Introduction
Wheat is commonly grown in rain-fed systems, where water supply limits production, often restricting potential yield by over 50% (Monneveux et al., Reference Monneveux, Jing and Misra2012). Among the regions with inadequate rainfall are Mediterranean-type climates, which are characterized by terminal drought – protracted water deficit during the crop's reproductive development stages (Tigkas and Tsakiris, Reference Tigkas and Tsakiris2015; Carter et al., Reference Carter, Hawes and Ottman2019). Besides reducing grain yield, terminal drought undermines grain quality (Sattar et al., Reference Sattar, Sher, Ijaz, Ullah, Ahmad and Umar2020). Climate change is expected to worsen the impact of drought on wheat, with changes in the location and frequency of dry periods (Bezner Kerr et al., Reference Bezner Kerr, Hasegawa, Lasco, Bhatt, Deryng, Farrell, Gurney-Smith, Ju, Lluch-Cota, Meza, Nelson, Pörtner, Roberts, Adams, Adler, Aldunce, Ali, Begum, Betts, Kerr, Biesbroek and Birkmann2022). Climate change adaptation requires techniques to improve crop production under drought (Daryanto et al., Reference Daryanto, Wang and Jacinthe2017). One such agronomic tactic is the use of antitranspirants – substances that are applied on leaves to reduce transpiration with a view to improving the plant water status and crop productivity (Mphande et al., Reference Mphande, Kettlewell, Grove and Farrell2020; Kociecka et al., Reference Kociecka, Liberacki and Strozecki2023).
Antitranspirants are grouped into three classes, based on their mode of action, as metabolic antitranspirants, reflective antitranspirants and film antitranspirants (Mphande et al., Reference Mphande, Farrell and Kettlewell2023). Metabolic antitranspirants, also known as stomata-closing antitranspirants, for example, exogenous abscisic acid (ABA), chitosan and fulvic acid, reduce transpiration by acting on guard cells to induce stomatal closure. Reflective antitranspirants (e.g., kaolin, calcium carbonate and magnesium silicate) reflect sunlight to lower the heat energy balance of the leaf and hence reduce water loss. Finally, film antitranspirants physically block stomata to reduce loss of water vapour from the plant (Xiang et al., Reference Xiang, Vickers, Hare and Kettlewell2022). Di-1-p-methene and acrylic polymers are examples of film antitranspirants (Allen and Allen, Reference Allen and Allen2021). Plant oils have also been shown to be effective film antitranspirants. For example, a study done by de Godoi and Kettlewell (Reference de Godoi and Kettlewell2023) found sunflower oil to be as effective as di-1-p-methene in reducing transpiration. Antitranspirants are being used commercially in horticulture, but the high cost has limited their use in arable crops (Mphande et al., Reference Mphande, Farrell and Kettlewell2023).
Nonetheless, the drought ameliorative potential of antitranspirants has been established in a wide range of crops, for example, rapeseed oil (Xiang et al., Reference Xiang, Vickers, Hare and Kettlewell2023), soya beans (Javan et al., Reference Javan, Tajbakhsh and Mandoulakani2013), sugar beet (Makhlouf et al., Reference Makhlouf, Khalil and Saudy2022) and wheat (Kettlewell et al., Reference Kettlewell, Heath and Haigh2010; Ouerghi et al., Reference Ouerghi, Ben-Hammouda, Teixeira Da Silva, Albouchi, Bouzaien, Aloui, Cheikh-M'hamed and Nasraoui2014). However, the ameliorative mechanisms involved have not been fully characterized. The direct effect of antitranspirants is to reduce transpiration and improve plant water status under drought conditions by blocking or closing stomata (Mphande et al., Reference Mphande, Kettlewell, Grove and Farrell2020), but an indirect effect is to reduce drought stress responses – including the production of ABA (Mphande et al., Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021).
It is known that drought stress elevates the concentration of endogenous ABA, and that the hormone is associated with reduced reproductive development and yield (Lamin-Samu et al., Reference Lamin-Samu, Farghal, Ali and Lu2021). Among the effects of elevated ABA concentration on reproductive development are suppression of anther development and increased pollen sterility (Yu et al., Reference Yu, Jiang and Guo2019; Wang et al., Reference Wang, Qiu, Yu, Zhang, Xiaoyi, Wang, Xufeng, Zhang and Yang2020). Pollen sterility is related to reduced starch availability due to repression of the cell wall invertase gene TaIVR1 in drought-stressed anthers, which is induced by elevated endogenous ABA (Ji et al., Reference Ji, Dong, Shiran, Talbot, Edlington, Hughes, White, Gubler and Dolferus2011; Yu et al., Reference Yu, Jiang and Guo2019). Genotypes that are more tolerant to drought stress have been found to accumulate lower levels of ABA and sustain higher grain number and yield than their more sensitive counterparts (Dong et al., Reference Dong, Zheng, Liu, Able, Yang, Zhao, Zhang, Qiao, Wang and Liu2017). In addition, tolerant genotypes are resilient in maintaining grain quality parameters under moderate drought conditions (Ozturk et al., Reference Ozturk, Erdem, Aydin and Karaoglu2021). This is because drought-tolerant genotypes have higher ABA catabolism (Ji et al., Reference Ji, Dong, Shiran, Talbot, Edlington, Hughes, White, Gubler and Dolferus2011). In drought sensitive genotypes, grain protein content can be significantly increased with negative rheological implications on bread wheat (Ozturk et al., Reference Ozturk, Erdem, Aydin and Karaoglu2021; Yang et al., Reference Yang, Yang, Liang, Marshall and Neibling2023). Thus, there is evidence suggesting that ABA may be responsible for loss of crop yield and grain quality during drought (e.g., reproductive organ abortion in chickpeas [Pang et al., Reference Pang, Turner, Khan, Du, Xiong, Colmer, Devilla, Stefanova and Siddique2017], suppression of seed set in maize [Setter et al., Reference Setter, Flannigan and Melkonian2001] and wheat [Bheemanahalli et al., Reference Bheemanahalli, Impa, Krassovskaya, Vennapusa, Gill, Obata and Jagadish2020]).
