 50 ± 10 %; 12 h light–dark cycle, 08.00–20.00 hours). After a 1-week acclimatisation period, the six-week-old SHRSP were divided into either a control group or two groups administered adenosine based on their body weight. All the rats were fed an AIN-93M-based diet supplemented with 20 % lard as the source of increased fat (Table 1). The rats in the control group were provided with distilled water for 8 weeks, while the rats in the adenosine (Wako Pure Chemical Co., Osaka, Japan) groups were administered distilled water containing either 10 mg/l adenosine (Ad10) or 100 mg/l adenosine (Ad100) over the same period. Water and food intakes were recorded every day. The systolic BP and body weight were measured every week during the experimental period. At the end of the experimental period, the rats were fasted for 16 h and were then sacrificed under light diethyl ether anaesthesia. Blood samples were collected and centrifuged at 1870 g for 15 min at 4°C in a centrifuge (CF7D2; Hitachi Co. Ltd, Tokyo, Japan). The livers were excised promptly and washed with ice-cold isotonic saline. Both plasma and liver tissue samples were stored at − 80°C until required for later analyses.
Table 1 Composition of the experimental diets based on the AIN-93 diet

* tert-Butylhydroquinone, l-cystine, choline bitartrate and soyabean oil were purchased from Wako Pure Chemical Co, and vitamin and mineral mixtures, cellulose, sucrose and casein were obtained from the Oriental Yeast Co. (Tokyo, Japan).
Ethical guidelines
The experiments did not involve human subjects or patients. The experimental plan for the present study was approved by the Animal Research-Animal Care Committee of Tohoku University. The entire experiment was carried out in accordance with the guidelines issued by this committee and the Japanese government legislation (2005). The same committee supervised the care and use of the rats used in the present study.
Blood pressure measurements
BP was measured by the tail-cuff method using a BP meter without warming (MK-2000; Muromachi Kikai, Tokyo, Japan) as described previously(Reference Ardiansyah, Shirakawa and Koseki15). A minimum of six BP measurements were obtained for each rat. The mean value of four consistent readings of systolic BP was regarded as the individual systolic BP.
Plasma and liver parameters
Plasma levels of blood urea nitrogen, creatinine, albumin, glucose, total cholesterol (TC), TAG, NEFA and HDL-cholesterol were determined by enzymatic colorimetric methods (Wako Pure Chemical Co.). Plasma adiponectin levels were measured using a rat adiponectin ELISA kit obtained from Otsuka (Otsuka Co., Tokyo, Japan), and plasma insulin levels were measured using a rat insulin ELISA kit purchased from Shibayagi (Shibayagi Co., Gunma, Japan). Plasma NO level was quantified by the Griess method [NO2/NO3 Assay kit-C II (Colorimetric) Dojindo, Kumamoto, Japan] asdescribed previously(Reference Ardiansyah, Shirakawa and Koseki15). Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) level was measured by an ELISA (New 8-OHdG Check; Institute for the Control of Aging, Shizuoka, Japan) as described previously(Reference Ardiansyah, Shirakawa and Koseki15). The concentration of LDL-cholesterol was calculated by Friedewald's formula(Reference Friedewald, Levy and Fredrickson16). Liver total lipids were measured according to the Folch method(Reference Folch, Lees and Stanley17), and liver TC and TAG concentrations were determined using a kit that was the same as that used for determining plasma TC and TAG concentrations.
Oral glucose tolerance test
Oral glucose tolerance tests were conducted on 11-week-old SHRSP after fasting for 16 h. Blood for glucose measurement was collected from the tail vein 30, 60 and 120 min before and after SHRSP were (1·8 g/kg body weight) via a gastric tube. Plasma glucose and insulin levels were measured as described earlier. The analysis of the incremental area under the curve of the plasma glucose and insulin response was done on the basis of the method of Wolever & Jenkins(Reference Wolever and Jenkins18).
