Introduction
Ischemic stroke is a condition caused by the occlusion of a cerebral vessel, leading to inadequate blood flow and subsequent cell death. Reference Zhao, Zhang, Chen and Wei1 In patients infected by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is the virus that causes coronavirus disease-2019 (COVID-19), stroke is a known and life-threatening complication that has been shown to affect up to 3% and 6% of patients in the hospital and intensive care units, respectively. Reference Iadecola, Anrather and Kamel2 This risk has been shown to be highest in patients with more severe disease and prior cardiovascular risk factors. Reference Nannoni, de Groot, Bell and Markus3 A meta-analysis of 50 studies on disease progression in patients with COVID-19 who experienced stroke showed a 9-day delay between COVID-19 symptom onset and stroke, and a majority of affected patients showed signs of pneumonia. Reference Nannoni, de Groot, Bell and Markus3 In a separate 11 study analysis comparing stroke features and outcomes between COVID-19 and non-COVID-19 patients, those with SARS-CoV-2-induced stroke were more likely to be younger males without a history of stroke and were associated with a greater risk of in-hospital mortality. Reference Nannoni, de Groot, Bell and Markus3 Additionally, COVID-19 related stroke has also been shown to be more prevalent in the Black and Hispanic community; a correlation likely related to cardiovascular risk factors and the disproportionate effect of COVID-19 on these communities. Reference Trifan, Goldenberg and Caprio4
SARS-CoV-2-Induced Ischemic Stroke
Infection with SARS-CoV-2 is associated with increased risk of ischemic stroke; however, the causal relationship remains still under investigation, with various proposed pathogenic pathways. Reference Mao, Jin and Wang5 Most of these proposed pathogenic pathways focus, in some way, on the interaction between the SARS-CoV-2 virus and the enzyme angiotensin-converting enzyme 2 (ACE2). Physiologically, ACE2 is a carboxypeptidase that plays an important role in vasoregulation and is highly expressed in human tissues, including vascular endothelial cells as well as the heart, gastrointestinal tract, and urinary system. Reference Fatehi, Hesam-Shariati, Abouzaripour, Fathi and Hesam Shariati6
Additional proposed mechanisms for the mechanism of ischemic stroke in COVID-19 are hypercoagulability, diminished activation of the alternative renin-angiotensin system (RAS) pathway, cardiac embolism, and endothelial cell dysfunction. Reference Sagris, Papanikolaou and Kvernland7 Though each of the mechanisms is discussed individually below, it is important to realize that they are likely fundamentally intertwined, working in a synergistic fashion to generate the thrombogenic state that, ultimately, results in ischemic stroke.
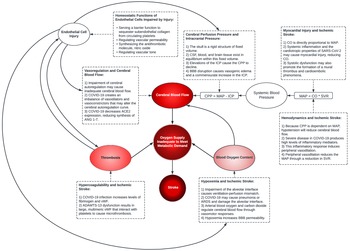
It is also important to recognize other factors may also influence the pathogenesis of ischemic stroke in COVID-19, particularly in severely ill patients, who are at higher risk of SARS-CoV-2-induced ischemic stroke. This includes the respiratory distress and subsequent hypoxemia that may be seen in severe cases of COVID-19. Reference Huang, Wang and Li8 Hypoxemia decreases the oxygen delivery to tissues, induces blood–brain barrier (BBB) disruption and permeability, and increases levels of hypoxia-inducible factor-1, driving cytokine release and promoting vascular changes that may increase cerebrovascular oxidative stress. Reference Yang and Rosenberg9 Severe illness from COVID-19 may also cause hypotension or shock. Reference Huang, Wang and Li8 These factors may prove detrimental in the setting of ischemic stroke by exacerbating the already inadequate oxygenation of brain tissue. An overview of the systemic manifestations of COVID-19 that interact to promote stroke is summarized in Figure 1.

