Dementia, the most common form being Alzheimer's disease, is characterised by progressive and profound loss of memory, cognitive function and ability to carry out daily functional activities of living. It has been associated with various genetic and environmental risk factors(Reference Blennow, de Leon and Zetterberg1). Its prevalence is increasing rapidly worldwide(Reference Ferri, Prince and Brayne2) and it represents a major public health concern. Therefore, any delay in its onset will have significant social and economic impacts, making early intervention an international imperative. It has been suggested that mild cognitive impairment (MCI) is possibly the earliest stage of detectable dementia(Reference Gauthier, Reisberg and Zaudig3) and may be the optimal time to intervene with preventive therapies(Reference Chertkow4). Furthermore, major and minor depression often occurs in patients with MCI(Reference Gabryelewicz, Styczynska and Pfeffer5), and this increases the risk of progressing to dementia(Reference Modrego and Ferrández6).
In Western populations, there has been a marked decrease in the dietary intake of n-3 PUFA along with a substantial increase in the consumption of n-6 PUFA(Reference Simopoulos7). The long-chain n-3 PUFA DHA (22 : 6n-3) is highly concentrated in the brain and has been associated with numerous structural and functional roles(Reference Assisi, Banzi and Buoinocore8–Reference Haag10). The long-chain n-3 PUFA EPA (22 : 5n-3) is also thought to have important functions in the brain(Reference Hibbeln, Ferguson and Blasbalg11) via the synthesis of benign eicosanoids that tend to counter the inflammatory, thrombotic and primarily vasoconstrictor properties of eicosanoids produced from the n-6 PUFA arachidonic acid (AA; 20 : 4n-6)(Reference Simopoulos12).
With ageing, neural membrane fluidity is compromised due to the increased presence of cholesterol, the reduced activity of desaturase enzymes, blockages to phospholipid pathways and increased oxidative stress(Reference Yehuda, Rabinovitz and Carasso13), all of which are inversely associated with PUFA. Brain autopsies of Alzheimer's disease patients have shown significantly higher saturated fat and lower n-3 PUFA content in the hippocampus and frontal lobes (governing memory and executive function, respectively) compared with age-matched controls(Reference Söderberg, Edlund and Kristensson14), consistent with reports of decreased hippocampus size and function in Alzheimer's disease patients(Reference Yehuda, Rabinovitz and Carasso13).
To date, few clinical trials evaluating the benefits of n-3 PUFA in patients with dementia have been published, although more are under way(Reference Sinn, Milte and Howe15). There are some reports of benefit(Reference Terano, Fujishiro and Ban16, Reference Yehuda, Rabinovitz and Carasso17). However, larger trials indicate that people with MCI are more likely to respond. These studies investigated supplementation of n-3 PUFA in patients with dementia but only found improvements in the subgroups with mild cognitive decline(Reference Chiu, Su and Cheng18–Reference Kotani, Sakaguchi and Warashina20). In elderly, cognitively healthy populations, there does not appear to be benefit(Reference Dangour, Allen and Elbourne21, Reference van de Rest, Geleijnse and Kok22); however, a recent study did report improvements following DHA supplementation in elderly people with age-related cognitive decline(Reference Yurko-Mauro23). This body of studies supports indications that intervention with n-3 PUFA may be more effective in early stages of cognitive decline, yet no studies have yet focused exclusively on people with MCI and therefore possibly in the early stages of dementia onset.
Depression is very common in older adults with MCI and increases the risk of developing dementia(Reference Modrego and Ferrández6). Clinical trials investigating the efficacy of n-3 supplementation in alleviating depressive symptoms have found positive results in about half of published studies(Reference Sinn, Milte and Howe15, Reference Sinclair, Begg and Mathai24). Among other methodological differences, studies have used varying ratios of EPA:DHA; some have used only DHA or ethyl-EPA.
The purpose of the present study was to compare the effects of high-EPA and high-DHA fish oils with n-6 PUFA linoleic acid (LA; 18 : 2n-6), a vegetable oil, on depressive symptoms, quality of life, memory and executive function in elderly people with MCI, to investigate the associations between increased erythrocyte DHA and EPA levels and any improved symptoms, and to determine whether those with lower baseline erythrocyte PUFA levels respond more readily to supplementation.
