Taurine (2-aminoethanesulfonic acid) is a sulphur-containing non-canonical amino acid, which is not incorporated into proteins(Reference Brosnan and Brosnan1, Reference Stapleton, Charles and Redmond2). It is the most abundant free amino acid in animal tissues, with three-quarters of the total body pool present in skeletal muscle(Reference Brosnan and Brosnan1, Reference Kendler3). Taurine has a role in numerous physiological processes, including intracellular osmoregulation, bile acid and salt formation, immunomodulation, stimulation of glycolysis and glycogenesis, as well as acting as an antioxidant, a membrane stabiliser and a neurotransmitter(Reference Brosnan and Brosnan1, Reference Stapleton, Charles and Redmond2, Reference Wesseling, Koeners and Joles4–Reference Jia, Yue and Chandra7).
In mammals, taurine is primarily synthesised from methionine and cysteine by cysteinesulfinic acid decarboxylase(Reference Sturman, Hayes and Draper8). However, the capacity to biosynthesise it varies among species, being high in rodents and low in human subjects(Reference Sturman, Hayes and Draper8, Reference Bouckenooghe, Remacle and Reusens9), while in cats taurine is an essential amino acid(Reference Hayes, Carey and Schmidt10, Reference Sturman, Messing and Gargano11). Taurine is thus conditionally essential in human subjects(Reference Kendler3, Reference O'Flaherty and Bouchier-Hayes5, Reference Gaull12), and its concentrations are depleted, for example, in association with diabetes mellitus(Reference Franconi, Bennardini and Mattana13) or following surgical trauma(Reference Ahlman, Ljungqvist and Andersson14).
Mammalian pregnancy is also associated with increased taurine requirements. The ability of the fetus to synthesise taurine appears to be severely limited(Reference Naismith, Rana and Emery15); this is why fetuses (and later suckling animals) rely on their mothers for an adequate supply(Reference Sturman, Hayes and Draper8). In the rat, taurine is transported from mother to fetus by specific transporters in the placenta (system β)(Reference Aerts and Van Assche16), and human taurine concentrations at term are at least 3·5-fold higher in fetal than maternal plasma(Reference Glendening, Margolis and Page17). In sheep, a specific taurine transporter has not been identified. Studies in mid-gestation(Reference Bell, Kennaugh and Battaglia18) and late-gestation(Reference Lemons, Adcock and Jones19) fetal lambs found no significant differences between umbilical arterial and venous taurine concentrations, thus suggesting either no transplacental flux or a flux that was too small to be detected without more sensitive methodology. We have previously found that in late-gestation singleton-bearing ewes maternal taurine concentrations were higher than those in the fetus(Reference Oliver, Hawkins and Breier20) and that concentrations in amniotic fluid are similar to those in fetal blood, with no detectable uptake across the fetal gut(Reference Bloomfield, Bauer and van Zijl21). Together, these data suggest that, at least in the last half of a normal ovine pregnancy, there is little net transfer of taurine from mother to fetus and, therefore, that taurine must be synthesised by the fetal sheep from amino acid precursors, rather than supplied by the mother(Reference Lemons, Adcock and Jones19). We are not aware of any study investigating taurine flux between mother and fetus in early pregnancy. This is not surprising, as contemporaneous sampling of mother and embryo would be technically very challenging. However, there are data from rodent studies demonstrating that taurine concentrations in reproductive fluid and the embryo are high in early gestation(Reference Miller and Schultz22, Reference Schultz, Kaye and McKay23) and that taurine supplementation can improve embryo development(Reference Dumoulin, Evers and Bras24, Reference McKiernan, Clayton and Bavister25). Taurine concentrations are also high in human uterine fluid(Reference Casslen26), and pregnant women demonstrate taurine conservation from the first trimester(Reference Naismith, Rana and Emery15). Thus, maternal concentrations of taurine in early pregnancy may be important for optimal pregnancy outcome.