Figure 1: Overview of the pathogenic mechanisms promoting stroke susceptibility in COVID-19. Stroke is the result of inadequate oxygen delivery to meet metabolic demand, resulting in brain cell death. Inadequate oxygen delivery is primarily related to a disruption in the cerebral blood supply; however, cerebral oxygen delivery is also dependent upon the oxygen content of blood. The direct effects of SARS-CoV-2 cellular invasion and the indirect effects of the host immune response increase stroke susceptibility through alterations in cerebral blood flow, alterations in blood oxygen content, and promotion of thrombosis. Cerebral blood flow is the volume of blood in cerebral circulation. Cerebral blood flow is driven by the net pressure gradient between systemic blood pressure and ICP: CPP = MAP – ICP. COVID-19 may precipitate low systemic blood pressures through inflammation-induced peripheral vasodilation, which causes inadequate circulatory volume. Low systemic pressures will reduce cerebral blood flow, exacerbating pre-existing cerebral circulation occlusions or causing stroke secondary to systemic hypoperfusion. To compensate for alterations in blood pressure, the cerebral vasculature is capable of autoregulation; however, this compensatory mechanism may be disrupted in COVID-19. Cerebral autoregulation dysfunction may precipitate ischemia or hyperemia. Additionally, COVID-19 may raise ICP – via vasogenic edema from disruption of the BBB or hyperemia – and this elevation will reduce CPP. Respiratory complications of COVID-19, including pneumonia and ARDS, cause ventilation-perfusion mismatch. This results in hypoxemia, and hypoxemia reduces cerebral oxygen delivery as well as induces BBB permeability. Thrombosis may also illicit stroke in COVID-19 by occlusion of a cerebral vessel. These mechanisms are diverse but should holistically analyzed to understand the pathogenesis of stroke in COVID-19. ARDS=acute respiratory distress syndrome; BBB=blood–brain barrier; CO=cardiac output; CPP=cerebral perfusion pressure; MAP=mean arterial pressure; ICP=intracranial pressure; SVR=systemic vascular resistance; vWF=von Willebrand factor.
Hypercoagulability
Evidence of hypercoagulability in patients infected with SARS-CoV-2 is supported by elevated levels of multiple pro-thrombotic biomarkers. Reference Esenwa, Cheng and Luna10–Reference Grobler, Maphumulo and Grobbelaar19 One of the elevated pro-thrombotic biomarkers seen in COVID-19 infection is fibrinogen, which is also known as factor I. Reference Panigada, Bottino and Tagliabue15,Reference Ladikou, Sivaloganathan and Milne16,Reference Grobler, Maphumulo and Grobbelaar19 Fibrinogen is an acute phase reactant – which means that is upregulated from various causes of inflammation – and its pro-sedimentation properties influence the erythrocyte sedimentation rate (ESR), which is non-specific measure of inflammation. Reference Grobler, Maphumulo and Grobbelaar19 Fibrinogen plays an essential role in the coagulation cascade, as it is activated to become fibrin, which serves as the basis for a mature fibrin clot. Reference Grobler, Maphumulo and Grobbelaar19 Additionally, other than the intrinsic pro-thrombotic effect of elevated fibrinogen due its role in the coagulation cascade, fibrinogen also serves as a ligand stimulating various inflammatory pathways that promote thrombosis. Reference Grobler, Maphumulo and Grobbelaar19 However, there also exists a regulatory mechanism to prevent excessive coagulation through which blood clots are degraded, producing fibrin degradation products. Reference Berger, Kunichoff and Adhikari13,Reference Grobler, Maphumulo and Grobbelaar19 One of these fibrin degradation products is D-dimer, and as healthy individuals demonstrate low serum levels of D-dimer, an elevated D-dimer level demonstrates evidence of coagulation and fibrinolysis. Reference Berger, Kunichoff and Adhikari13,Reference Grobler, Maphumulo and Grobbelaar19 Therefore, D-dimer serves a biomarker for thrombosis, Reference Berger, Kunichoff and Adhikari13,Reference Grobler, Maphumulo and Grobbelaar19 and elevated serum levels of D-dimer have been demonstrated in patients with COVID-19 infection. Reference Berger, Kunichoff and Adhikari13–Reference Panigada, Bottino and Tagliabue15,Reference Lopez-Castaneda, Garcia-Larragoiti and Cano-Mendez17–Reference Grobler, Maphumulo and Grobbelaar19 Studies also suggest D-dimer is correlated with disease severity. Reference Berger, Kunichoff and Adhikari13,Reference Panigada, Bottino and Tagliabue15,Reference Lopez-Castaneda, Garcia-Larragoiti and Cano-Mendez17–Reference Grobler, Maphumulo and Grobbelaar19 Furthermore, the pro-thrombotic marker von Willebrand factor (vWF) is elevated in COVID-19 infection and may also correlate with disease severity. Reference Panigada, Bottino and