RNA preparation and quantitative RT-PCR
Total RNA was isolated from the liver and perirenal fat with a guanidine isothiocyanate-based reagent, Isogen (Nippon Gene Co., Tokyo, Japan), according to the instruction manual. Measurement of the wavelength ratio at 260 and 280 nm and agarose gel electrophoresis were performed for quantitative and qualitative analyses of the isolated RNA. Due to the low value of the ratio of perirenal fat mRNA at 260 and 280 nm, we performed a RNA cleanup procedure based on RNeasy (Qiagen, Tokyo, Japan). Five micrograms of total RNA were used as a template to synthesise the cDNA. The RNA was denatured with oligo-dT/random primers, followed by incubation in 10 mmol/l dNTP (Amersham Biosciences) and distilled water at 65°C for 5 min. The RNA was then incubated in 50 mmol/l Tris–HCl buffer (pH 8·3), 0·1 m DTT containing 50 units of SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA, USA) and 20 units of RNaseOUT RNase inhibitor (Invitrogen) in a total volume of 20 μl at 25°C for 5 min, 50°C for 60 min and 70°C for 15 min. Aliquots of the cDNA were used as a template for the following quantitative PCR using an Applied Biosystems 7300 Real-Time PCR System (Foster City, CA, USA) according to the manufacturer's instructions. The genes listed in Table 2 were amplified by cDNA-specific primers using the SYBR Premix Ex Taq solution (Takara Bio Inc., Shiga, Japan). The relative levels of gene expression were normalised against the mRNA expression level of eukaryotic elongation factor-1α1(Reference Shirakawa, Ohsaki and Minegishi19).
Table 2 Sequences of the PCR primers for amplification

Ef-1, eukaryotic elongation factor-1α1; Fasn, fatty acid synthase; Pparγ, PPARγ; Srebp1c, sterol regulatory element-binding protein-1c, Sod, superoxide dismutase; Gshpx, glutathione peroxidase; Adipoq, Adiponectin; Adipor1, adiponectin receptor 1; Adipor2, adiponectin receptor 2; Adora2a, adenosine A2A receptor; Adora2b, adenosine A2B receptor; Adora3, adenosine A3 receptor.
Statistical analysis
Values are presented as means with their standard errors. The differences between the groups studied were evaluated using one-way ANOVA followed by the Fisher post hoc test. Statistical Analysis Systems software (StatView-J 5.0; 1998 SAS Institute Inc., Cary, NC, USA) was used for all the statistical analyses. Probability values of P < 0·05 were considered as statistically significant.
Results
Body weight and daily intake
The consumption of a high-fat diet for 8 weeks resulted in a further increase in body weight in SHRSP, with an approximate 20 % increase compared with that observed in our previous studies on normal diets; the final body weight of SHRSP fed AIN-93M standard diet was 255·6 (sem 3·7) g(Reference Ardiansyah, Shirakawa and Koseki15). At the end of the experimental period, the final body weights were 297·0 (sem 4·2), 290·3 (sem 3·3) and 284·5 (sem 8·6) g for the control, Ad10 and Ad100 groups, respectively. There was no difference in the gain in body weight, food or water intake and relative liver weight between the three groups (data not shown). We found that the relative perirenal fat weights for the control, Ad10 and Ad100 groups were 1·8 (sem 0·1), 1·5 (sem 0·1) and 1·7 (sem 0·1) g/100 g body weight, respectively. A low dose of adenosine (Ad10) did not show a significant difference from the other two groups. The mean daily adenosine intake (mg/d) was 0·18 (sem 0·02) in the Ad10 group and 2·1 (sem 0·4) in the Ad100 group.