Experimental methods
Participants and procedure
Eligibility criteria considered discussions by the International Working Group on MCI(Reference Gauthier, Reisberg and Zaudig3, Reference Winblad, Palmer and Kivipelto25). Identification of MCI is generally defined as signs of cognitive decline beyond those expected for age but not dementia. Therefore, subjective complaints of memory loss but otherwise normal daily functioning are recommended, along with cognitive assessment scores ≥ 1·5 sd below the population mean. We screened elderly people aged over 65 years who had self-reported memory loss, were able to maintain normal daily functioning and gave informed consent, did not eat fish more than once per week, and had not taken any fish oil supplements within the previous 3 months. They had to be prepared to attend a screening, complete two rounds of assessments, take daily supplements, keep a weekly diary of fish intake and not to consume fish more than once per fortnight during the study. Those who met the following criteria after screening were invited to take part: standardised Mini-Mental State Examination scores ≥ 22 (a score ≤ 20 indicates possible dementia), recall memory (Verbal Paired Associates Task(Reference Wechsler26)) age-standardised scores ≥ 1·5 sd below the population mean and/or age- and education-adjusted DemTect scores between 9 and 12(Reference Kalbe, Kessler and Calabrese27) (these tests are described further below).
According to Cohen's f, the required n to detect a medium effect size (d = 0·50) in a repeated-measures within–between interaction design for three groups with a power of 0·80 is sixty-six participants (twenty-two per group). Recruitment took place in Adelaide and Brisbane, Australia, via newspaper, magazine and radio advertisements, retirement villages (talks and letterbox drops), Alzheimer's Association, shopping malls and television. A flow chart of participants through the study is depicted in Fig. 1. After 6 months of extensive screening, fifty eligible volunteers were recruited: forty-four in Adelaide and six in Brisbane (see Table 1 for baseline characteristics), of whom forty completed the 6-month assessments. This provided adequate power to detect an effect size of d = 0·64, or d = 0·56 with all cases (n 50) included in the analyses (assuming a balanced dataset). The intervention took place between March 2009 and March 2010.

Fig. 1 Flow of participants through the study. UniSA, University of South Australia; QUT, Queensland University of Technology; MCI, mild cognitive impairment; LA, linoleic acid.
Table 1 Baseline sample characteristics (n 50)*
(Mean values, standard deviations, number of participants and percentages)

LA, linoleic acid; AD, Alzheimer's disease; MMSE, Mini-Mental State Examination; VPA, Verbal Paired Associates (age- and sex-adjusted standardised score, M = 10); GDS, Geriatric Depression Scale (scores >4 may indicate clinical depression); bpm, beats per minute; RAVLT, Rey Auditory Verbal Learning Test
* Mean values were not significantly different between the groups for any of these variables (one-way ANOVA or χ2 tests).
† Education: 1, years 8–10; 2, year 11; 3, year 12; 4, technical and further education (or 13–14 years of total education); 5, university; 6, postgraduate.
‡ Current smoker: ‘yes/no’.
§ Frequency of consuming more than two alcoholic drinks per d (1, never/rarely; 2, occasionally; 3, once per week; 4, few days per week; 5, daily). The SF-36 scales are reverse-scored, where necessary, so that a higher score always represents improved health; each scale has a different scale and number of items and therefore different possible total scores.
As participants were enrolled, they were independently allocated to one of three conditions (EPA-rich oil, DHA-rich oil or placebo) using the process of minimisation based on age, Geriatric Depression Scale (GDS) scores and sex, respectively. Altman & Bland(Reference Altman and Bland28) recommended this simple process for small trials with progressive enrolment in order to achieve balanced treatment groups without investigator bias. Of the participants, eighteen (36 %) had GDS scores of 4 or above, indicating possible depression. All researchers involved with participants, data entry or analysis and participants were blinded to treatment conditions. Supplements were coded and labelled independently; participants were asked at 6 months which condition they thought they were in (and why) and these observations were recorded.