Tagliabue15–Reference Lopez-Castaneda, Garcia-Larragoiti and Cano-Mendez17,Reference Grobler, Maphumulo and Grobbelaar19 Physiologically, vWF promotes platelet aggregation and stabilization of factor VIII of the coagulation cascade. Reference Grobler, Maphumulo and Grobbelaar19 Therefore, high levels of vWF may promote spontaneous platelet adhesion, contributing to a hypercoagulable state. Reference Lopez-Castaneda, Garcia-Larragoiti and Cano-Mendez17,Reference Grobler, Maphumulo and Grobbelaar19 Notably, vWF is normally cleaved into a smaller protein by the enzyme ADAMTS-13 to reduce vWF’s ability to capture platelets; however, patients with severe COVID-19 infection demonstrate lower activity of ADAMTS-13. Reference McAlpine, Zubair and Maran20 Dysfunction of the physiologic role of ADAMTS-13 results in increased levels of large, multimeric vWF and an increased ability for vWF to capture platelets and cause thrombotic sequela, particularly ischemic stroke. Reference McAlpine, Zubair and Maran20 Furthermore, elevation in the levels P-selectin, which supports platelet-leukocyte heteroaggregate formation and thrombus stabilization, has been demonstrated in COVID-19 infection. Reference Lopez-Castaneda, Garcia-Larragoiti and Cano-Mendez17,Reference Grobler, Maphumulo and Grobbelaar19 Ultimately, the elevation of these various pro-thrombotic biomarkers in patients with COVID-19 provides considerable evidence of its association with a hypercoagulable state. The etiology of this hypercoagulable state found in patients with COVID-19, and concomitant elevation of these pro-thrombotic biomarkers, may be explained by an uncontrolled and abundant release of pro-inflammatory cytokines, known as cytokine storm. Reference Nishiga, Wang, Han, Lewis and Wu21 It is worth noting that hypercoagulability in COVID-19 may be integrally related to endothelial cell dysfunction, Reference Grobler, Maphumulo and Grobbelaar19 which is further discussed below, and the thrombophilia seen in COVID-19 is likely multi-factorial and related to the cross-interaction of multiple processes (Figure 2).

Figure 2: Hypercoagulability in COVID-19. Hypercoagulability in COVID-19 stems from a series of interactions that include the direct and indirect results of SARS-CoV-2 cellular invasion. SARS-CoV-2 invades cells displaying the ACE2 receptor; this results in direct cellular damage. Damaged cells release cytokines, which stimulate the host immune response. Immune cells release additional cytokines – stimulating apoptosis – and reactive oxygen species to cause further cell damage. These cytokines also stimulate host production of acute phase reactants. Some acute phase reactants, such as fibrinogen and vWF, promote thrombophilia, whereas other acute phase reactants, such as CRP, promote further endothelial cell damage. The endothelial cell layer acts to prevent thrombosis by sequestering subendothelial collagen underneath a cellular barrier to prevent platelet adhesion and producing anticoagulants, principally nitric oxide. These mechanisms work synergistically to promote hypercoagulability. ACE2=angiotensin-converting enzyme II; CRP=C-reactive protein; vWF=von Willebrand factor.
It is also noteworthy that some studies have also found low or even normal levels of these pro-thrombotic biomarkers in patients with COVID-19 infection, demonstrating variability of these pro-thrombotic biomarkers that may be influenced by severity of infection or dysregulation of the coagulation cascade. Reference Grobler, Maphumulo and Grobbelaar19
Despite the exact etiology, evidence of hypercoagulability is associated with ischemic stroke in patients with COVID-19. Reference Esenwa, Cheng and Luna10–Reference Topcuoglu, Pektezel and Oge12 In two studies, D-dimer has shown to be independently associated with an increased risk of ischemic stroke. Reference Esenwa, Cheng and Luna10,Reference Goyal, Sodani, Jain and Ram11 In a cohort of 5652 patients with COVID-19 infection, those in the fourth quartile demonstrated an eight-fold higher risk of ischemic stroke than patients in the first quartile. Reference Esenwa, Cheng and Luna10 Additionally, a prediction model for ischemic stroke created using 7938 SARS-CoV-2 positive patients demonstrated that a D-dimer cut-off >441.8 ng/mL provides a sensitivity of 90.0% and specificity of 76.6% for an area under the receiver operating characteristics (AUROC) of 0.876. Reference Goyal, Sodani, Jain and Ram11 An additional predictor model was developed using an ESR cut-off of > 19 mm/h that provides sensitivity of 91.7% and specificity of 53.3% for an AUROC of 0.740. Reference Goyal, Sodani, Jain and Ram11 Therefore, these biomarkers may identify patients with COVID-19-induced hypercoagulability who are at increased risk of ischemic stroke and allow providers to guide management accordingly.