Effects of adenosine on blood pressure and plasma nitric oxide levels
The changes in the systolic BP of the rats during the experimental period are shown in Fig. 1(a). Compared with the control group, administration of adenosine at 10 and 100 mg/l was associated with significant decreases in BP starting from 7 weeks of age until the end of the experimental period. At the end of the experimental period, mean systolic BP was 209·5 (sem 5·5), 191·5 (sem 2·6) and 184·3 (sem 4·2) mmHg for the control, Ad10 and Ad100 groups, respectively. We also observed that adenosine administration had an effect on plasma NO levels in the rats. The plasma NO levels of both the adenosine groups were significantly higher (P < 0·05) than that of the control group (Fig. 1(b)). This result corresponded well with the hypotensive effect of adenosine shown in Fig. 1(a).

Fig. 1 Effect of adenosine administration on systolic blood pressure (a) and plasma nitric oxide production (b) in rats (mean values with their standard errors of four rats per group). *Mean values were significantly different from control group (P < 0·05). –♦–, Control; –■–, adenosine 10 mg/l (Ad10); –▲–, adenosine 100 mg/l (Ad100).
Effects of adenosine on plasma parameters
Table 3 summarises the changes in lipid profile and plasma parameters of kidney function recorded in the present study. After consumption of a high-fat diet, the adenosine groups had significantly lower plasma TAG, blood urea nitrogen and creatinine levels (P < 0·05) and increased plasma HDL-cholesterol levels compared with the control group. However, there was no difference in the liver lipid levels between the three groups. Administration of 10 mg/l adenosine caused significant decreases in the level of TC and LDL-cholesterol compared with the controls and the 100 mg/l adenosine group. After 8 weeks of adenosine intake, the rats showed significant decreases in plasma glucose and insulin levels (P < 0·05) compared with the control animals (Table 4). Analysis of plasma adiponectin levels revealed that both the adenosine groups had significant increases compared with the control group (Fig. 2). Furthermore, adenosine treatment (10 μM) resulted in significant increases in adiponectin levels in the culture medium of 3T3-L1 adipocyte cells after 6 h. The adiponectin level was 155·5 (sem 5·3) and 118·8 (sem 10·5) ng/ml in the test and the control groups, respectively.
Table 3 Effect of adenosine on plasma total cholesterol, HDL-cholesterol, LDL-cholesterol, TAG, NEFA, blood urea nitrogen (BUN), creatinine, BUN/creatinine and liver total lipid, total cholesterol and TAG levels
(Mean values with their standard errors of four rats per group)
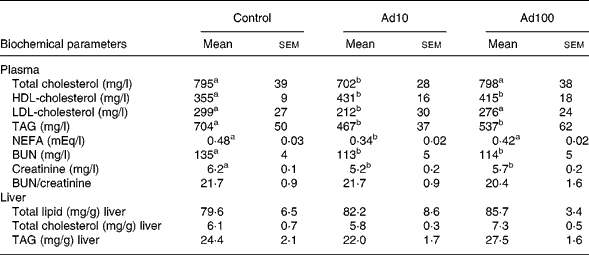
Ad10, Adenosine 10 mg/l; Ad100, Adenosine 100 mg/l.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 4 Effect of adenosine on plasma glucose and insulin levels
(Mean values with their standard errors of four rats per group)
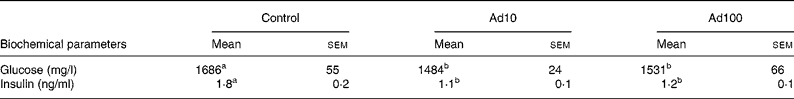
Ad10, Adenosine 10 mg/l; Ad100, Adenosine 100 mg/l.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).

Fig. 2 Effect of adenosine administration on plasma adiponectin in rats (mean values with their standard errors of four rats per group). * Mean values were significantly different from control group (P < 0·05). Ad10, Adenosine 10 mg/l; Ad100, Adenosine 100 mg.
Urinary 8-OHdG has been used widely as a sensitive biomarker of oxidative DNA damage and total systemic oxidative stress. The effect of adenosine on urinary 8-OHdG level is shown in Fig. 3. Both the adenosine groups had significant decreases in urinary 8-OHdG levels compared with the control group.