In a recent study, Mphande et al. (Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021) reported that di-1-p-menthene (DpM), a film antitranspirant, induced yield improvement in spring wheat and that this was associated with reduced endogenous ABA. Here, the extent to which drought-induced yield losses were driven by changes in ABA was tested by independently manipulating water status and ABA through the use of a film antitranspirant, exogenous ABA and fluridone (1-methyl-3-phenyl-5-[3-(trifluoromethyl)phenyl]-4(1H)-pyridinone), an ABA-inhibitor. Therefore, the objective of the current study was to determine whether drought mitigation with the film antitranspirant (di-1-p-menthene) was solely mediated through the reduction of spike ABA. It was hypothesized that DpM improved yield of droughted spring wheat, at least in part, by downregulating the concentration of endogenous spike ABA. A combination of treatments was used to test the hypothesis in field-grown plants. DpM was used to reduce water loss and spike ABA concentration; while application of fluridone was used to reduce the accumulation of spike ABA. The application of exogenous ABA was used to increase spike ABA. The effects of these products on physiological and agronomic traits were compared including relative water content (RWC), photosynthesis, transpiration, yield and yield components.
Materials and methods
Experimental site description
Two separate field experiments, one in spring and another in summer, were conducted in 2020 at the Birds' Nest Field, Harper Adams University, Shropshire, UK (52°46′N, 2°25′W, 70 m a.s.l.). The soil textural classification at the site was determined to be a sandy loam (62% sand, 21% silt and 17% clay) in the top 60 cm. The field capacity (FC) and permanent wilting point (PWP) were determined using the pressure plate method as 19 and 9% (volumetric water content, VWC), respectively. This means that the plant available water (PAW) was 10%. To simulate a Mediterranean-type climate, polythene rain shelters (approximately 26 by 8 m in size) were used. The land was watered to FC before ploughing, and the plants were grown under stored soil moisture. The experimental plots were all under progressive drought, which was sustained until crop maturity and harvest (to simulate terminal water deficit – with no rainfall during the reproductive growth stages).
Experimental design and treatments
The experiments were arranged in a randomized complete block design, with all blocks maintained under terminal drought. There were four treatments per block. In the spring experiment, these were the control (hereafter referred to as ‘Water’; was the spray application of water equivalent to the water volume used for the other treatments, i.e., 200 l water/ha), DpM at 1 l/ha, fluridone at a concentration of 10 μM (designated as F10) and a combination of DpM and 100 μM exogenous ABA (DpM + ABA). The experimental unit size was 1.0 by 1.5 m with four plots per block and six replicate blocks. In summer, the treatments included Water, DpM and two dose rates of fluridone (at 20 and 50 μM, designated as F20 and F50, respectively). The increase in the fluridone level used in the summer experiment was implemented to allow a fuller assessment of this spray. The plot dimensions were 1.2 by 1.2 m with four plots per block and eight replicate blocks. Treatments were randomly assigned to experimental units using GenStat 20th Edition (VSN International, Hemel Hempstead, UK). A set of benchmark plots was included within each rain shelter to provide a well-watered reference point, these were manually irrigated to FC throughout the season. The benchmark plots did not receive any of the experimental sprays (outlined above) but provided a reference point illustrating the potential performance of unstressed, untreated plants at the site. Benchmark plots were located at the end of the rain shelters (not at random) to eliminate the possibility of water movement to the droughted plots. As such, the benchmark plots could not be included in the statistical design or analysis but are herein presented alongside the other data as a reference point.
Plant establishment
Primary tillage was done using a two-furrow Kverneland tractor-drawn plough (Kverneland Group, UK Ltd). Secondary tillage using a rotary harrow (Lely UK, Cambourne), followed immediately after. This was particularly necessary for the benchmark plots which were under irrigation throughout the growing seasons. Nitrogen was applied at a rate of 100 kg N/ha, just after primary tillage, but before harrowing to incorporate the fertilizer, in accordance with fertilizer recommendations.
A spring wheat variety, Chilham (supplied by KWS UK Ltd), dressed with the fungicide (active substance 40 g/l tefluthrin plus 10 g/l fludioxonil), was sown by hand drilling in both spring and summer. Hand-drilling was required to ensure comparable sowing rates between plots and experiments. The seed rate was 400 seeds/m2, and with the germination and establishment percentages being 99 and 90%, respectively, the target plant population was ~356 plants/m2. The amount of seed sown per plot was determined by weight after counting the number of seeds in weighed subsamples picked from the seedlot.
A herbicide mixture of 143 g/kg metsulfuron-methyl, 143 g/kg tribenuron-methyl and 600 g/l mecoprop-P was applied in the morning at 10:00 h to control young weeds and when the crop was at GS23. The solution was prepared by dissolving 42 mg of each of metsulfuron-methyl and tribenuron-methyl and 20 ml of mecoprop-P in two litres (2 l) of water.
Experimental spray application
The chemical sprays were applied onto the plants at Zadoks et al. (Reference Zadoks, Chang and Konzak1974) crop growth stage 37 (GS37) using Hypro flat fan nozzles (110° at a pressure of 2.0 bars) mounted on a hand-held boom sprayer (Lunch Box Sprayer, Trials Equipment (UK) Ltd, Essex CM7 4EH), with the water volume equivalent of 200 l/ha. In a previous experiment, Mphande et al. (Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021) found that the highest yield response to DpM application under drought of the spring wheat genotype being studied was at GS37, hence the selection of this growth stage. For spray application and endogenous ABA sampling dates, see Table SI-1 in the supplementary information file.
Data collection and analysis
Meteorological data
Temperature and relative humidity inside the rain shelters were monitored using Tinytag View 2 (Gemini Data Loggers UK Ltd, Chichester, England) data loggers. The mean daily values were calculated, and graphs plotted (Fig. SI-1).
Soil moisture
Soil moisture content in the top 60 cm was determined using a Time Domain Reflectometry (TDR) probe (TRIME-TDR, IMKO Micromodultechnik GmbH, Ettlingen, Germany). TDR access tubes were inserted into the soil just after sowing. Soil moisture was monitored at regular intervals from 42 DAP (GS31) in spring and 26 DAP (GS24) in summer (Fig. 1) and ended at GS87 (physiological maturity), which was 123 DAP and 97 DAP in spring and summer, respectively. Readings were recorded as volumetric water content (%).

Figure 1. Changes in soil moisture content between 42 days after planting (DAP, growth stage 31, GS31) and 123 DAP (GS87) in spring (a); and 26 DAP (GS24) and 97 DAP (GS87) in summer (b). DpM, DpM + ABA, F10, F20 and F50 stand for di-1-p-menthene, a mixture of di-1-p-menthene and abscisic acid, and fluridone concentrations at 10, 20 and 50 μM, respectively. BM indicates the benchmark plots which were fully irrigated throughout the growing period. Arrows mark days after planting when spray types were applied, and spike abscisic acid (ABA) sampled.