Diminished Alternative RAS Pathway
The RAS is made up of two counter-regulatory pathways: the classical RAS pathway and the alternative RAS pathway. Reference Chappell22 The classical RAS pathway involves interactions between ACE and angiotensin II (ANG2), whereas the alternative RAS pathway consists of interactions between ACE2 and ANG2. Reference Chappell22 The alternative RAS pathway appears to be neuroprotective and has an antithrombotic effect by decreasing levels of pro-inflammatory cytokines, Reference Flores-Munoz, Godinho, Almalik and Nicklin23 while the classical RAS pathway promotes inflammation and cerebral endothelial dysfunction. Reference Sashindranath and Nandurkar24 As previously mentioned, the SARS-CoV-2 virus utilizes ACE2 for cellular invasion; Reference Hoffmann, Kleine-Weber and Schroeder25 thus, the downregulation of ACE2 in SARS-CoV-2 infection results in a relative imbalance of these two pathways, shifting toward the pro-inflammatory classical pathway and increased levels of ANG2. Reference Li, Zhang and Zhuo26 The classical pathway, subsequently, becomes exaggerated leading to vasoconstriction, inflammatory cytokine release, and profibrotic effects. Reference Sardu, Gambardella, Morelli, Wang, Marfella and Santulli27 This RAS imbalance and its downstream effects – such as dysregulation of anticoagulation pathways and elevation of inflammatory cytokines – creates a pro-thrombotic environment in patients infected with SARS-CoV-2. Reference Sardu, Gambardella, Morelli, Wang, Marfella and Santulli27
Endothelial Dysfunction
The vascular endothelium is essential for regulating coagulation, synthesizing the potent antithrombotic nitrous oxide and serving as a barrier to subendothelial collagen. Reference Ladikou, Sivaloganathan and Milne16,Reference Sashindranath and Nandurkar24,Reference Wang, Yang and Liang28,Reference Zubair, McAlpine, Gardin, Farhadian, Kuruvilla and Spudich29 The vascular endothelium also functions in vasoregulation, maintaining homeostasis between vasodilators (e.g., nitric oxide and prostacyclin) and vasoconstrictors (e.g., endothelin-1, ANG2, and reactive oxygen species). Reference Topcuoglu, Pektezel and Oge12,Reference Ladikou, Sivaloganathan and Milne16,Reference McAlpine, Zubair and Maran20,Reference Liu, Li and Chen30 As vascular endothelium, including the vascular endothelium that composes the BBB, expresses the ACE2 receptor, it is susceptible to direct viral invasion by the SARS-CoV-2 virus. Reference Hoffmann, Kleine-Weber and Schroeder25,Reference Zubair, McAlpine, Gardin, Farhadian, Kuruvilla and Spudich29 This direct infection by SARS-CoV-2 damages the vascular endothelium, causing endothelial cell dysfunction and disruption of its regulatory functions. Reference Mobayen, Dhutia and Clarke31 Breakdown of this regulatory barrier allows subendothelial collagen to be exposed, reduces synthesis of nitrous oxide, and impairs vasoregulation. Reference Ladikou, Sivaloganathan and Milne16,Reference Sashindranath and Nandurkar24,Reference Wang, Yang and Liang28,Reference Zubair, McAlpine, Gardin, Farhadian, Kuruvilla and Spudich29 This endothelial cell dysfunction is amplified by the acute inflammatory response. Reference Sashindranath and Nandurkar24,Reference Pasceri, Willerson and Yeh32 For example, SARS-CoV-2 infection has been shown to induce the unregulated formation of membrane attack complexes, which damage vascular endothelial cells. Reference Merrill, Erkan, Winakur and James33 The acute inflammatory response stimulates C-reactive protein (CRP) production, and CRP, in turn, downregulates the expression of nitric oxide synthase on vascular endothelial cells, promoting vasoconstriction and thrombosis. Reference Sardu, Gambardella, Morelli, Wang, Marfella and Santulli27 CRP also promotes the creation of reactive oxygen species – which exert vasoconstrictive effects – and further creates a pro-thrombotic environment through the increased expression of molecules that promote inter-cellular adhesion events, including ICAM-1, VCAM-1, and E-selectin. Reference Pasceri, Willerson and Yeh32 Finally, endothelial cell dysfunction increases the expression of pro-inflammatory cytokines that increase levels of various aforementioned pro-thrombotic biomarkers, such as vWF and fibrinogen. Reference Ladikou, Sivaloganathan and Milne16,Reference Grobler, Maphumulo and Grobbelaar19,Reference Sashindranath and Nandurkar24,Reference Liu, Li and Chen30
Cardiac Embolism