Fig. 3 Effect of adenosine administration on urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) in rats (mean values with their standard errors of four rats per group). * Mean values were significantly different from control group (P < 0·05). Ad10, Adenosine 10 mg/l; Ad100, Adenosine 100 mg.
In the present study, we carried out an oral glucose tolerance test at a dose of 1·8 g glucose solution/kg body weight (Figs. 4(a) and 5(a)) to determine whether adenosine administration improved glucose clearance after intake of a high-fat diet. During the oral glucose tolerance test, blood glucose and insulin levels at 30 and 60 min were decreased in both the adenosine groups compared with the control group. In addition, in the adenosine groups, the incremental areas under the curve of plasma glucose and insulin concentrations were significantly lower than those in the control group (Figs. 4(b) and 5(b)).

Fig. 4 Changes in plasma glucose (a) and incremental area under the curve (iAUC) (b) determined by the oral glucose tolerance test (OGTT) in rats (mean values with their standard errors of four rats per group). Mean values were significantly different from those of the control group: *P < 0·05, **P < 0·01. –♦–, Control; –■–, adenosine 10 mg/l (Ad10); –▲–, adenosine 100 mg/l (Ad100).

Fig. 5 Changes in plasma insulin (a) and incremental area under the curve (iAUC) (b) determined by the oral glucose tolerance test (OGTT) in rats (mean values with their standard errors of four rats per group). * Mean values were significantly different from control group (P < 0·01). –♦–, Control; –■–, Ad10; –▲–Ad100.
Gene expression levels
We investigated the effect of adenosine administration on mRNA expression levels in the liver and perirenal fat using the quantitative RT-PCR method (Table 5). The mRNA expression level of genes related to anti-oxidative activity such as superoxide dismutase was significantly upregulated in Ad10 group, and tended to be higher in Ad100 group than in the control group. In accordance with our finding that plasma adiponectin levels were increased after adenosine administration (Fig. 2), we confirmed that mRNA expression of adiponectin and adiponectin receptor 1 in perirenal fat was also upregulated compared with the control. In addition, we found that the mRNA expression level of the adiponectin receptor 2 was higher in the adenosine groups than in the control group. Furthermore, we determined the mRNA expression level of adenosine receptors in the liver, and found that the adenosine receptor A2B was significantly downregulated in the Ad10 group and tended to be lower in the Ad100 group than in the control group. There was, however, no difference in the mRNA expression level of the adenosine receptors A2a and A3 between the three groups.
Table 5 Effect of adenosine on liver and perirenal fat mRNA expression levels expressed as relative increases or decreases as determined by quantitative RT-PCR
(Mean values with their standard errors of six rats per group)
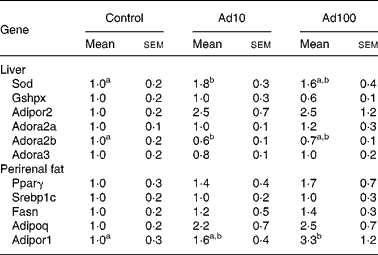
Ad10, Adenosine 10 mg/l; Ad100, Adenosine 100 mg/l; Sod, superoxide dismutase; Gshpx, glutathione peroxidase; Adipor2, adiponectin receptor 2; Adora2a, adenosine A2A receptor; Adora2b, adenosine A2B receptor; Adora3, adenosine A3 receptor; Pparγ, PPARγ; Srebp1c, sterol regulatory element-binding protein-1c; Fasn, fatty acid synthase; Adipoq, Adiponectin; Adipor1, adiponectin receptor 1.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
We demonstrated, for the first time to our knowledge, that oral administration of low (Ad10)- and high (Ad100)- dose adenosine in SHRSP fed a high-fat diet improved hypertension after 1 week and hyperlipidaemia and hyperglycaemia after 8 weeks of administration. Our results were consistent with those of previous reports(Reference Fukumori, Maeda and Takeda7, Reference Fukumori, Takeda and Fujisawa8), and also support the findings of our previous study in SHRSP fed a normal diet(Reference Ardiansyah, Shirakawa and Shimeno11). We chose to study SHRSP fed a high-fat diet consisting of lard as these rats exhibit a spontaneous hypertension, hyperlipidaemia and insulin resistance(Reference Aitman, Gotoda and Evans20, Reference Collison, Glazier and Graham21). The concentration of adenosine in cells and tissue fluids was estimated between 10 and 100 nm. Adenosine is mainly formed by the breakdown of intracellular or extracellular adenine nucleotides(Reference Zimmermann6). Two main enzymes, adenosine deaminase and adenosine kinase, play a key role in the catabolisation of adenosine. When adenosine enters the blood circulation, it is cleaved by adenosine deaminase, which is present in blood vessel wall(Reference Hershfield22). Future study is needed to explain the underlying bioavailability of adenosine in the present study.