Eligible volunteers completed assessments at baseline and 6 months at the University of South Australia (Adelaide, SA, Australia) or the Institute of Health and Biomedical Innovation (Brisbane, QLD, Australia) in the morning following an overnight fast. Height, weight and blood pressure were measured and blood samples collected. Volunteers were then offered breakfast (toast, cereal, tea, orange juice and water) and underwent 45 min of cognitive assessments. The same instructions and protocol were used for each volunteer's assessments.
The project was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki, and all procedures involving human participants were approved by the Human Research Ethics Committees at the University of South Australia and Queensland University of Technology. Informed written consent was obtained from all participants.
Supplements
Participants were randomly allocated to EPA-rich fish oil, providing 1·67 g EPA+0·16 g DHA/d, DHA-rich fish oil, providing 1·55 g DHA+0·40 g EPA/d, or control (safflower oil), providing 2·2 g LA (n-6 PUFA)/d for 6 months. The oils were taken in four capsules daily. Participants were asked to return all of their jars at the end of the study and capsules were counted to assess compliance. Adverse events were recorded on the 6-month questionnaires and via regular phone calls throughout the study.
Assessment tools
All of the following assessments were conducted at baseline and 6 months.
Depression and quality of life
Geriatric depression scale
The fifteen-item short form of the GDS(Reference Sheikh and Yesavage29) was our primary outcome measure of depressive symptoms. Respondents circle ‘yes’ or ‘no’ in response to a series of statements, e.g. ‘Are you in good spirits most of the time’, ‘Do you feel that your life is empty’. A score of one point is allocated for each response that is indicative of possible depression. Therefore, a higher score indicates greater symptom severity; scores of 4 or above possible depression. The highest possible score is 15.
SF-36 health survey
This survey assesses eight domains of health and quality of life: physical functioning, limitations in usual role due to physical health problems (role – physical), bodily pain, general health, vitality, social functioning, limitations in usual role due to emotional problems (role – emotional) and mental health. By combining these domains, two further subscales are derived: physical health and mental health. We used the Australian adaptation(Reference Sanson-Fisher and Perkins30). Where necessary, scales are reverse-scored so that on all the scales, a higher score indicates better health. Each subscale varies in the length of items; each item response is given from a selection varying from ‘excellent’ to ‘poor’, ‘yes, limited a lot’ to ‘no, not limited at all’, ‘all of the time’ to ‘none of the time’, and so on, depending on the nature of the question.
Cognition
Subjective and objective tests were selected that can predict memory loss or dementia and may therefore be sensitive to meaningful improvements in or alleviations of cognitive decline. We selected specific subtests that may be sensitive to the effects of the intervention on memory and executive function and have been used previously in drug trials for MCI(Reference Raschetti, Albanese and Vanacore31). Where necessary, alternate versions of the tests were used for re-test purposes.
Memory
Memory Functioning Questionnaire
This questionnaire(Reference Gilewski and Zelinski32) assesses four domains of subjective perception of memory loss in the recent and more distant past: general frequency of forgetting, seriousness of forgetting, retrospective functioning and mnemonics usage.
Rey Auditory Verbal Learning Test
This test(Reference Lezak33) measures immediate, delayed recall and recognition memory(Reference Gilewski and Zelinski32). It requires participants to recall a list of fifteen words learned over five trials, immediately, then after a distracter list and again 20 min later. To test recognition memory, they are then given a list of words containing the target words and asked to select those that were on the list. The immediate memory score is calculated by adding trials 1–5 together (total 75), the delayed recall by totalling the number of words recalled on that trial (total 15) and recognition memory by totalling the number of words correctly recognised (total 15).
Digits Forward
It is a subtest from the Wechsler Adult Intelligence Scale (WAIS III)(Reference Wechsler34) that requires participants to recall a random series of numbers starting with 2 and increasing to 9. The task is discontinued following failure on both items of the same length. The maximum score that can be obtained is 16 (two on each trial).
Boston Naming Task
This task(Reference Lezak33) tests verbal expression and may predict dementia(Reference Kent, Bryan and Clark35). It consists of sixty large pictures of items ranging in familiarity from common items such as ‘pencil’ to less common objects such as ‘sphinx’ and is discontinued after eight consecutive failures. A score of one point is given for each correct response; the maximum score that can be obtained is 60.