Taurine deficiency during pregnancy may affect a number of aspects of fetal development, and pancreatic and neurological development in particular. Fetuses of rats fed with a low-protein diet during pregnancy have smaller pancreatic islets with reduced rates of islet-cell proliferation and higher rates of apoptosis(Reference Boujendar, Reusens and Merezak27). These effects can be prevented if the mothers' drinking water is supplemented with taurine(Reference Boujendar, Reusens and Merezak27). Conversely, in normally nourished 4-week-old mice, supplementing the drinking water with 0·05 % taurine for 4 weeks resulted in a significant increase in the size and number of islets in the pancreas(Reference El Idrissi, Boukarrou and L'Amoreaux28).
Taurine is also critical for the development of the central nervous system(Reference Stapleton, Charles and Redmond2, Reference Kendler3). In man and rhesus monkeys, taurine concentrations are 4- to 5-fold higher in the brains of fetuses than in those of adults(Reference Sturman and Gaull29). Taurine acts as an inhibitory amino acid during development by both γ-aminobutyric acid and glutamate receptor pathways(Reference El Idrissi and Trenkner30). In rats, during physical/emotional stress (e.g. forced swimming) taurine is released from the glial cells within the supraoptic nucleus, where it acts as a tonic inhibitor of arginine vasopressin release, which in turn enhances adrenocorticotropic hormone secretion(Reference Engelmann, Ludwig and Singewald31, Reference Engelmann, Landgraf and Wotjak32). Further, elevated fetal plasma taurine concentrations were associated with markers of impaired fetal well-being after placental embolisation in late-gestation sheep(Reference de Boo and Harding33). Thus, it seems that fetal taurine status is intimately related to regulation of fetal hypothalamic–pituitary–adrenal axis function and stress responses.
We have previously shown in sheep that maternal periconceptional undernutrition from 60 d before conception until 30 d gestational age ( − 60 to 30 dGA) alters many aspects of fetal development, including growth(Reference Harding34, Reference Oliver, Hawkins and Harding35), pancreatic function(Reference Oliver, Hawkins and Breier20) and hypothalamic–pituitary–adrenal axis maturation(Reference Bloomfield, Oliver and Hawkins36–Reference Kumarasamy, Mitchell and Bloomfield38). The fetal effects appear to be physiologically important, since they persist into postnatal life, with adult offspring of periconceptionally undernourished mothers showing impaired glucose tolerance(Reference Todd, Oliver and Jaquiery39) and suppressed hypothalamic–pituitary–adrenal axis function(Reference Oliver, Todd and Bloomfield40). Furthermore, the timing of the periconceptional undernutrition appears to be important, since some effects are seen if the undernutrition is confined to the period before, but not after conception(Reference Rumball, Bloomfield and Oliver41, Reference Jaquiery, Oliver and Rumball42).
We have also described altered plasma taurine concentrations in association with some of these experiments. In one experiment, maternal plasma taurine concentrations were reduced during periconceptional undernutrition, while fetal concentrations were similar to those of controls at 50 dGA(Reference Jaquiery43). In another experiment, both maternal and fetal plasma taurine concentrations were increased in late gestation (119 dGA) after periconceptional undernutrition(Reference Oliver, Hawkins and Breier20). We therefore hypothesised that altered maternal taurine status during early pregnancy may be one mechanism contributing to the long-term effects of periconceptional undernutrition on fetal development that we have described previously. To address this question, we have investigated the effects of mild undernutrition before and/or in early pregnancy on maternal taurine economy in sheep. Since the body taurine pool is normally regulated by the kidney(Reference Chesney6), we measured plasma and urine taurine concentrations longitudinally in ewes undernourished before, after or both before and after pregnancy.
Experimental methods
Animals
Institutional and national guidelines for the care and use of animals were followed, and all experimental procedures were approved by the University of Auckland Animal Ethics Committee. Multiparous 4- to 5-year-old Romney ewes were acclimatised for a week to indoor conditions, and to a concentrate feed (65 % lucerne, 30 % barley, with the remainder consisting of limestone, molasses and trace element supplements (CamTech, Cambridge, New Zealand)). The animals were then randomly divided into four groups: control (ad libitum feeds at 3–4 % of body weight/d; n 11), undernutrition from 60 d before until mating (PreC; n 10), from 2 d before mating until 30 d after (PostC; n 11) or from 60 d before until 30 d after mating (Pre+PostC; n 11) (Fig. 1). Undernutrition comprised a 2-d fast, and then feed concentrates individually adjusted to achieve and maintain 10–15 % body weight reduction. Feed intake during undernutrition was initially 1–2 % of body weight/d, increasing to approximately 80 % of controls. All ewes were fed ad libitum when not undernourished. After ultrasound scanning at 55 dGA, only singleton-bearing ewes were retained in this study.