Endogenous abscisic acid concentrations
To determine the effects of drought and spray applications on endogenous ABA concentration, three spikes were taken from randomly selected wheat plants and placed into plastic tubes at GS68 (Table SI-1), these were immediately flash frozen in liquid nitrogen contained in a Dewar flask. Samples were then stored at −80 ˚C, according to the ABA ELISA protocol. The assay was performed using Cusabio ABA ELISA protocol, code CSB-E09159Pl, found at http://www.cusabio.com, whose detection range was 0.156–10 μg/ml.
Gas exchange
Net photosynthesis, transpiration and leaf temperature were measured simultaneously using an infrared gas analyser (LC pro-SD, ADC BioScientific Ltd, England) on dates indicated in Table SI-2. The photosynthetic photon flux density (PPFD) was set to 1500 μmol/m2/s. Measurements were carried out on a minimum of three fully expanded flag leaves per plot. This was done between 10:00 am and 2:00 pm, which is within the time stomata are fully open and conductance is highest (Correia et al., Reference Correia, Rodrigues, Ferreira and Pereira1997; Grassi et al., Reference Grassi, Ripullone, Borghetti, Raddi and Magnani2009). Readings were logged when the system reached a steady state, which on average was after five minutes.
Relative water content
To determine the effect of drought on plant water status, determination of RWC was included in the summer experiment. RWC was determined according to the method described by Mullan and Pietragalla (Reference Mullan, Pietragalla, Pask, Pietragalla and Mullan2012) and calculated according to the mathematical expression: RWC (%) = [(FW – DW)/(TW – DW)] × 100, where FW, DW and TW represent the fresh weight, oven-dry weight and turgid weight of the leaf, respectively. To allow leaves to attain turgidity, which was not achievable using the Mullan and Pietragalla (Reference Mullan, Pietragalla, Pask, Pietragalla and Mullan2012) method, the protocol was modified by making several incisions in each leaf lamina, about 30 mm apart. The incisions were made from one side, across the midrib and close to the other margin, to allow for better absorption of water. The leaves were then incubated in distilled water under light at room temperature for 18 h before refrigeration at 4°C for 24 h. Due to restrictions associated with the COVID-19 pandemic, RWC sampling in spring was not done but in summer (Table SI-2) only.
Yield and yield components
Data on fertile spike density, grain number per m2, fertile tillers per plant (tillering ratio), grain number per spike, thousand grain weight (TGW) and grain yield per hectare were collected and/or calculated. Determination of fertile spike density per m2 and number of fertile tillers per spike were done at physiological maturity (GS87). Fertile spike density per m2 was determined by counting grain-bearing spikes in three random samples per plot, using a 33 × 33 cm quadrat. Spikes bearing one or more grains were counted as fertile. The number of fertile tillers per plant was determined by systematically sampling 20 plants per plot (following a ‘W’ or ‘M’ pattern across a plot) away from the borders and counting the number of grain-bearing tillers per plant in the sample. Grain number per spike was also determined using systematic sampling, like for tillers, to obtain 10 main spikes and 10 primary tiller spikes. After harvesting (at GS97), the crops were oven-dried at 65°C to enhance threshing efficiency. For bulk samples, an electrical threshing machine (F. Walter & H. Wintersteiger KG, Austria) was used, but smaller samples (for determining the number of grains per spike) were threshed and counted manually. TGW was obtained by weighing 40 g of grain oven-dried at 105°C, as described by Sylvester-Bradley et al. (Reference Sylvester-Bradley, Grills, Roebuck and Tottman1985). Samples for TGW were collected from the bulk grain of each plot and counted manually. The number of grains in this weight, minus the weight of broken grain, was also used in the calculation of number of grains per m2 (Moeller and Rebetzke, Reference Moeller and Rebetzke2017). Finally, grain yield per plot was determined by bulking and weighing all grain oven-dried at 105°C for each sample.
Statistical analyses
Analysis of variances (ANOVA) of endogenous ABA concentration, gas exchange, RWC and yield and yield components were performed using GenStat 20th Edition (VSN International Hemel Hempstead, UK) to determine any statistically significant differences between the spray treatments (at 5% significance). Post hoc analysis was done with the Tukey test (at 5% significance). Relationships between ABA and yield and yield components were analysed using simple linear regression. Multiple regression analysis was used to investigate spike ABA and transpiration as explanatory variates against yield and yield components, with season as the grouping factor. Due to a strong collinearity between spike ABA and transpiration (r = 0.93), a simple linear regression was used. Accumulated analysis of variance showed that the grouping factor was not significant for any response variate. Only one interaction factor, number of tillers per plant × spike ABA, was significant showing a weak interaction (P = 0.045), compared to the stronger explanatory variate effect (P < 0.001). All the data were therefore best fitted with a common regression line. Curve and model fitting were performed using Microsoft Excel, but with constants in the models generated from GenStat (as the values in Microsoft Excel were slightly inflated).
Results
Temperature, relative humidity and vapour pressure deficit
The mean daily temperature inside the rain shelters between the day of planting and attainment of physiological maturity (GS87) for summer was significantly greater (P < 0.001) than for spring (Fig. SI-1, a). The minimum and maximum temperatures were 2.6 and 20.2°C in spring and 9.4 and 28.9°C in summer, respectively. The average mean daily temperature for spring was 14.9°C, while for summer it was 19.6°C. For relative humidity, the converse was true, with spring having a significantly higher (P < 0.001) mean daily value, 80.2%, than summer, 73.5% (Fig. SI-1, b). The minimum and maximum relative humidity were 65.4 and 96.4% in spring and 52.8 and 100% in summer, respectively. The vapour pressure deficit inside the rain shelters was significantly higher (P < 0.001) in summer than in spring. During the growing seasons, the mean daily vapour pressure deficit for spring and summer were 0.322 and 0.531 kPa, respectively. The vapour pressure deficit graph (see supplementary information, Fig. SI-2) followed a similar trend to the temperature graph.
Soil moisture
Soil moisture readings (%VWC) were taken at five different times between GS31 and GS87 in spring; and GS24 and GS87 in summer. In spring, soil moisture declined from 14.3%, at 42 DAP, to 10%, at 123 DAP, which was just above the pre-determined PWP (Fig. 1). The readings in summer were 13.3%, at 26 DAP and 8.92% at 97 DAP, this was slightly below PWP. At GS37, when the chemical treatments were sprayed, in spring, the PAW was depleted by 60% approximately; and in summer by 72%. At physiological maturity (GS87), 90% of PAW was depleted in the spring experiment, and 110% (10% lower than the pre-determined PWP) of PAW was depleted in the summer experiment. Benchmark plots were kept between 17.7% and 20.4%, close to the FC.