Executive function/working memory
Letter-Number Sequencing
It is a subtest of the WAIS III(Reference Lezak33) that measures working memory and executive function. It requires participants to recall strings of digits and letters, reorganising them so that digits are recalled first in numerical order and then letters in alphabetical order, starting with two and increasing up to eight consecutive letters/numbers with two trials of each. It is discontinued following failure on both items of the same length. The maximum score that can be obtained is 14 (two on each trial).
Digits Backward
It is another subtest from the WAIS III that measures working memory and executive function(Reference Wechsler34). Following Digits Forward, participants are asked to recall number sequences in reverse order, starting with two and increasing to eight numbers and discontinued following failure on both items of each trial. The maximum score that can be obtained is 14 (two on each trial).
Trail-Making Task
This task(Reference Lezak33) is a measure of scanning and visuomotor tracking, divided attention, and cognitive flexibility. It requires volunteers to draw lines as quickly as they can to connect consecutively numbered circles on one worksheet (part A) and then connect consecutively numbered and lettered circles on another worksheet by alternating between the two sequences (part B). The score is derived by dividing the time taken (in s) during part B by the time taken during part A.
Stroop Colour-Word Test
This test(Reference Sachs, Clark and Pols36) measures the ability to ignore distracting information. Part A requires participants to read a sheet of colour names. In part B, they are asked to name the colours (which are incongruent with the words; for example, the word blue may be printed in the colour red) and ignore the words. The score is derived by dividing the time taken (in s) during part B by the time taken during part A.
Verbal fluency
Strategic retrieval of verbal material was assessed by tests of initial and excluded letter fluency(Reference Bryan, Luszcz and Crawford37, Reference Rosen38). These tests require participants to produce as many words as possible within 60 s either beginning with a designated letter (e.g. F, S) or not containing a designated letter (e.g. E, A), respectively. A score of one point is given for each correct word that is generated within 60 s for each respective trial.
Potential confounders
A background questionnaire assessed potential confounders: highest level of education, frequency of consuming more than two alcoholic drinks/d, whether or not they smoke, medications, medical conditions and family history of dementia (see Table 1).
Physiological parameters
We tested heart rate, BMI and blood pressure as potential confounders. Height and weight were measured by a stadiometer (Seca, Hamburg, Germany) and scales (Tanita, Tokyo, Japan), respectively. Blood samples were collected into 6 ml K3EDTA tubes, 5 ml serum tubes and 4 ml lithium heparin tubes (Interpath Services Pty Ltd, Heidelberg West, VIC, Australia) by venepuncture. Following the blood sample, volunteers were invited to rest quietly in a darkened room for a minimum of 10 min before three measures of blood pressure and heart rate were taken using an automatic blood pressure monitor (model IA1B; Omron, Kyto, Japan), with 5 min rest between each measure.
Assessment of fatty acid profiles
Relative proportions of individual fatty acids in erythrocytes were assessed using a method adapted from previously established methods(Reference Bligh and Dyer39–Reference Lepage and Roy41). Erythrocytes were isolated within 2 h of collection by centrifugation, washed in isotonic saline and stored at − 80°C. They were subsequently thawed and the lipids were extracted with chloroform and isopropanol (2:1). The organic phase containing the lipid was evaporated to dryness under a stream of N2 gas. The lipids were then transesterified with acetyl chloride in methanol toluene (4:1, v/v) at 100°C for 1 h. The resultant fatty acid methyl esters were extracted with 10 % potassium carbonate. Fatty acid methyl esters were separated and quantified using a Shimadzu 2010 gas chromatograph equipped with a 50 m capillary column (0·32 mm, inner diameter) coated with BPX-70 (0·25 μm film thickness; SGE Analytical Science Pty Limited, Ringwood, VIC, Australia). The injector temperature was set at 250°C and the detector (flame ionisation) temperature at 260°C. The initial oven temperature was 130°C and was programmed to rise to 220°C at 5°C/min. H2 was used as the carrier gas at a velocity of 36·4 cm/s. Fatty acid methyl esters were identified based on the retention time to authentic lipid standards (GLC-463; Nu-Chek Prep, Inc., Elysian, MN, USA).