Infection with SARS-CoV-2 has been associated with myocarditis, vascular inflammation, and resulting ventricular dysfunction. Reference Lee and Yoon34,Reference Imaeda, Kabata and Shiraishi35 This ventricular dysfunction results in reduced contractility and impaired systolic cardiac function, which may precipitate the formation of a left ventricular thrombus (LVT). Reference Lee and Yoon34,Reference Imaeda, Kabata and Shiraishi35 Once formed, an LVT may become dislodged and embolize into the cerebral arteries, causing ischemic stroke. Reference Lee and Yoon34,Reference Imaeda, Kabata and Shiraishi35 Preliminarily literature suggests those with pre-existing myocardial dysfunction are at greater risk. Reference Lee and Yoon34,Reference Imaeda, Kabata and Shiraishi35
Brain Fog and Ischemic Stroke
Recent studies have suggested that SARS-CoV-2-infected astrocytes can lead to cognitive dysfunction, including confusion and forgetfulness. Reference Marshall36 This phenomena is often termed “brain fog” or long-haul COVID. Reference Marshall36 Authors in one study report that as many as 47% of patients with prolonged COVID-19 have associated cognitive dysfunction. Reference Frontera, Lewis and Melmed37 However, these episodes of cognitive dysfunction may present a diagnostic dilemma, as the early symptoms of ischemic stroke may be misappropriately classified as “brain fog.” As time until stroke intervention is crucial for restoring vascularization and optimizing patient outcomes, it is imperative to be cognizant of possible anchoring biases when evaluating neurological changes in patients with COVID-19.
Treatment of SARS-CoV-2 Ischemic Stroke
As ischemic stroke results in necrosis of brain tissue due to inadequate cerebral blood flow, rapid reperfusion of the brain tissue is the most effective way to avoid permanent brain damage and death. Reference Zhao, Zhang, Chen and Wei1 The primary methods of revascularization include IV thrombolysis and endovascular thrombectomy. Reference Zhao, Zhang, Chen and Wei1
IV Thrombolysis
The current standard of care for acute ischemic stroke is intravenously infused tissue plasminogen activator (tPA). Reference Herpich and Rincon38 Alteplase is a form of tPA that is used frequently; however, Alteplase must be administered within 3–4.5 hours of symptom onset. Reference Gonzales and Grotta39 For this reason, tPA is only used in 3.2%–5.2% of ischemic stroke patients, marking a substantial limitation. Reference Del Zoppo, Saver, Jauch and Adams40 Though treatment with tPA in patients suffering from COVID-19-induced stroke has not been explicitly studied, it seems reasonable without any obvious safety concerns, Reference Hess, Eldahshan and Rutkowski41 and its use has been described in previous literature. Reference Co, Yu, Laxamana and David-Ona42,Reference Lodigiani, Iapichino and Carenzo43
Mechanical Thrombectomy
Mechanical thrombectomy is the removal of a clot from a blood vessel under imaging guidance through a catheter-based therapy. Reference Herpich and Rincon38 Thrombectomy is typically done after IV infusion of tPA and more than doubles the chance of functional outcome improvement in ischemic stroke patients. Reference Gonzales and Grotta39 Conversely, tPA administration before thrombectomy can dislodge the occlusion and cause it to migrate to a distal, previously unaffected region of the brain. Reference Goyal, Menon and van Zwam44
Mechanical thrombectomy treatment in patients with SARS-CoV-2-induced ischemic stroke is complicated by the prevalence of multiple clots, the fragility of the clot(s), and the patients’ hypercoagulable state. Reference Wang, Mandigo, Yim, Meyers and Lavine45 These complexities are highlighted in a retrospective study on five patients who underwent mechanical thrombectomy for SARS-CoV-2-induced ischemic stroke. Reference Wang, Mandigo, Yim, Meyers and Lavine45 Successful revascularization was achieved in one patient, while immediate re-occlusion of the vessel was observed in two patients, which was attributed to hypercoagulability. Reference Wang, Mandigo, Yim, Meyers and Lavine45 Similar outcomes were seen in a ten-patient cohort from France, where zero patients saw early neurological improvement, four patients experienced early cerebral re-occlusion, and six died in the hospital. Reference Escalard, Maier and Redjem46 Therefore, although mechanical thrombectomy may improve outcomes in patients with ischemic stroke, the increased complexity of SARS-CoV-2-induced ischemic stroke may dictate exploration of a different approach in this unique patient population.