It is well established that NO produced by endothelial cells plays a pivotal role in the maintenance of vascular function and health(Reference Kibbe, Billiar and Tzeng23) by elevating intracellular cyclic GMP levels, resulting in smooth muscle relaxation(Reference Moncada, Higgs and Palmer24). Several studies have also reported that impaired NO release from endothelial cells(Reference Malinski, Kapturczak and Dayharsh25) is related to the increase in BP that occurs in SHRSP. In the present study, we observed that administration of low- or high-dose adenosine resulted in increased plasma NO levels (Fig. 1(b)), and that this change corresponded closely with the hypotensive effect in SHRSP (Fig. 1(a)). We consider that the enhanced plasma NO level observed was a consequence of the vasodilatory effect of adenosine administration, and that the hypotensive effect of adenosine may be due to the potent vasodilation induced by adenosine following the activation of adenosine receptors (A2) in vascular smooth muscle(Reference Fullerton, Agrafojo and McIntyre26). We therefore propose that the mechanism underlying the hypotensive effect of adenosine is based on increased plasma NO levels, with this increase enhancing NO-mediated vasodilatory tone and accounting for the observed amelioration of hypertension. We consider that low- and high-dose adenosine administration may therefore improve depressed vasodilation in SHRSP.
We also investigated the hepatic mRNA expression level of adenosine receptors in order to elucidate the mechanism by which adenosine administration improved lipid metabolism. In line with previous studies(Reference Guinzberg, Laguna and Zentella27–Reference Gonzalez-Benitez, Guinzberg and Diaz-Cruz30), we confirmed that adenosine A1, A2A, A2B and A3 receptors are clearly expressed on hepatocytes, and showed that the hepatic mRNA expression levels of adenosine A2A, A2B and A3 receptors were altered by adenosine administration (Table 5). Previous studies indirectly provide evidence to support the role of adenosine and its receptors in the pathogenesis of fatty liver(Reference Muroyama, Murosaki and Yamamoto31, Reference Murosaki, Lee and Muroyama32). In the present study, we found that Ad10 significantly downregulated and Ad100 tended to downregulate the mRNA expression level of the adenosine A2B receptor. A recent study showed that the adenosine A2B receptor regulates the phosphorylation of a critical signalling molecule that controls the pathways of hepatic fatty acid oxidation(Reference Peng, Borea and Varani33). From this viewpoint, our results suggest that regulation of lipid metabolism associated with the administration of low-dose adenosine (Ad10) is mediated by adenosine receptors. Our observations also suggest that lipid metabolism in SHRSP may be regulated by a different mechanism compared with the animals administered adenosine in drinking water, although further study is required to clarify this.