Statistical analysis
Data analysis was conducted using SPSS Statistics (version 17.0; SPSS, Inc., Chicago, IL, USA). Baseline one-way ANOVA were used for parametric data and χ2 analyses for non-parametric data to examine whether demographic, physiological and dependent variables (mood, quality of life and cognition) were evenly distributed between the groups. Linear mixed model analyses were planned to investigate the effects of DHA and EPA v. LA on outcome variables (assuming data were missing at random). Regressions were used to investigate the correlations between increased PUFA levels and improved outcomes and baseline PUFA levels with improved outcomes. Due to the relatively small sample size, P values were set at 0·05 to avoid the possibility of a type II error.
Results
Adverse events
The treatments were well tolerated apart from a few gastrointestinal-related complaints. From the LA (control) group, three complained of bowel problems, one of nausea and one had reflux. From the EPA-rich oil group, there was one case reported for each of the following: flatulence, nausea, reflux and upset stomach, and one participant withdrew due to diverticulitis. No adverse events were reported in the DHA group.
In the LA, DHA and EPA groups, one, eight and six persons, respectively, guessed that they had been taking fish oil (P = 0·09); six, three and two individuals, respectively, guessed they were on placebo and two, five and three did not know. However, of the people who guessed the condition they were in, whether correctly or not, one, three and five from each group said it was because of a fishy taste; seven, seven and three said it was because they thought they were either not deriving or were deriving benefits (mostly physical, e.g. less aches and pains) from the treatment. Therefore, treatment blinding was successful on the whole.
Compliance
Capsule counts indicated excellent compliance, with an average supplement consumption of 93 % across the groups (DHA 97 %, EPA 94 % and LA 82 %). This is confirmed by changes in erythrocyte n-3 and n-6 PUFA levels after 6 months for each treatment group (see Table 2). As shown in Table 2, PUFA levels corresponded to the respective treatments (e.g. DHA levels increased in the DHA treatment group, etc.).
Table 2 Erythrocyte PUFA levels at baseline and 6 months in each treatment group (as percentage of fatty acids)*
(Mean values and standard deviations)
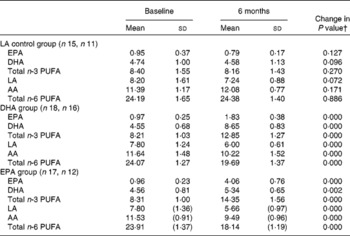
LA, linoleic acid; AA, arachidonic acid.
* Mean values were not significantly different between the groups for baseline PUFA levels (one-way ANOVA).
† Changes from baseline to 6 months were analysed using paired-samples t tests.
Primary analysis: effect of the supplement
There were eleven missing observations in the follow-up data; however, demographic data were complete. Logistic regression, with missingness as the dependent variable and demographic variables (sex, age, condition, history of dementia and Alzheimer's disease) as predictors, showed that none of the predictors were significant in predicting missingness, and the number of dropouts between the groups was not significantly different (P = 0·39). Therefore, it was reasonable to assume that the data were missing at random at worst. The application of a linear multilevel model was appropriate for this missing pattern. All information could be used and predicted values could be obtained for all participants at each time point(Reference Hedeker and Gibbons42). However, for the Memory Functioning Questionnaire, there were only twelve cases without missing data. Data imputation resulted in unacceptable data variability, so the Memory Functioning Questionnaire was not included in the analyses. Baseline comparisons between the groups showed no significant differences between the groups for outcome variables (data not shown). Treatment effects on outcome variables were assessed via visit (baseline and 6 months) × treatment interactions for the DHA and EPA groups compared with the LA group using linear mixed model analysis (Table 3).