Relative water content
In the summer experiment, the RWC of droughted and well-watered plants was determined (Fig. 2). Compared with the water-sprayed plants, DpM increased RWC while F50 depressed it, but F20 had no significant effect.

Figure 2. Spray type effects on wheat leaf relative water content under progressive drought in summer (P < 0.001) at GS73. DpM, F20 and F50 stand for di-1-p-menthene, a combination of di-1-p-menthene and abscisic acid and fluridone concentrations at 20 and 50 μM, respectively. Error bars are common standard errors of means from the ANOVA table. Different letters indicate significant differences between spray treatments (Tukey test; P < 0.05). BM indicates the benchmark plots which were fully irrigated throughout and were not included as treatments in the ANOVA. No relative water content data was collected in spring due to logistical restrictions associated with the COVID-19 pandemic.
Spike abscisic acid
Drought increased spike ABA concentration by an average of 55% for the two seasons. The increase in summer (57%) was slightly greater than in spring (53%). Comparing the seasonal difference for the water-sprayed treatment, the accumulation was more than twofold greater in summer than in spring (Fig. 3). In spring, application of F10 and DpM significantly curtailed the accumulation of spike ABA by the same percentage (25%). In summer, DpM and F50 reduced the ABA concentration by 25 and 23%, respectively. Fluridone at the lowest concentration (F10) used in spring was more effective in suppressing the accumulation of spike ABA than at the greater concentrations in summer. The DpM + ABA treatment was not significantly different from the water-sprayed or the DpM-sprayed treatments, but intermediate between them. For information on spike ABA at GS54, see Fig. SI-3 in the supplementary information file.
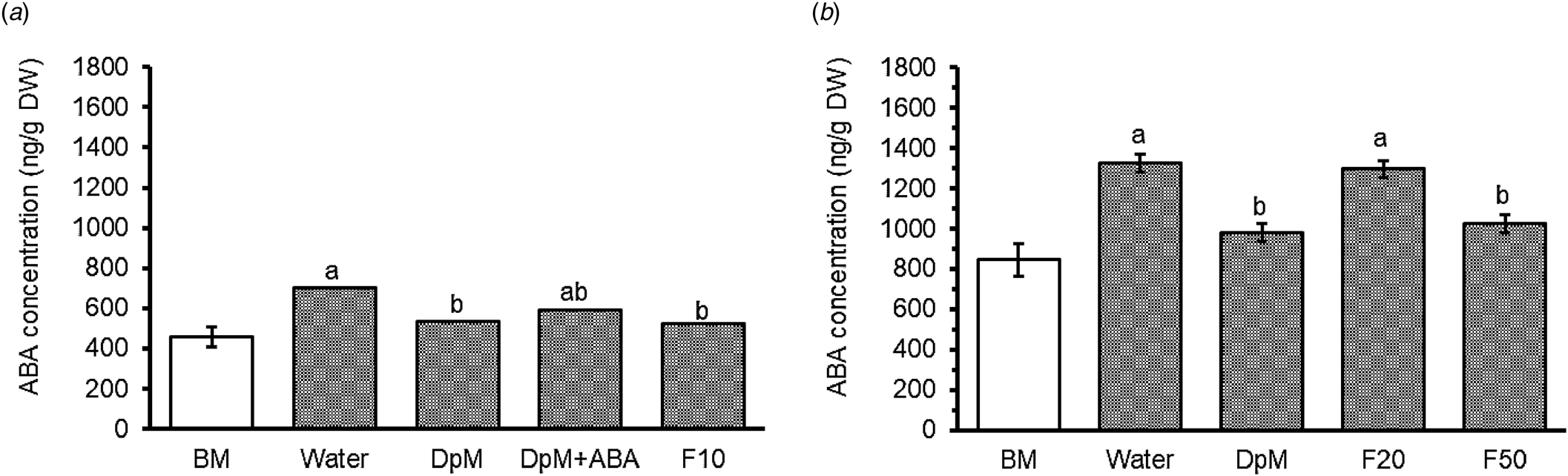
Figure 3. Spray type effects on wheat spike abscisic acid (ABA) concentration under progressive drought in (a) spring (P = 0.017) at GS68 and (b) summer (P < 0.001) at GS68. DpM, DpM + ABA, F10, F20 and F50 stand for di-1-p-menthene, a mixture of di-1-p-menthene and exogenous abscisic acid and fluridone concentrations at 10, 20 and 50 μM, respectively. Error bars are common standard errors of means from the ANOVA table. Different letters indicate significant differences between spray treatments (Tukey test; P < 0.05). BM stands for the benchmark plots which were fully irrigated throughout and were not included as treatments in the ANOVA. Figure 3(a) was constructed from back transformed data; the spray type means on the natural log scale in the order water, fluridone at a concentration of 10 μM (F10), di-1-p-menthene (DpM), and DpM + ABA being 6.55, 6.26, 6.29 and 6.39 and s.e.m. (df,18): 0.063.
Gas exchange
Drought stress reduced transpiration rates in spring and in summer by 17 and 23%, respectively (Fig. 4). Di-1-p-menthene further significantly suppressed transpiration in spring and in summer, by an average of 21%. The application of fluridone had no significant effect on transpiration compared to water-sprayed plants, although at the higher concentration fluridone increased transpiration above that of the water-sprayed plants at 11 days after spraying (Fig. SI-4). Surprisingly, the application of DpM + ABA resulted in significantly higher transpiration compared to DpM alone, with transpiration similar to water-sprayed plants (Fig. 4).

Figure 4. Spray type effects on wheat transpiration under progressive drought in (a) spring (P < 0.001) at GS75 and (b) summer (P = 0.001), at GS73. DpM, DpM + ABA, F10, F20 and F50 stand for di-1-p-menthene, a mixture of di-1-p-menthene and exogenous abscisic acid and fluridone concentrations at 10, 20 and 50 μM, respectively. Different letters indicate significant differences between spray treatments (Tukey test; P < 0.05). BM indicates the benchmark plots which were fully irrigated throughout and were not included as treatments in the ANOVA. Measurements in summer were taken twice, at GS54 and GS73 (see Figs SI-4 and SI-5 in supplementary information).
Drought consistently reduced net photosynthesis, particularly in summer. Application of DpM in spring and DpM, F20 and F50 in summer further reduced net photosynthesis, significantly (Fig. 5). Di-1-p-menthene was the most potent spray type in suppressing photosynthetic activity at GS73.