Fig. 1 Timeline of periconceptional undernutrition. Pre+PostC, group undernourished from 60 d before until 30 d after mating; PostC, group undernourished from 2 d before mating until 30 d after mating; PreC, group undernourished from 60 d before until mating. ![]() , Undernutrition; ■, ad libitum.
, Undernutrition; ■, ad libitum.
Assays
Blood samples were taken by jugular puncture at 61, 29, 16 and 2 d before mating; on the day of mating (0 d); and at 13, 30, 69 and 97 d after mating (term = 147 d). Urine samples were collected in a plastic container placed underneath individual animals while being restrained at 0, 30, 69 and 97 d. Plasma and urine taurine concentrations were measured by HPLC using the Waters AccQ.Tag method (Waters Associates, Milford, CT, USA) as described previously(Reference Bloomfield, van Zijl and Bauer44), except that a Luna 3 μ C18(2) 100A 250 × 4·6 mm column (Phenomenex, Auckland, New Zealand) and Waters 2475 fluorescence detector were used. Plasma and urine creatinine concentrations were measured on a Hitachi 902 autoanalyser by enzymatic colorimetric assay (Roche, Mannheim, Germany). Urine taurine excretion was corrected for urinary dilution or concentration by calculating fractional excretion(Reference Zelikovic, Chesney and Friedman45):

Statistical analysis
Data were analysed separately for each period (preconception ( − 60 to − 2 d), mating (0 d), early gestation (0–30 dGA) and post-treatment (30–97 dGA)) by repeated-measures ANOVA, with treatment group and animal identity (nested within group) as independent variables and with a P value of < 0·05 considered statistically significant. Data were further investigated using Tukey's post hoc multiple comparison tests. The Johnson transformation was used where necessary to stabilise the variance.
Results
We studied forty-three singleton-bearing ewes: eleven control, ten PreC, eleven PostC and eleven Pre+PostC. Plasma taurine concentrations were not different among groups at the start of the experiment (Fig. 2; Table 1). In control ewes, neither plasma taurine concentration nor urine taurine excretion changed significantly throughout the experiment (Fig. 2; Tables 1 and 2).
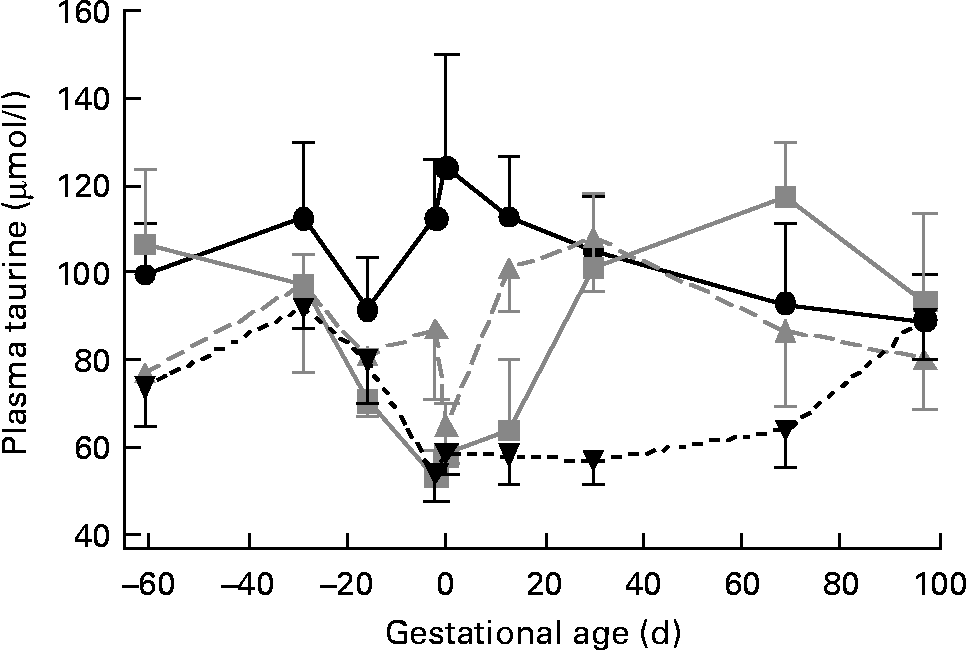
Fig. 2 Changes in plasma taurine concentrations in response to undernutrition and refeeding. Control (●, n 11), group undernourished from 60 d before until mating (![]() , n 10), group undernourished from 2 d before mating until 30 d after mating (
, n 10), group undernourished from 2 d before mating until 30 d after mating (![]() , n 11) and group undernourished from 60 d before until 30 d after mating (