Table 3 Treatment effects (time×treatment interactions) for linear mixed model analysis of all cases as randomised to treatment (EPA v. linoleic acid (LA), DHA v. LA) on depression, health-related quality of life and cognitive assessments over 6 months (n 50; LA n 15, DHA n 18, EPA n 17)
(Estimates, standard errors and 95 % confidence intervals)
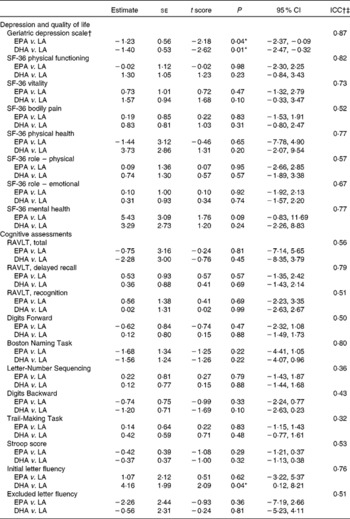
ICC, intra-class correlations; RAVLT, Rey Auditory Verbal Learning Test.
* Values were significantly different (P < 0·05).
† Geriatric Depression Scale results shown with a statistical outlier removed from the placebo group.
‡ ICC gives the percentage of variance explained by individual subject differences; over 50 % indicates reasonable goodness of fit.
Depressive symptoms
Following inspection of data distributions, one statistical outlier was removed from the placebo group in Brisbane as the GDS change score was >3 sd from the mean(Reference Tabachick and Fidell43) (nine at baseline and three at 6 months). According to Osborne & Overbay(Reference Osborne and Overbay44), ‘the presence of outliers can lead to inflated error rates and substantial distortions of parameter and statistic estimates when using either parametric or nonparametric tests’. Following the removal of this outlier, there was a significant improvement in depression (GDS) scores in the EPA (P = 0·04) and DHA (P = 0·01) groups compared with the LA group (Fig. 2; Table 3). The amount of variance explained was 87 %, indicating a very good fit of the model to the data. The magnitude of change was slightly less than 0·5 sd in the EPA group and greater than 0·5 sd in the DHA group, which is accepted as a meaningful improvement in health-related quality of life(Reference Norman, Sloan and Wyrwich45). Paired-samples t tests found that within-group changes within each condition were only significant in the DHA group (P = 0·02).
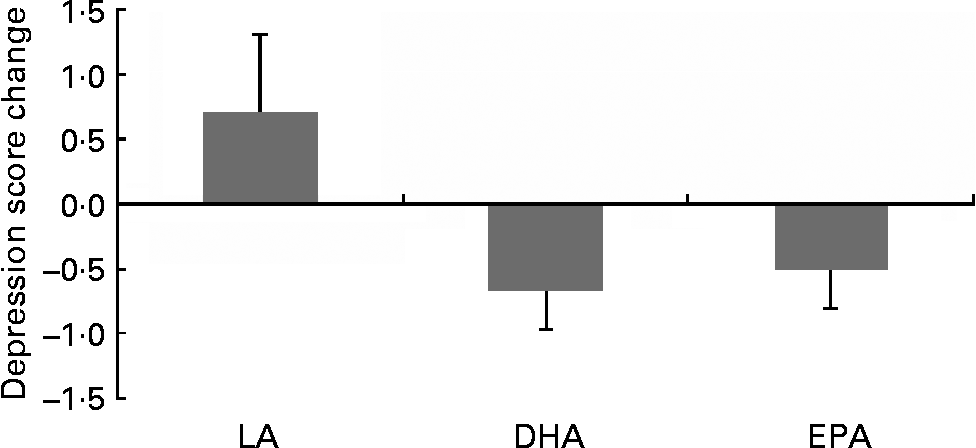
Fig. 2 Comparison of changes in depression scores in the treatment groups: DHA and EPA significantly different from linoleic acid (LA; P = 0·01, P = 0·03, respectively). Values are means, with standard deviations represented by vertical bars.
Quality of life
There were no significant treatment effects on outcomes using mixed model analysis for physical or mental quality of life parameters (Table 3).
Memory and cognitive function
From assessments of memory and cognitive function, only Initial Letter Fluency scores significantly improved in the DHA treatment group compared with the LA group (P = 0·04), explaining 76 % of the variance and therefore a very acceptable goodness of fit (Fig. 3; Table 3). The Digits Backward score also showed improvement in the DHA group, although not statistically significant (P = 0·10). The intra-class correlations in Table 3 indicate that the majority of outcome variables showed an acceptable fit of the data to the model and yet no other treatment effects.