SARS-CoV-2-Induced Hemorrhagic Stroke
Hemorrhagic stroke refers to bleeding into the cerebral parenchyma due to the rupturing of blood vessels. This class of strokes can be further subdivided into intracerebral hemorrhage (ICH) and subarachnoid hemorrhage (SAH). ICH is bleeding into the brain parenchyma, while SAH is defined as bleeding into the subarachnoid space. While hemorrhagic stroke in general is linked to severe morbidity and high mortality, ICH particularly has a higher risk of early mortality and long-term disability. Reference van Asch, Luitse, Rinkel, van der Tweel, Algra and Klijn47
In SARS-CoV-2-induced stroke, there is a lower incidence of hemorrhagic stroke than ischemic stroke, and hemorrhagic stroke accounts for only approximately 21.7%–25.7% of SARS-CoV-2-induced stroke. Reference Rajdev, Lahan, Klein, Piquette and Thi48 The presentation of hemorrhage is markedly varied in COVID-19 patients and may be massive with substantial hemispheric involvement, and/or with multiple hematomas in both supra and infra-tentorial areas. Reference Vogrig, Gigli, Bna and Morassi49 Multi-focal and multi-compartmental ICH was more likely to present in patients with severe pulmonary COVID-19 infection. Reference Altschul, Unda and de La Garza Ramos50 Unlike intraparenchymal hemorrhage, it appears that COVID-19 does not provoke SAH with the same frequency. Reference Altschul, Unda and de La Garza Ramos50 Reports have shown that most patients affected by hemorrhagic stroke during COVID-19 infection have underlying chronic disease, such as hypertension or diabetes, which are key risk factors for hemorrhagic stroke in the general population. Reference Wang, Tang and Fan51 It is also important to note that patients can present with cerebral hemorrhage without the primary pulmonary symptoms for which COVID-19 infection is known. Reference Altschul, Unda and de La Garza Ramos50
Interestingly, the reported rates of mortality for COVID-19 patients having experienced hemorrhagic stroke widely vary. Reference Altschul, Unda and de La Garza Ramos50 Mortality rates for hemorrhagic stroke can vary depending on the type, location, etiology, acuity, and severity, ranging between 12% and 15% for subdural hematoma and 35% at 7 days to 59% at 1 year for intraparenchymal hemorrhage. Reference Altschul, Unda and de La Garza Ramos50 However, the specific impact of COVID-19 on the outcome of patients with cerebral hemorrhage is currently unknown. Reference Altschul, Unda and de La Garza Ramos50
The pathogenesis of hemorrhagic stroke during COVID-19 infection is intricate and not yet fully understood, but it has been hypothesized to result from a vicious amalgamation of virus-related factors, characteristics of the host, and virus–host interaction. Reference Vogrig, Gigli, Bna and Morassi49
Intraparenchymal hemorrhage can spontaneously occur in critically ill patients, especially in the settings of multi-organ failure or circulation instability. Reference Vogrig, Gigli, Bna and Morassi49 Intraparenchymal hemorrhage may also result as sequelae of other pathologic conditions. Reference Vogrig, Gigli, Bna and Morassi49 This can include hemorrhagic transformation of acute ischemic stroke, ruptures of pseudoaneurysm, or secondary to the increased venous pressure – such as in the setting of cerebral venous thrombosis. Reference Vogrig, Gigli, Bna and Morassi49 Disseminated intravascular coagulopathy could also theoretically predispose to brain hemorrhage, especially when combined with iatrogenic use of anticoagulation. Reference Vogrig, Gigli, Bna and Morassi49
Neuroinvasion
It is possible that the interaction of SARS-CoV-2 on the surface of endothelial and arterial smooth muscle cells allows the virus to damage cerebral arteries and cause arterial wall dissection or rupture with hemorrhage. Reference Vogrig, Gigli, Bna and Morassi49 It has also been postulated that neuronal tissue invasion by SARS-CoV-2 may result in hemorrhagic stroke. Reference Jha, Ojha and Jha52 This hypothesis is based on the detection of various proteins and genetic material of different viruses in tissue samples of the nervous system, including the cerebrospinal fluid (CSF) and brain. Reference Jha, Ojha and Jha52 Detection of SARS-CoV-2 RNA in the CSF fluid specimen of a patient with COVID-19 provides