Figure 5. Spray type effects on wheat net photosynthesis under progressive drought in (a) spring (P < 0.001) at GS 75 and (b) summer (P < 0.001) at GS73. DpM, DpM + ABA, F10, F20 and F50 stand for di-1-p-menthene, a mixture of di-1-p-menthene and abscisic acid and fluridone concentrations at 10, 20 and 50 μM, respectively. Error bars are common standard errors of means from the ANOVA table. Different letters indicate significant differences between spray treatments (Tukey test; P < 0.05). BM indicates the benchmark plots which were fully irrigated throughout and were not included as treatments in the ANOVA.
Yield and yield components
All yield component quantities in spring were higher than in summer (Tables 1 to 3). Drought reduced all yield components except the number of grains per spike in spring and the thousand grain weight in both seasons. Compared to the benchmark plots, drought reduced the number of fertile tillers per plant (74%), fertile spike density (54%), grains per m2 (63%) and yield (42%). Plants in spring developed fertile secondary tillers in all plots but the number of grains per spike was not significantly different between treatments (results not shown), however, there were much fewer tertiary tillers. Only a few plots had plants with secondary tillers in summer. In both seasons, application of DpM improved the number of tillers per plant (75%), fertile spike density (37%), number of grains per m2 (26%) and grain yield (27%), relative to the water-sprayed plants, averaged across the two seasons. DpM also improved the number of grains per spike in main stems (20%) in summer only. The only yield component significantly improved with a spray type other than DpM was the number of tillers per plant, which was higher in summer, with F20. Overall, the effects of fluridone on grain yield and yield components were negligible.
Table 1. Spray type effects on wheat under progressive drought for number of fertile tillers per plant and fertile spike density

1 DpM, DpM + ABA, F10, F20 and F50 stand for di-1-p-menthene, a mixture of di-1-p-menthene and abscisic acid and fluridone concentrations at 10, 20 and 50 μM, respectively. Different letters indicate significant differences between spray treatments (Tukey test; P < 0.05). Benchmark plots were fully irrigated throughout and were not included as treatments in the ANOVA.
Table 2. Spray type effects on wheat under progressive drought for number of grains per spike of primary tillers (T1), number of grains per spike of main stems (MS) and thousand grain weight
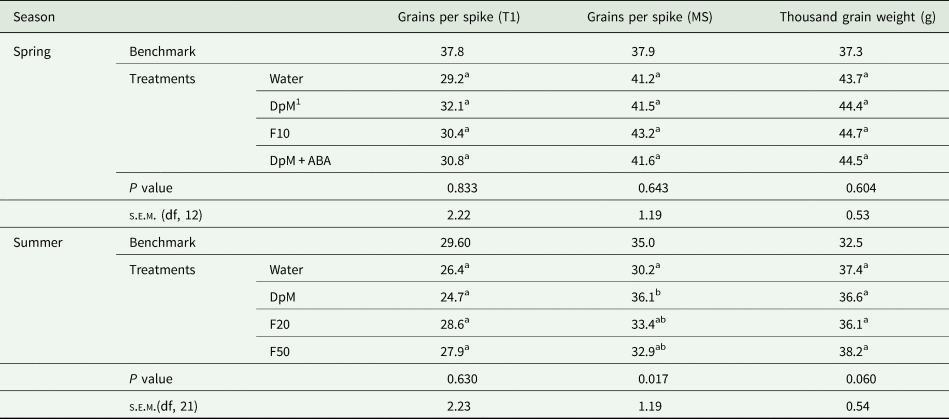
1 DpM, DpM + ABA, F10, F20 and F50 stand for di-1-p-menthene, a mixture of di-1-p-menthene and abscisic acid and fluridone concentrations at 10, 20 and 50 μM, respectively. Different letters indicate significant differences between spray treatments (Tukey test; P < 0.05). Benchmark plots were fully irrigated throughout and were not included as treatments in the ANOVA.
Table 3. Spray type effects on wheat under progressive drought for number of grains per m2 and yield per ha (tonnes)
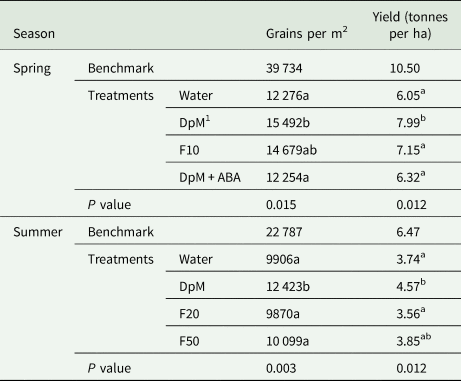
1 DpM, DpM + ABA, F10, F20 and F50 stand for di-1-p-menthene, a mixture of di-1-p-menthene and abscisic acid and fluridone concentrations at 10 μM, 20 μM and 50 μM, respectively. Different letters indicate significant differences between spray treatments (Tukey test; P < 0.05). Benchmark plots were fully irrigated throughout and were not included as treatments in the ANOVA.
Regression analysis
Figure 6 summarizes the significant associations between spike ABA and yield and yield components. The associations between transpiration and yield components were also significant (Fig. 7). The similarity of the associations between spike ABA and yield and yield components on one hand, and transpiration and yield and yield components on the other reflects the collinearity between these explanatory variates. TGW was left out of the regression analysis, because it was not significant. Grain number per spike was also left out of the regression analysis, being significant only in summer. Overall, both spike ABA concentration and transpiration showed significant negative associations with tillers per plant, fertile spike density, grains per m2 and yield per ha.

Figure 6. Regression analysis of wheat spike abscisic acid (ABA) concentration with (a) tillers per plant, (b) fertile spike density, (c) grains per m2 and (d) grain yield. Plants were grown under progressive drought and treated with different spray types. Data combines results from experiments performed in spring and summer. Water, DpM, DpM + ABA and F10, stand for spring water spray, di-1-p-menthene, a mixture of di-1-p-menthene, exogenous abscisic acid and fluridone at 10 μM; Water1, DpM1, F20 and F50, stand for summer water spray, di-1-p-menthene and fluridone at 20, 50 μM, respectively.

Figure 7. Relationship between tillers per plant (a), fertile spike density (b), grains per m2 (c) and grain yield (d) and transpiration of wheat under progressive drought treated with different spray types. Data combines results from experiments performed in spring and summer. Water, DpM, DpM + ABA and F10, stand for spring Water spray, di-1-p-menthene, a mixture of di-1-p-menthene and exogenous abscisic acid, and fluridone at 10 μM; Water1, DpM1, F20 and F50, stand for summer water spray, di-1-p-menthene and fluridone at 20 50 μM, respectively.