Our data show that urinary 8-OHdG concentration was higher in the control group than in the adenosine groups, suggesting that increased oxidative stress may have a primary pathogenic role in SHRSP (Fig. 3). It has been reported that 8-OHdG level is increased significantly in obese diabetic KKAy mice in comparison with C57BL mice(Reference Kanaya, Doi and Sasaki34), and that 8-OHdG occurs in multiple tissues of streptozotocin-induced diabetic rats(Reference Hsieh, Lien and Lin35). We also observed that mRNA expression level of genes related to anti-oxidative activity such as superoxide dismutase was upregulated significantly by adenosine administration (Table 5). Our results imply that adenosine ameliorates oxidative capacity-related factors along with improving diabetic condition in SHRSP. On the other hand, it has been demonstrated that high-fat diet decreases insulin sensitivity(Reference Friedman36). As seen in Table 4, adenosine administration may improve insulin sensitivity despite consumption of a high-fat diet. The results of the oral glucose tolerance test clearly showed that adenosine administration suppressed the rapid increase in plasma glucose and insulin levels and the incremental areas under the curve (Figs. 4 and 5). Taken together, these results suggest that adenosine contributes to improving the regulation of glucose metabolism in SHRSP fed a high-fat diet.
It is well known that adiponectin plays a central role in antagonising the metabolic parameters involved in obesity, hepatic lipid deposition and inflammation that ultimately lead to systemic insulin resistance(Reference Kim, van de Wall and Laplante37). Our previous results suggest that an enhanced plasma adiponectin level alleviates hyperinsulinaemia, and may prevent the development of hypertension in SHRSP fed a normal diet(Reference Ardiansyah, Shirakawa and Shimeno11). In the present study, we also found that adenosine administration increased plasma adiponectin levels (Fig. 2) and improved hyperinsulinaemia in rats fed a high-fat diet (Table 4). Furthermore, increased adiponectin expression may have an impact on lipid levels in obese mice, as it has been reported that HDL-cholesterol levels are increased by adiponectin in genetic mouse models of obesity (ob/ob and db/db)(Reference Silver, Jiang and Tall38–Reference Gruen, Plummer and Zhang40). In the present study, low- and high-dose adenosine administration resulted in significantly lower total plasma TC and increased HDL-cholesterol levels compared with the controls (Table 3). We therefore consider that enhanced plasma adiponectin level resulting from daily intake of adenosine may be caused by the upregulation of the mRNA levels of adiponectin and its receptors in perirenal fat and liver (Table 5). As adiponectin and adiponectin receptors represent potential versatile therapeutic targets to combat obesity-related diseases characterised by insulin resistance(Reference Kadowaki, Yamauchi and Kubota41) and to ameliorate hyperglycaemia and hyperinsulinaemia thereby protecting against diabetes(Reference Yamauchi, Kamon and Waki42), we suggest that adenosine is effective for improving metabolic syndrome-related parameters in SHRSP.
In conclusion, the present results are what we consider to be novel findings on the effects of low- and high-dose adenosine administration in SHRSP fed a high-fat diet. Our results clearly indicate that dietary adenosine is effective in increasing insulin sensitivity and plasma adiponectin levels and also in upregulating mRNA levels of adiponectin and its receptors in perirenal fat and liver and in downregulating hepatic mRNA levels of adenosine A2B receptor. We propose that these effects mediate the effect of adenosine on metabolic activity including the lowering of BP and improvement in glucose and lipid metabolism. The mechanism by which the oral administration of adenosine mediates this process, however, requires further investigation. In this regard, we plan to perform clinical studies to confirm the anti-metabolic syndrome activity of adenosine in human subjects, in addition to carrying out further experiments in animal models.
Acknowledgements
This research was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science to A. and H. S. (no. 19.07 166), and a Grant for the City Area Program from the Ministry of Education, Culture, Sports, Science and Technology of Japan to T. K. There are no conflicts of interest in relation to the present paper. All authors are responsible for the content of the manuscript. A. A. carried out majority of the analytical work, designed the experiment and wrote the manuscript. Y. S. contributed to the in vitro experimental work. T. K. was involved in the experiments and discussion of the experimental results. H. S. and M. K. contributed to the supervision and drafting of the manuscript and to the design of some of the experiments.