, n 11) and group undernourished from 60 d before until 30 d after mating (![]() , n 11). Data were analysed by repeated-measures ANOVA, with Tukey's post hoc multiple comparison tests. Values are means with their standard errors represented by vertical bars, but error bars are only shown in one direction per data point.
, n 11). Data were analysed by repeated-measures ANOVA, with Tukey's post hoc multiple comparison tests. Values are means with their standard errors represented by vertical bars, but error bars are only shown in one direction per data point.
Table 1 Changes in plasma taurine concentrations in response to undernutrition and refeeding‡
(Mean values with their standard errors)
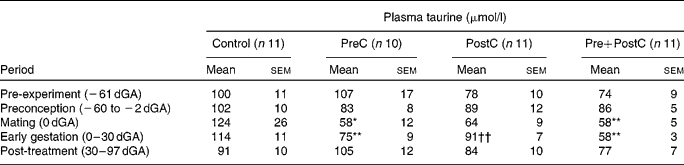
PreC, group undernourished from 60 d before until mating; PostC, group undernourished from 2 d before mating until 30 d after mating; Pre+PostC, group undernourished from 60 d before until 30 d after mating; dGA, gestational age (d).
Mean values were significantly different from those of controls, within the same time period: * P < 0·05; ** P < 0·01
Mean values were significantly different from those of Pre+PostC, within the same time period: †† P < 0·01.
‡ Mean values of all samples were taken within the time period, to allow comparison with Table 2.
Table 2 Changes in urine fractional excretion of taurine in response to undernutrition and refeeding
(Mean values with their standard errors)
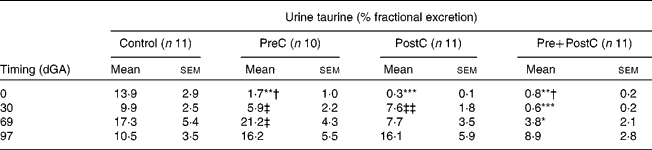
PreC, group undernourished from 60 d before until mating; PostC, group undernourished from 2 d before mating until 30 d after mating; Pre+PostC, group undernourished from 60 d before until 30 d after mating; dGA, gestational age (d).
Mean values were significantly different from those of controls, within the same time period: * P < 0·05; ** P < 0·01; *** P < 0·001.
Mean values were significantly different from those of PostC, within the same time period: † P < 0·05.
Mean values were significantly different from those of Pre+PostC, within the same time period: ‡ P < 0·05; ‡‡ P < 0·01.
In PreC ewes, plasma taurine concentrations were similar to those in controls during the first 20 d of undernutrition, but fell during the second half of the undernutrition period to reach approximately half those of the controls by the time of mating (Fig. 2; Table 1). By this time, urine taurine excretion was at very low levels and significantly less than that of controls (Table 2). After refeeding, both plasma concentration and urine excretion of taurine recovered, and were not different from control values by 30 dGA remaining relatively unchanged thereafter (Tables 1 and 2; Fig. 2).