Discussion
In the current study, a film antitranspirant (di-1-p-menthene), applied to droughted spring wheat during flag leaf emergence (GS37), was effective in reducing the endogenous ABA concentration and improving yield. The application of a film antitranspirant was effective in mitigating the impact of terminal drought on yield in both seasons. In each season, yield improvement was associated with decreased spike ABA, lower transpiration and improved water status (measured in summer only) during the reproductive growth stage. The use of an ABA inhibitor produced varied results, but overall had no significant impact on yield despite a similar reduction in spike ABA. The results confirm that yield under drought can be increased using an antitranspirant (Weerasinghe et al., Reference Weerasinghe, Kettlewell, Grove and Hare2016) and that this yield increase is in part due to improved spike fertility, but suggest that the increased fertility is not solely dependent on reduced spike ABA. Furthermore, the observed improvement in spike fertility is not exclusively contingent on the downregulation of spike ABA concentration.
Drought treatments and drought impacts
Although drought has damaging effects at any growth stage, reproductive-stage water deficit has the worst impact (Yu et al., Reference Yu, Jiang and Guo2019). Drought-induced ABA accumulation in anthers represses pollen development (Ji et al., Reference Ji, Dong, Shiran, Talbot, Edlington, Hughes, White, Gubler and Dolferus2011; Bheemanahalli et al., Reference Bheemanahalli, Impa, Krassovskaya, Vennapusa, Gill, Obata and Jagadish2020). Mediterranean-type drought was successfully simulated in both spring and summer rain shelter experiments, with no additional irrigation following pre-planting flooding. The level of drought achieved through progressive soil drying was sufficient to induce terminal water deficit stress. Some of the soil moisture readings in summer were below the PWP, suggesting excessive drought, but all readings remained within the target range given the precision of the instruments used.
Averaged across the two experiments, drought stress reduced grain yield by 42% for the droughted plots compared to the fully irrigated benchmark plots. This is comparable to the yield reduction seen when rainfed wheat is grown in marginal areas (Monneveux et al., Reference Monneveux, Jing and Misra2012; Farooq et al., Reference Farooq, Hussain and Siddique2014; Figueroa-Bustos et al., Reference Figueroa-Bustos, Palta, Chen, Stefanova and Siddique2020). The RWC results (≈ 80% RWC at GS73 in the water-sprayed plots) are also consistent with moderate to severe drought values observed in other studies (e.g., Ghulam et al., Reference Ghulam, Ahmed, Zeng, Yang, Arslan, Zeeshan, Shahid, Ikram, Ullah, Mohammed and Alghanem2020; Sharma et al., Reference Sharma, Brar and Knipfer2023). By physically blocking stomata and suppressing transpiration, DpM significantly increased RWC (≈ 86% RWC at GS73 in the DpM plots). Improved plant water status under drought conditions is desirable for sustaining plant metabolism especially during reproductive growth (Farooq et al., Reference Farooq, Wahid, Kobayashi, Fujita and Basra2009; Kettani et al., Reference Kettani, Ferrahi, Nabloussi, Ziri and Brhadda2023). This is consistent with past studies in which film antitranspirants were used to improve plant water status at the reproductive stage (Weerasinghe et al., Reference Weerasinghe, Kettlewell, Grove and Hare2016; Mphande et al., Reference Mphande, Kettlewell, Grove and Farrell2020).
Drought, di-1-p-menthene and gas exchange
Drought stress downregulates both transpiration and photosynthesis (Maghsoudi et al., Reference Maghsoudi, Emam and Pessarakli2016). In droughted crops, the objective of applying antitranspirants is to protect the sensitive reproductive growth stages by further reducing transpirational water loss (Faralli et al., Reference Faralli, Grove, Hare, Boyle, Williams, Corke and Kettlewell2016). Regardless of the mode of action, antitranspirants suppress both transpiration and photosynthesis, in the case of DpM, this is achieved by physically blocking stomata (Mphande et al., Reference Mphande, Kettlewell, Grove and Farrell2020). Antitranspirant application also reduces gas exchange, but in certain cases has been shown to mitigate drought stress despite the inhibiting effects on photosynthesis (Xiang et al., Reference Xiang, Vickers, Hare and Kettlewell2023). Here, drought reduced transpiration by 20% and photosynthesis by 20% on average compared to the benchmark plots. Di-1-p-menthene further inhibited both transpiration and photosynthesis by 21 and 26%, respectively. Despite the considerable inhibition of photosynthesis, reduced transpiration with DpM during the reproductive growth stage drought was associated with higher yields, in agreement with past research (e.g., in wheat [Abdullah et al., Reference Abdullah, Aziz, Siddique and Flower2015] and rapeseed oil [Xiang et al., Reference Xiang, Vickers, Hare and Kettlewell2022]).
Under progressive drought, reduced transpiration can be beneficial for maintaining plant development and yield (Faralli et al., Reference Faralli, Grove, Hare, Boyle, Williams, Corke and Kettlewell2016; Mphande et al., Reference Mphande, Farrell, Grove, Vickers and Kettlewell2022). This is corroborated by the regression analysis as transpiration was negatively associated with yield and most of the yield components under drought stress conditions. This suggests that the positive linear relationship between transpiration and grain yield (Acevedo et al., Reference Acevedo, Silva, Silva, Curtis, Rajaram and Macpherson2002; Velazquez et al., Reference Velazquez, Alberdi, Paz, Aguirrezabal and Irujo2017; Vadez et al., Reference Vadez, Choudhary, Kholova, Hash, Srivastava, Kumar, Prandavada and Anjaiah2021) under well-watered conditions does not hold under prolonged drought stress, as Siahpoosh and Dehghanian, (Reference Siahpoosh and Dehghanian2012) observed in wheat. This is in part because some photosynthesis is retained even with closed stomata, as stomatal closure does not account for all of the drought-induced inhibition of photosynthesis (Saud et al., Reference Saud, Yajun, Fahad, Hussain, Na, Xin and Alhussien2016). Generally, under water-limited soil conditions, reducing transpiration to conserve water for reproductive processes is a desirable drought tolerance trait (Kholova et al., Reference Kholova, Hash, Kakkera, Koova and Vadez2010; Sinclair et al., Reference Sinclair, Devi, Shekoofa, Choudhary, Sadok, Vadez, Riar and Rufty2017), e.g., a maize genotype with a transpiration-limiting trait under drought stress improved yield by 6.5% (Gaffney et al., Reference Gaffney, Schussler, Loffler, Cai, Paszkiewicz, Messina, Groeteke, Keaschall and Cooper2015).