Fig. 3 Comparison of changes in Initial Letter Fluency: DHA significantly different from linoleic acid (LA; P = 0·04).
Associations between increased erythrocyte PUFA and changes in outcome variables
Table 4 shows correlations between increased erythrocyte PUFA levels and improvements in outcome variables. Increased DHA plus EPA was correlated with improved depressive symptoms on the GDS (P = 0·02). A reduced AA:EPA ratio was also associated with reduced depressive symptoms, further supporting the mixed model analysis results. Similarly, increased AA was associated with declines in self-reported mental health and a reduced AA:EPA ratio was correlated with improved self-reported mental health and vitality.
Table 4 Correlations between change in PUFA and outcome variables from baseline to 6 months†

E+D, EPA+DHA; AA, arachidonic acid; n-6, total n-6 PUFA; A:E, AA:EPA ratio; GDS, Geriatric Depression Scale; RAV, Rey Auditory Verbal Learning Test, total recall; Rcl, Rey Auditory Verbal Learning Test, delayed recall; Rec, Rey Auditory Verbal Learning Test, recognition; BNT, Boston Naming Task; Strp, Stroop Colour-Word Test; ILF, Initial Letter Fluency; ELF, Excluded Letter Fluency; LNS, Letter-Number Sequencing; TM, Trail Making; DF, Digits Forward; DB, Digits Backward; PF, physical functioning; PH, physical health; MH, mental health; BP, bodily pain; Vit, vitality.
* P < 0·05, ** P < 0·01.
† All change variables are calculated so that any change in a positive direction indicates a positive change: i.e. for PUFA, an increase in PUFA levels; for AA:EPA, a decreased ratio; for all other variables, an improvement in the outcome (e.g. positive change in GDS scores, reduction in depressive symptoms; positive change in bodily pain, reduction in bodily pain, etc.).
Increased DHA was significantly associated with improved self-reported physical functioning on the SF-36, and of those subscales, improved bodily pain was associated with improved depressive symptoms (P < 0·05); therefore, this may to some degree have contributed to improved mood.
Baseline erythrocyte PUFA levels and improved outcome measures
There were no significant correlations between baseline erythrocyte PUFA levels and improved outcome measures. However, comparisons between those with EPA plus DHA levels below and above the 50th percentile indicate that those with lower levels tended to show greater improvement in GDS scores (P = 0·066), physical functioning (P = 0·069), physical health (P = 0·088) and bodily pain (P = 0·058).
Discussion
We investigated the effects of 6 months' supplementation with fish oils rich in either EPA or DHA v. a LA-rich control (safflower oil) on memory and executive function, depressive symptoms and health-related quality of life in elderly people with MCI. A third of the sample had scores that indicated possible depression, significantly greater than a healthy control group recruited at baseline(Reference Milte, Sinn and Street46), and they were equally assigned to each treatment condition. The treatment was well tolerated overall, with no adverse effects in the DHA group and a handful of gastrointestinal complaints in each of the LA and EPA groups.
Depressive symptom scores were significantly reduced after 6 months of high-EPA and high-DHA supplementation compared with LA and increased erythrocyte levels of EPA plus DHA were associated with improved depressive symptoms, as was a reduced ratio of AA:EPA. Increased erythrocyte DHA levels were associated with reduced bodily pain, and, although not significantly, with other items associated with physical health. Therefore, reduced depressive symptoms may to a small degree have been accounted for by reduced bodily pain resulting from improved physical functioning with DHA supplementation. Although depressive symptoms can increase the risk of developing dementia in elderly people with cognitive impairment, to our knowledge, this has not been addressed in previous studies.