direct evidence to support this theory of neurotropic involvement. Reference Fraiman, Freire, Moreira-Neto and Godeiro-Junior53 Currently, this neuronal invasion is believed to occur via one of two pathways. Reference Baig54,Reference Eliezer, Hautefort and Hamel55 The first pathway is retrograde axonal transport via the cribriform plate, supported by the high proportion of COVID-19 patients experiencing anosmia. Reference Eliezer, Hautefort and Hamel55,Reference Li, Bai and Hashikawa56 The second pathway is hematogenous spread following respiratory tract infection and subsequent direct BBB infiltration. Reference Baig54 This BBB infiltration may occur through three mechanisms: transcellular migration, paracellular migration, and the Trojan horse strategy. Reference Achar and Ghosh57 The transcellular mechanism is the direct viral invasion of the endothelial cells that make up the BBB via the ACE2. Reference Huang, Wang and Li8 The paracellular mechanism involves viral invasion of tight junctions or a leaky BBB. Reference Huang, Wang and Li8 This is primarily due to a strong post-infection systemic inflammatory response driven by pro-inflammatory cytokines and acute phase reactants – such as IL-1β, IL-6, TNF-α, IFN-γ, CCL2, and CRP – causing subsequent BBB disruption. Reference Huang, Wang and Li8 The third mechanism, the Trojan horse, is when an infected immune cell can cross the BBB: a mechanism that has been well described in the HIV literature. Reference Berth, Leopold and Morfini58 Similar to the paracellular mechanism, the acute inflammatory response incited by COVID-19 may disrupt the BBB and allow for infiltration of infected immune cell into the central nervous system (CNS). Reference Steardo, Steardo, Zorec and Verkhratsky59
Impaired ACE2 Function
Similar to the pathogenesis of SARS-CoV-2-induced ischemic stroke, growing evidence has suggested that patients with COVID-19 are at an increased risk of hemorrhagic stroke due to a downregulation of ACE2 expression and activity. Reference Fatehi, Hesam-Shariati, Abouzaripour, Fathi and Hesam Shariati6 This is particularly relevant in patients with hypertension, as baseline levels of ACE2 are likely lower. Reference Wang, Tang and Fan51 As previously mentioned, ACE2 also serves to inactivate ANG2, a pro-thrombotic and pro-inflammatory peptide hormone, by converting ANG2 into ANG 1-7 (Figure 3). Reference Sweid, Hammoud and Bekelis60 Therefore, increases in ANG2 conversely mean decreases in ANG 1-7. Reference Sweid, Hammoud and Bekelis60 Once synthesized, ANG 1-7 has a downstream effect on endothelial cells, stimulating the release of prostaglandin and nitric oxide to promote vasodilatation. Reference Sweid, Hammoud and Bekelis60 ANG 1-7 also induces a decrease in tyrosine hydroxylase expression, which is the rate-limiting enzyme in catecholamine biosynthesis. Reference Sweid, Hammoud and Bekelis60 This decreases brain catecholaminergic activity, and, as such, ANG 1-7 is neuroprotective. Reference Sweid, Hammoud and Bekelis60 The decrease in ANG 1-7 synthesis during SARS-COV-2 infection may inhibit its neuroprotective properties. Reference Sweid, Hammoud and Bekelis60 Ultimately, the downregulation of ACE2 during SARS-CoV-2 infection mediates a loss of vasoregulation, disruption of the BBB, cerebral hypoperfusion, impaired neuroprotection, and vasogenic edema. Reference Vogrig, Gigli, Bna and Morassi49 These dysfunctions concomitantly increase a patient’s risk of hemorrhagic stroke episodes. Reference Vogrig, Gigli, Bna and Morassi49

Figure 3: Impact of SARS-CoV-2-induced ACE2 receptor downregulation on stroke. SARS-CoV-2 cellular invasion is mediated by spike protein interactions with membrane-bound ACE2. As a response, ACE2 is downregulated, which increases angiotensin II relative to angiotensin 1-7. This favors angiotensin II receptor type I activation and initiates a pro-inflammatory cascade that subsequently impairs vasoregulation. Cerebral vasoregulation dysfunction causes aberrant vasoconstriction, which increases the risk of intraparenchymal hemorrhage. This is compounded by the downstream effects of angiotensin II receptor type I activation on vascular remodeling and oxidative stress. ACE=angiotensin-converting enzyme; ACE2=angiotensin-converting enzyme II; BBB=blood–brain barrier.