PostC ewes were fasted from − 2 dGA; by mating (0 dGA) plasma taurine concentrations began to fall and there was practically no taurine excretion in urine (Tables 1 and 2; Fig. 2). However, despite the ongoing undernutrition, plasma taurine concentrations recovered by approximately 2 weeks after mating and onset of undernutrition to reach levels similar to controls, and were not different from controls thereafter. Urinary taurine excretion had also recovered to control levels by 30 dGA (Tables 1 and 2; Fig. 2).
In Pre+PostC ewes, as in the PreC ewes that experienced the same initial undernutrition regime, maternal plasma taurine concentrations were initially similar to controls but fell during the second half of the undernutrition period, and by the time of mating there was very little excretion of taurine in urine (Tables 1 and 2; Fig. 2). These changes persisted with continued undernutrition after mating. However, after refeeding at 30 dGA, both plasma concentrations and urine excretion of taurine recovered only slowly, and did not reach control levels until the end of the experiment at 97 dGA (Tables 1 and 2; Fig. 2).
Discussion
This study shows that mild undernutrition in sheep results in substantial changes in maternal taurine economy. Both plasma concentrations and urinary excretion of taurine fell transiently after a 2-d fast, but then they were relatively preserved for the first 30 d of undernutrition. When the undernutrition was prolonged beyond 30 d in the PreC and Pre+PostC groups, plasma taurine concentrations fell despite almost complete cessation of urinary taurine excretion. Refeeding after 60 d in the PreC group restored plasma concentrations and urinary excretion of taurine over 30 d, but after 90 d of undernutrition in the Pre+PostC group this recovery was much slower, with control concentrations not reached for almost 10 weeks after refeeding. Studies in cats have shown that switching from a standard taurine-containing diet to a taurine-free diet resulted in an 80 % reduction in plasma taurine concentration after 6 weeks, and it took a similar time period to replenish taurine-deficient animals(Reference Pacioretty, Hickman and Morris46). Our less severe undernutrition paradigm also suggests gradual taurine depletion induced by extended mild undernutrition, and a period of several weeks required for recovery after refeeding.
Taurine is not found in plants(Reference Bouckenooghe, Remacle and Reusens9), and herbivores cannot obtain it from the diet, therefore it has to be synthesised from methionine and cysteine. Under conditions of increased protein accretion, consumption of these two amino acids reduces the pool of taurine precursors, thus limiting taurine synthesis(Reference Waterfield, Jairath and Asker47, Reference Bastos, Carvalho and Remião48). Conversely, when protein accretion is inhibited, cysteine and methionine availability is increased, and these amino acids are diverted into other metabolic pathways including taurine synthesis(Reference Waterfield, Turton and Scales49, Reference Waterfield, Asker and Timbrell50). Indeed, treatment of rats with drugs that inhibit or enhance protein synthesis led to increased and decreased taurine urine excretion, respectively(Reference Waterfield, Jairath and Asker47, Reference Waterfield, Turton and Scales49).
It seems unlikely that the initial decrease we observed in plasma taurine concentrations in undernourished animals was due to increased protein accretion and consequent reduction of taurine synthesis. Rather, it seems more likely that undernutrition resulted in reduced supply of precursor amino acids from the diet and decreased bacterial production of precursor amino acids within the rumen(Reference Alison51). However, despite continued undernutrition, plasma concentrations and urinary excretion of taurine recovered and were maintained at close to control levels for several weeks. Possible mechanisms for this recovery may include: reduction in urinary taurine excretion, mobilisation of stored taurine, maternal protein catabolism releasing taurine precursor amino acids, and decreased fetal and placental growth to reduce demand for taurine.