Research has independently indicated that n-3 PUFA supplementation may assist with both cognitive impairment and depression, and it is possible that combined depression and cognitive impairment is indicative of common underlying biological mechanisms that may in some cases be attributed to suboptimal n-3 PUFA levels(Reference Sinn and Howe47). To our knowledge, other studies have also not yet reported comparisons of high-EPA and high-DHA supplementation for depressive symptoms or cognitive impairment. Although high EPA or ethyl-EPA has been reportedly effective in some studies with depression(Reference Nemets, Stahl and Belmaker48, Reference Peet and Horrobin49), we cannot rule out the effect of DHA. For instance, most studies have not correlated increased erythrocyte PUFA levels with outcomes. In one study that used a supplement containing DHA plus EPA, significant correlations between increased DHA and improved depressive symptoms were found(Reference Meyer, Grenyer and Crowe50) (although this study had a large placebo effect as both groups received counselling, masking differences between groups)(Reference Grenyer, Crowe and Meyer51). Another study that used a supplement with an EPA:DHA ratio of 2:1 reported baseline levels of erythrocyte DHA at 2·4 % and this increased to 5·8 %(Reference Su, Huang and Chiu52); therefore, it is possible that DHA contributed to the positive findings. Other methodological considerations include the fact that one of the pure DHA studies was also the shortest study on n-3 PUFA and depression (6 weeks)(Reference Marangell, Martinez and Zboyan53). Furthermore, only three studies in depression have used DHA compared with ten or more that have focused on EPA(Reference Sinn, Milte and Howe15). The present study indicates that DHA may be equally, if not more, effective than EPA for improving mood. This needs to be investigated further in clinical depression. Given the relative ratios of EPA and DHA in brain tissue, it may be that EPA is not required, nor effective, in as large proportions as DHA.
Of the cognitive outcomes, significant improvements were only detected in the DHA group for Initial Letter Fluency, a test of fluid thinking ability. Scores on Digits Backward also showed trends for improvement in the DHA group. These tests measure executive function, which is impaired in dementia. Increased DHA was correlated with improved Initial Letter Fluency, although not statistically significant. It should be noted that our sample had baseline EPA plus DHA levels over 5 % of erythrocyte fatty acids, whereas Chiu et al. (Reference Chiu, Su and Cheng18) had an average combined level of 4·2 %. Therefore, it is possible that our MCI sample had higher levels than those that have derived cognitive benefits from n-3 PUFA. This may also be attributed in part to the small sample size, the outcome measures and/or a lower n-6:n-3 ratio in our population. Our final numbers in each group were smaller than the numbers in MCI subgroups from previous studies that detected reduced cognitive decline with 2 g n-3 PUFA over 6 months(Reference Chiu, Su and Cheng18, Reference Freund-Levi, Basun and Cederholm19). There is also variation in an individual's performance on cognition from day to day, and it is possible, as discussed previously(Reference Sinn, Milte and Howe15) that the failure of this and many other studies that employ a range of cognitive tests to measure outcomes is due to this daily variation being larger than nutritional treatment effects, thereby potentially rendering the tests largely insensitive to such effects.
In conclusion, these results indicate that DHA-rich and EPA-rich fish oils may be effective for depressive symptoms and health parameters, exerting variable effects on cognitive and physical outcomes. Reducing n-6 PUFA intake as well as increasing n-3 PUFA intake for a more balanced ratio may be beneficial, and this should also be explored further. It is possible that reductions in mood and verbal fluency in the LA group represented a normal decline in this population, although we did observe significant correlations between a decreased ratio of AA:EPA and improved mood. The present findings suggest that pure EPA supplements employed in some mental health studies may not be the optimal choice. Future research should further investigate this in larger, clinically depressed samples of people with MCI and conduct longitudinal follow-up to assess whether the risk of progression to dementia is reduced in these populations. Research with n-3 PUFA and mental health in elderly populations should also factor in favourable influences of fish oil on physical function, particularly joint pain, which could also have an impact on mood.
Acknowledgements
This study was funded by an Australian Research Council Linkage grant in partnership with Novasel Australia. The authors acknowledge the assistance of all volunteers in the present study, plus the support of the Australian Centre for Metabolic Fitness, and Professors Andrew Hills and Karen Sullivan at the Queensland University of Technology. N. S. and P. R. C. H. designed the research; N. S., C. M. M., J. D. B., A. M. C. and S. J. S. conducted the research; J. P., N. S., C. M. M. and P. R. C. H. analysed the data; N. S. prepared the manuscript. All authors read, edited and approved the final manuscript. We declare no conflicts of interest.