Acute Stress Response
Increased catecholamine release due to anxiety, stress, and depression is also postulated to play a role in aggravating CNS symptoms of COVID-19 infection. Reference Wang, Tang and Fan51 Research has shown that adrenergic stimulation by catecholamines could lead to potential vasospasms and microcirculation disturbances, thereby increasing the risk of hemorrhagic stroke. Reference Wang, Tang and Fan51 SARS-CoV-2 infection has been shown to cause sympathetic system dysregulation and even sympathetic storm, Reference Al-Kuraishy, Al-Gareeb and Mostafa-Hedeab61 and this unregulated sympathetic response may contribute to the development of hemorrhagic stroke.
Treatment of SARS-CoV-2-Induced Hemorrhagic Stroke
As the outcomes in patients with hemorrhagic stroke are poor, it is imperative for physicians to assess patients for potential risk throughout hospitalization, especially as many patients with COVID-19 receive prophylactic anticoagulation. Reference Fatehi, Hesam-Shariati, Abouzaripour, Fathi and Hesam Shariati6,Reference Dogra, Jain and Cao62 Due to its connection to hemorrhagic episodes and its relationship to cerebral hemorrhage prognosis, aggressive blood pressure control is the cornerstone of management. Reference Wang, Tang and Fan51,Reference Hemphill, Greenberg and Anderson63 Additionally, all anticoagulants should be immediately ceased, and any active anticoagulants should be reversed. Reference Hemphill, Greenberg and Anderson63 Intracranial pressure monitoring and seizure prophylaxis should be employed if appropriate. Reference Hemphill, Greenberg and Anderson63
Conclusions
The COVID-19 pandemic has stressed stroke services worldwide in unprecedented ways. Although the exact pathways underlying increased risk of ischemic and hemorrhagic strokes have yet to be defined, several pathways have been identified. The hypercoagulable state and endothelial damage following infection appear to play key roles in the pathogenesis of both ischemic stroke and, more commonly, brain fog that we see in infected patients. Similarly, direct neuroinvasion, hypertension, and vascular damage are implicated in the formation of hemorrhagic strokes. Immediate intervention utilizing traditional stroke treatments is crucial for achieving optimal outcomes. Though specific treatment modalities aimed at SARS-CoV-2-induced stroke have yet to be implemented, novel treatments – including risk reduction and targeting inflammatory and vascular change – are currently being investigated. Management of these patients requires high-quality care and coordination between multiple healthcare teams, providing important lessons for future pandemics. This includes protected pathways for COVID-19 infected patients with suspected stroke from pre-hospital stroke care to the emergency department and appropriate stroke intervention with minimal delay.
Disclosures
Brandon Lucke-Wold has an R25 grant, was awarded the American Association of Neurological Surgeons (AANS) Dempsey cerebrovascular award, and the Bryan Robinson endowment award. No specific funding was received to support this project. All other authors have no interests and declare no disclosures.
Statement of Authorship
CS and BL have given substantial contributions to the conception or the design of the manuscript. All authors have participated in drafting the manuscript and critical revisions. All authors read and approved the final version of the manuscript.