The kidney regulates the body's taurine pool, conserving taurine during periods of need and excreting it in times of excessive availability(Reference Chesney6, Reference Bellentani, Pecorari and Cordoma52, Reference Suliman, Barany and Filho53). Taurine stored in tissues is released into the plasma during times of depletion(Reference Pacioretty, Hickman and Morris46, Reference Sturman54). Studies in cats have shown that tissue and plasma taurine concentrations are markedly reduced following 6 months on a taurine-free diet; the offspring of these animals are also significantly taurine deficient. In our study, urinary taurine excretion was almost completely abolished within 2 d of the onset of undernutrition, suggesting very rapid changes in maternal taurine economy in response to dietary change. This reduced excretion, together with mobilisation of body taurine stores, appeared to be sufficient to restore plasma taurine concentrations and some urinary taurine excretion during the initial period of undernutrition. The recovery of plasma concentrations and urinary excretion of taurine during the first 30 d of pregnancy in the PreC group after refeeding (and even in the PostC group while still undernourished) suggests that the additional pregnancy-related demand for taurine can be initially met by the pregnant sheep. However, after the first 30 d of undernutrition, plasma concentrations could no longer be maintained, even though urinary excretion effectively ceased, indicating that the mother may be entering a taurine-depleted state.
During a nutritional insult, protein catabolism can result in release of amino acids for oxidation and other metabolic pathways. This would potentially release precursor amino acids to support taurine synthesis, contributing to the initial maintenance of plasma taurine concentrations. We have previously reported that plasma ketone and fatty acid concentrations were increased in ewes during similar periods of undernutrition, but that plasma urea concentrations, reflecting amino acid oxidation, remained low(Reference Rumball, Bloomfield and Oliver41). Thus, it seems that these ewes were mobilising fat rather than protein to meet the increased demands for oxidative fuels during undernutrition, and that release of taurine precursors from muscle is unlikely to have contributed significantly to maintaining plasma taurine concentrations.
We expected that the increased demand for all amino acids (including taurine) during early pregnancy might further aggravate the effects of undernutrition on maternal taurine status. Placental taurine concentrations are high, and taurine requirements for fetal growth could also contribute to the decrease in plasma concentrations in pregnant animals. In humans, it is postulated that taurine is actively transported into the placenta by a sodium-dependent transporter, then down a concentration gradient into the fetal circulation(Reference Battaglia and Regnault55). Previous studies in mid- and late-gestation sheep found no significant umbilical arterio-venous concentrations differences for taurine, suggesting no or minimal net taurine transport across the placenta in the second half of pregnancy(Reference Bell, Kennaugh and Battaglia18, Reference Lemons, Adcock and Jones19). Nonetheless, embryonic and fetal taurine uptake from maternal sources, both histiotrophic and haemotrophic, is likely to occur earlier in gestation, particularly when the liver is insufficiently mature to allow taurine biosynthesis and precursor amino acid supply to the fetus is limited during undernutrition. Indeed, taurine concentrations in maternal fluids that provide histiotrophic nutrition, and in amniotic and allantoic fluid(Reference Waterfield, Turton and Scales49), have been found to be high in early gestation supporting the likelihood of maternal–fetal taurine transfer.
This study also shows that the pattern of recovery of taurine status after undernutrition varied among undernutrition groups. In PreC ewes, refeeding at the time of mating resulted in recovery of taurine status 2–4 weeks later, so that by 30 dGA both plasma concentrations and urinary excretion were similar to controls. However, Pre+PostC ewes took much longer to recover, with plasma concentrations and urinary excretion still low (4 weeks after refeeding; 69 dGA), only reaching those of controls at approximately 97 dGA (almost 10 weeks after refeeding). This longer recovery period may reflect greater whole-body taurine depletion after the more extensive period of undernutrition(Reference Pacioretty, Hickman and Morris46). It is also possible that the taurine requirements of the fetus during the period after 30 dGA may have delayed recovery of maternal taurine status in Pre+PostC animals, because of greater demand for precursor amino acids. Consistent with this, we showed that maternal plasma taurine concentrations were low, while fetal concentrations were normal at 50 dGA after a similar period of undernutrition(Reference Jaquiery43), suggesting that fetal taurine status is restored at the expense of maternal taurine replenishment over this period.