Drought, di-1-p-menthene, exogenous abscisic acid and spike abscisic acid
The DpM + ABA treatment was designed to replicate the positive impacts of DpM on water status while retaining high ABA levels comparable to that of the droughted control plants. However, the treatment did not significantly increase ABA, which was intermediary between the droughted control and the DpM spray. Combining DPM with exogenous ABA (the DpM + ABA treatment) eliminated any significant benefit to yield due to DpM. Under progressive drought, Mphande et al. (Reference Mphande, Farrell, Grove, Vickers and Kettlewell2022) observed that exogenous ABA was ineffective in increasing spike ABA concentration; although it consistently increased the accumulation of the hormone in well-watered plants. Once ABA reaches an upper limit, negative feedback mechansims prevent its further accumulation (Liu et al., Reference Liu, Li, Li, Su, Ge, Li, Li and Liu2016).
The levels of spike ABA in the spring experiment for the current study were slightly higher than what Mphande et al. (Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021) reported in an experiment also established in spring (e.g., for the water-sprayed treatment 700 ng/gDW with sampling at GS68 against just below 569 ng/gDW with sampling at GS55, respectively). However, the concentration in the summer experiment for the same treatment was much higher, being 1325 ng/gDW. These differences can be attributed to growth stage and seasonal effects. It is known that endogenous ABA increases during grain filling to enhance senescence and remobilization of water-soluble carbohydrates stored in stems and leaf sheafs (Kondhare et al., Reference Kondhare, Farrell, Kettlewell, Hedden and Monaghan2015; Mphande et al., Reference Mphande, Nicolas, Seneweera, Bahrami and Bbebe2016; Zhang et al., Reference Zhang, Liu, Li, Ma, Wang, Su and Yang2020). The findings in the current study are consistent with past reports, which also linked elevated endogenous ABA to reduced crop productivity (e.g., suppression of seed set and grain yield in maize [Setter et al., Reference Setter, Flannigan and Melkonian2001; Xu et al., Reference Xu, Qian, Li, Zhou, Tian, Yuan, Wang and Yang2023]; and wheat [Saini and Aspinall, Reference Saini and Aspinall1982; Westgate et al., Reference Westgate, Passioura and Munns1996]; and abortion of reproductive organs in chickpeas [Pang et al., Reference Pang, Turner, Khan, Du, Xiong, Colmer, Devilla, Stefanova and Siddique2017]). Wheat genotypes that are drought-tolerant prevent spike ABA accumulation under drought conditions through repression of ABA biosynthesis genes and upregulation of catabolic genes (Ji et al., Reference Ji, Dong, Shiran, Talbot, Edlington, Hughes, White, Gubler and Dolferus2011). The regression analysis in the current study supports these reports, as elevated ABA, induced by drought stress, was negatively associated with fertile tillers per plant, fertile spike density and grain number per m2, which are the foundation of grain yield. Nonetheless, the regression analysis figures suggest that overall, the spike ABA concentration was not the sole or key factor in determining yield formation, as inhibition of ABA with fluridone did not significantly improve yield. This implies that yield improvement with antitranspirant application under drought conditions is not solely dependent on reducing the damaging effects of elevated spike ABA.
Drought, di-1-p-menthene, fluridone and spike ABA
ABA-inhibitors can be used to counteract plant stress responses that are driven by ABA. Typically, soil drying induces increased ABA biosynthesis, which causes stomatal closure allowing plants to conserve water (Saradadevi et al., Reference Saradadevi, Bramley, Siddique, Edwards and Palta2014). Fluridone, an aquatic herbicide whose mode of action involves the inhibition of chlorophyll and carotenoid biosynthesis (Caudill et al., Reference Caudill, Jones, Anderson, Madsen, Gilbert, Shuler and Heilman2019), inhibits ABA biosynthesis, interfering with the plant's ability to close the stomata, so that when drought occurs the plant water status declines, (Kondhare et al., Reference Kondhare, Hedden, Kettlewell, Farrell and Monaghan2014; Vysotskaya et al., Reference Vysotskaya, Arkhipova, Kudoyarova and Veselov2018; Zou et al., Reference Zou, Zou, Zhao, Xia, Qian, Wang and Yin2018). Fluridone is widely used in research where inhibition of ABA biosynthesis is desired (Zou et al., Reference Zou, Zou, Zhao, Xia, Qian, Wang and Yin2018). For example, fluridone has been found to downregulate endogenous ABA in wheat (Yang, et al., Reference Yang, Zhang, Liu, Wang and Liu2006); increase transpiration rates in rice (Hsu and Kao, Reference Hsu and Kao2005) and peanuts (Liu et al., Reference Liu, Peng, Wang, Lian and Shen2010); and reduce RWC in maize (Zhang et al., Reference Zhang, Gao, Hu, Zhang, Wang and Ashraf2012) and rice (Wei et al., Reference Wei, Wang, Yang, Wang, Guo and Kang2015). In the current study, the highest concentration of fluridone (F50) significantly reduced ABA and increased transpiration, however, no increase was seen in RWC and there was no significant change in yield or yield components.
Suppression of photosynthetic activity and curtailing of the accumulation of spike ABA were common to both fluridone and DpM, but only DpM improved water status and yield. This suggests that the improved water status during grain development is the more important mechanism in mitigating yield losses under drought. The regression curves show that yield was negatively associated with both spike ABA and transpiration. Indeed, reduction in endogenous ABA concentration can only come with improved plant water status resulting from decreased transpiration, which conserves plant water (Oyedeji, Reference Oyedeji, Tripathi, Bhadouria, Srivastava, Singh and Devi2024). It is not surprising that ABA and transpiration were correlated in this study. Reduced transpiration with DpM was expected to drive a reduction in ABA concentration in droughted plants. In a study conducted by Faralli et al. (Reference Faralli, Grove, Hare, Boyle, Williams, Corke and Kettlewell2016), involving drought-affected rapeseed plants sprayed with a foliar antitranspirant, it was noted that a reduction in transpiration coincided with an improvement in the plant water potential. Consequently, it can be anticipated that the improvement in plant water status results in the breakdown of endogenous ABA. This sequential relationship is underscored by the fact that the elevation in plant water potential acts as the trigger for the decline in ABA concentration, as shown by Davies et al. (Reference Davies, Kudoyarova and Hartung2005) and Rossdeutsch et al. (Reference Rossdeutsch, Edwards, Cookson, Barrieu, Gambetta, Delrot and Ollat2016). The strong collinearity (r = 0.93) between spike ABA and transpiration observed is further evidence of the close relationship between these variables.