Recovery of plasma taurine concentrations may also be achieved in part due to suppression of protein synthesis. The onset of severe maternal undernutrition in late gestation results in rapid cessation of fetal growth in sheep(Reference Oliver, Hawkins and Harding35). Reduced growth, with the associated reduced rates of protein synthesis would potentially result in increased supply of precursor amino acids for taurine synthesis. This may provide a possible explanation for elevated maternal and fetal plasma taurine concentrations that we have previously observed in late gestation after periconceptional undernutrition(Reference Oliver, Hawkins and Breier20). Not only was fetal growth reduced in these animals(Reference Oliver, Hawkins and Harding35), but maternal weight was also lower and blood glucose concentrations were decreased(Reference Rumball, Bloomfield and Oliver41), even though these ewes actually consumed more feed. Hence, inhibition of maternal protein accretion may also be a response to extended undernutrition in pregnant ewes, and this inhibition may continue well into the refeeding period while body stores are being replenished.
The pattern of late-gestation growth that we have previously reported of fetuses from the ewes reported here also suggests that the timing of the change in maternal taurine status affects fetal development. Fetuses of control ewes or those on a stable plane of undernutrition around the time of conception displayed similar growth trajectories and size in late gestation, whereas PreC fetuses grew more slowly in late gestation and had reduced fetal weight compared with the controls(Reference Rumball, Bloomfield and Oliver41). Data on concentrations of taurine in other maternal compartments, such as uterine and oviductal fluid, and concentrations in embryonic compartments, such as the embryo itself or the embryonic fluid compartments, could assist further with understanding the role of taurine in early fetal development. However, as we wished to investigate maternal taurine status throughout the first half of pregnancy, and also fetal and postnatal development, as described elsewhere, these additional samples were not available.
These substantial changes in maternal taurine economy during and after periconceptional undernutrition may be one mechanism explaining some of the long-term effects on the physiology of the offspring. Taurine is particularly important in the development of the fetal pancreas and brain(Reference Boujendar, Reusens and Merezak27, Reference Sturman and Gaull29), although the timing of any developmental requirements is not understood. All undernourished ewes in this study experienced reduced plasma taurine concentrations, at least transiently, during early pregnancy, so that their offspring underwent early development in a maternal low-taurine environment. For offspring of Pre+Post ewes, this low-taurine environment persisted throughout the first half of gestation. We have previously reported that offspring of ewes undernourished in a similar manner to the Pre+Post group had impaired pancreatic function when subjected to a glucose tolerance test at 10 months of age(Reference Todd, Oliver and Jaquiery39). They displayed higher plasma glucose concentrations and a lower rise in plasma insulin(Reference Todd, Oliver and Jaquiery39), indicating inadequate insulin production or secretion. These animals also had suppressed hypothalamic–pituitary–adrenal axis function at 4 and 10 months of age, with a 15 % lower cortisol response to a corticotropin-releasing hormone/arginine vasopressin challenge(Reference Oliver, Todd and Bloomfield40). The changes in maternal taurine economy reported here, which persist for many weeks after refeeding, may be one mechanism explaining these long-term effects of periconceptional undernutrition on the offspring. Although the present study data in sheep cannot be extrapolated to humans, the recognition that many women have inadequate nutritional intakes before and during early pregnancy(Reference Inskip, Crozier and Godfrey56, Reference Crozier, Robinson and Godfrey57) and the fact that pregnant women also appear to conserve taurine in early pregnancy suggest that maternal taurine status should be considered as a possible contributor to the long-term effects of undernutrition in early human pregnancy on the health of the offspring.
We conclude that mild undernutrition in ewes for longer than 30 d results in onset of taurine deficiency that can take several weeks to recover. Importantly, ewes undernourished both before and after conception experienced taurine deficiency throughout the first half of pregnancy. Fetuses of these ewes develop in a low maternal taurine environment; this may be one mechanism by which maternal periconceptional undernutrition alters the development of the offspring with implications for adult health.
Acknowledgements
We thank members of the Liggins Institute's Fetal and Neonatal Physiology Group for invaluable contributions to this study. This work was funded by the Health Research Council of New Zealand. The authors have no conflicts of interest to declare. M. H. O., A. L. J., J. E. H. and F. H. B. were responsible for the design and execution of the research; E. B. T. analysed all the samples; J. G. B. D. carried out the statistical analyses; E. B. T. and J. G. B. D. wrote the manuscript, with input from the other authors.