Under ideal soil moisture conditions, the rate of transpiration is positively correlated with biomass accumulation but not under water deficit when higher water use efficiency becomes more important (Velazquez et al., Reference Velazquez, Alberdi, Paz, Aguirrezabal and Irujo2017; Vadez et al., Reference Vadez, Choudhary, Kholova, Hash, Srivastava, Kumar, Prandavada and Anjaiah2021; Saudy et al., Reference Saudy, Salem and Abd El-Momen2023). Reduced stomatal conductance mediated through an increase in drought-induced ABA (Lee and Luan, Reference Lee and Luan2012) contributes to improving the intrinsic water use efficiency (Sikder et al., Reference Sikder, Foulkes, West, De Silva, Gaju, Greenland and Howell2015). The application of an ABA inhibitor under drought conditions would be expected to increase transpiration and overtime reduce water content (Dodd, Reference Dodd2013; Schippers et al., Reference Schippers, Schmidt, Wagstaff and Jing2015). Application of fluridone did not consistently alter transpiration or plant water status, however at the highest concentration it was associated with a reduction in water content. It is likely that high residual ABA levels or the restricted water supply limited the potential for increased transpiration in this case (despite the reduction in ABA).
Drought, di-1-p-menthene and yield
Application of DpM, which was associated with reduced spike ABA and transpiration, improved grain yield by 27%, averaged for the experiments. This is comparable to Mphande et al. (Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021) who found that DpM improvement of grain yield was correlated with reduced endogenous ABA. Many previous studies have shown that DpM improves grain yield of droughted wheat. The reported percentage improvements vary (e.g., 30% (Abdullah et al., Reference Abdullah, Aziz, Siddique and Flower2015), 42% (Kettlewell and Holloway, Reference Kettlewell and Holloway2010), 29% (Mphande et al., Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021) and 10% (Weerasinghe et al., Reference Weerasinghe, Kettlewell, Grove and Hare2016)). In the current study, DpM significantly improved grain yield by 27% (average for the two experiments) compared to the control (water-sprayed) treatment.
Agronomically, DpM improvement of yield was achieved through gains in the number of grain-bearing tillers per plant, fertile spike density and grain number per m2. Under given environmental conditions, the grain number per spike declines with increasing plant density beyond the optimum (Li et al., Reference Li, Cui, Ni, Zheng, Yang, Jin, Chen, Wang and Yin2016). There was limited evidence that DpM improved grain number per spike, as this only occurred in main stem spikes in the summer experiment. In three of the previous field experiments, which were all established in spring, DpM did not have a significant effect on grain number per spike. This suggests that DpM does not have a large mitigative effect on meiotic-stage drought, which influences grain number per spike (Westgate et al., Reference Westgate, Passioura and Munns1996; Ji et al., Reference Ji, Dong, Shiran, Talbot, Edlington, Hughes, White, Gubler and Dolferus2011). Therefore, grain number per spike may be a less important trait in the DpM amelioration of drought in spring wheat. Di-1-p-menthene was associated with a significantly higher number of grain-bearing tillers per plant, fertile spike density and grain number per m2. Severe drought coinciding with reproductive development is known to induce abortion of flowering heads due to elevated ABA (Pang et al., Reference Pang, Turner, Khan, Du, Xiong, Colmer, Devilla, Stefanova and Siddique2017), undermining fertile spike density (Katerji et al., Reference Katerji, Mastrorilli, van Hoorn, Lahmer, Hamdy and Oweis2009). Reproductive development phase is bounded by end of vegetative development, which is marked by floral initiation, and anthesis (Slafer et al., Reference Slafer, Kantolic, Appendino, Tranquilli, Miralles, Savin, Sadras and Calderini2015). Some of the key processes in between are spikelet morphogenesis, spike growth and differentiation of floret primordia into florets (Sreenivasulu and Schnurbusch, Reference Sreenivasulu and Schnurbusch2012; Gol et al., Reference Gol, Tome and Von Korff2017). Specific timings of these events can be found in Mphande et al. (Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021). It is also known that drought stress during these reproductive phases in wheat shortens the growth cycle; and decreases the number of spikes per m2 and grain yield (Day and Intalap, Reference Day and Intalap1970). Considering the current study and the findings of Mphande et al. (Reference Mphande, Farrell, Grove, Vickers and Kettlewell2021), it is plausible to suggest that DpM amelioration of drought in spring wheat is connected to spike morphogenesis and fertility. This effect is mediated directly via improved plant water status and perhaps indirectly by ABA levels in the spikes.
The current study demonstrates the potential of DpM as a drought mitigation technique that can contribute significantly to reducing yield losses in spring wheat in Mediterranean-type climates and semi-arid regions where drought events are predictable. For the cost-benefit analysis of using DpM to improve yield of droughted spring wheat, see the supplementary information file.
Conclusion
The current study examined drought-induced yield losses by manipulating water status and ABA levels using a film antitranspirant, exogenous ABA, and fluridone and showed that di-1-p-menthene and fluridone had similar effects on spike ABA but opposing effects on transpiration. Regression analysis highlighted the importance of manipulating both spike ABA and transpiration in explaining grain yield under water deficit stress. Di-1-p-menthene decreased transpiration and improved grain yield, while fluridone increased transpiration but with no effect on yield, emphasizing the need to reduce both spike ABA and transpiration for improved grain yield in droughted spring wheat. The study suggests that amelioration of drought stress with di-1-p-menthene in spring wheat is primarily mediated through transpirational water loss with secondary effects on the accumulation of spike ABA.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0021859624000157
Acknowledgements
We thank Telford Seventh-Day Adventist Church for helping with manually counting hundreds of envelopes of grain for determining number of grains per spike and thousand grain weight. We also thank Dominic Scicchitano of the Miller Chemical and Fertilizer Corp. for free supplies of di-1-p-menthene. We also thank technical staff at Harper Adams University for assistance rendered in various ways. This work was based on the PhD thesis of the first author: The Role of Di-1-p-menthene and Drought Signalling in Yield Formation in Spring Wheat, Wiza Mphande, 2021, Ph.D. thesis, Harper Adams University, UK.
Author contributions
PK conceived this research. WM, AF, LV, IG and PK designed the research. WM conducted experiments. WM analysed data. WM wrote the manuscript. PK, AF, LV and IG assisted in reading and correcting the manuscript.
Funding statement
There was no funding exclusively targeted to support this research, rather the work was supported as part of the sponsorship by the Commonwealth Scholarship Commission, UK, awarded to Wiza Mphande.
Competing interests
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the production of this work.
Ethical standards
Not applicable.
Availability of data and material
All data generated and analysed in support of the findings of this research have been included in this published article and its associated supplementary information file.













