Global obesity rates have been steadily rising over the last 30 years and are reaching epidemic proportions. Although weight gain generally occurs over time, some periods are considered especially problematic. The winter holiday season, a traditional feasting time ingrained in many Western cultures, is one of them. Depending on sample composition and study design, several studies conducted during the holiday season in US adults consistently reported increases in body weight of 0·37 kg(Reference Yanovski, Yanovski and Sovik1) and 0·78 kg(Reference Stevenson, Krishnan and Stoner2) between mid-November and early/mid-January, 0·4 kg over the Christmas period(Reference Andersson and Rossner3), 0·5 kg during a 13-d period over Thanksgiving(Reference Hull, Radley and Dinger4), and 0·6 kg between Christmas and New Year(Reference Helander, Wansink and Chieh5). Evidence shows weight gain during the 6-week holiday season between Thanksgiving and the New Year is a significant contributor to annual weight gain for many(Reference Yanovski, Yanovski and Sovik1). Similar trends have been described in other countries. In the UK, studies found mean 0·9 kg weight gains between Christmas and early/end January(Reference Rees, Holman and Turner6, Reference Reid and Hackett7). A study involving wireless digital scales with 760 participants in Germany reported a 0·8 kg weight gain between Christmas and New Year(Reference Helander, Wansink and Chieh5). This weight gain is often maintained into the summer months and beyond(Reference Yanovski, Yanovski and Sovik1, Reference Helander, Wansink and Chieh5), and, if repeated every year, may result in cumulative weight gain over time. Data also show that overweight individuals tend to gain more weight than normal-weight individuals(Reference Yanovski, Yanovski and Sovik1, Reference Hull, Radley and Dinger4). Steady weight gain over the years may lead to obesity, which is a risk factor for many chronic diseases and increased morbidity and mortality(Reference Leung, Carlsson and Colditz8–Reference Abdelaal, le Roux and Docherty12). Evidence also shows that obesity greatly impacts quality of life and mental health(Reference Taylor, Forhan and Vigod13). Importantly, even a modest 5 % weight loss is enough to produce substantial health improvements(Reference Wing, Lang and Wadden14, Reference Magkos, Fraterrigo and Yoshino15). Therefore, effective dietary strategies need to be implemented to prevent weight gain during the sensitive winter holiday season. Yet, very few studies have investigated such strategies. Some have explored cognitive–behavioural treatment and self-monitoring interventions or supplementation with conjugated linoleic acid(Reference Watras, Buchholz and Close16, Reference Boutelle, Kirschenbaum and Baker17), but none has evaluated the benefits of intermittent fasting.
The winter holiday season is a time for celebration often associated with an overindulgence of delicious, highly palatable, and readily available foods usually rich in fats and sugars. Along with a more sedentary lifestyle during the cold autumn and winter months, increased energy intake and decreased energy expenditure may promote weight gain if this episode of energy imbalance is maintained. Many different strategies have been developed over the years that could be effective approaches for managing holiday weight gain. Long-term energy restriction is challenging and unrealistic during the holiday season for many individuals. On the other hand, intermittent fasting – short periods of energy intake reduction significantly below the amount normally consumed between longer periods of habitual energy intake – has become increasingly popular in the lay press and has been shown to result in weight loss and numerous health benefits(Reference Golbidi, Daiber and Korac18–Reference Wei, Brandhorst and Shelehchi28). A well-known short-term intermittent fasting approach is the popular 5:2 diet. Clinical studies have demonstrated the many health benefits associated with consuming 500–650 kcal/d (2090–2720 kJ/d) on two scheduled fasting days a week, and ad libitum eating for the other 5 d in overweight and obese adults(Reference Harvie, Pegington and Mattson22, Reference Conley, Le Fevre and Haywood24, Reference Sundfør, Svendsen and Tonstad29). This is an attractive strategy for weight management during the holiday season, as reduced energy intake during fasting days may offset excessive energy intake during ad libitum days, without ‘calorie counting’ or food restriction. Yet, no study has addressed the benefits of a 5:2 intermittent fasting approach during the winter holiday season.
Based on the above considerations, this pilot, single-centre, parallel-group, randomised and controlled study was designed to evaluate the effects of a modified 5:2 intermittent energy restriction nutrition programme to prevent weight gain in overweight healthy adults over the winter holiday period, compared with a control group following their habitual diet.
Materials and methods
Participants
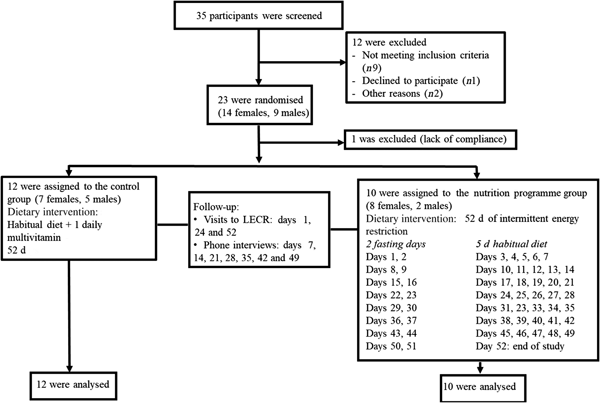
Subjects were recruited by means of posters and flyers, advertisement on social media, and email blasts to potential volunteers in the Life Extension database. We did not perform an a priori sample size calculation as results from this pilot trial will be used to conduct power analyses for a future larger study. Eligible participants were overweight healthy males and females, aged 21-65 years with a BMI between 25 and 29·9 kg/m2. Having a stable weight (±3 kg) over the past 6 months preceding the study, no known or suspected gastrointestinal disorders, and no difficulty swallowing (dysphagia) or difficulty chewing were among the inclusion criteria. Exclusion criteria included having been diagnosed with, received medical treatment for, or taking daily medication for type 2 diabetes, dyslipidaemia, hypertension, impaired glucose tolerance, impaired fasting glucose, and other metabolic diseases. A signed informed consent was obtained from each participant. Of thirty-five subjects assessed for eligibility, twenty-three were randomised. One female subject in the nutrition programme group was excluded after the first week due to lack of compliance; therefore, twenty-two participants (thirteen females, nine males) completed the study and were included in the final analyses (Fig. 1). The study was conducted according to the Declaration of Helsinki. The study was approved by IntegReview Institutional Review Board and registered at ClinicalTrials.gov (NCT03372109).
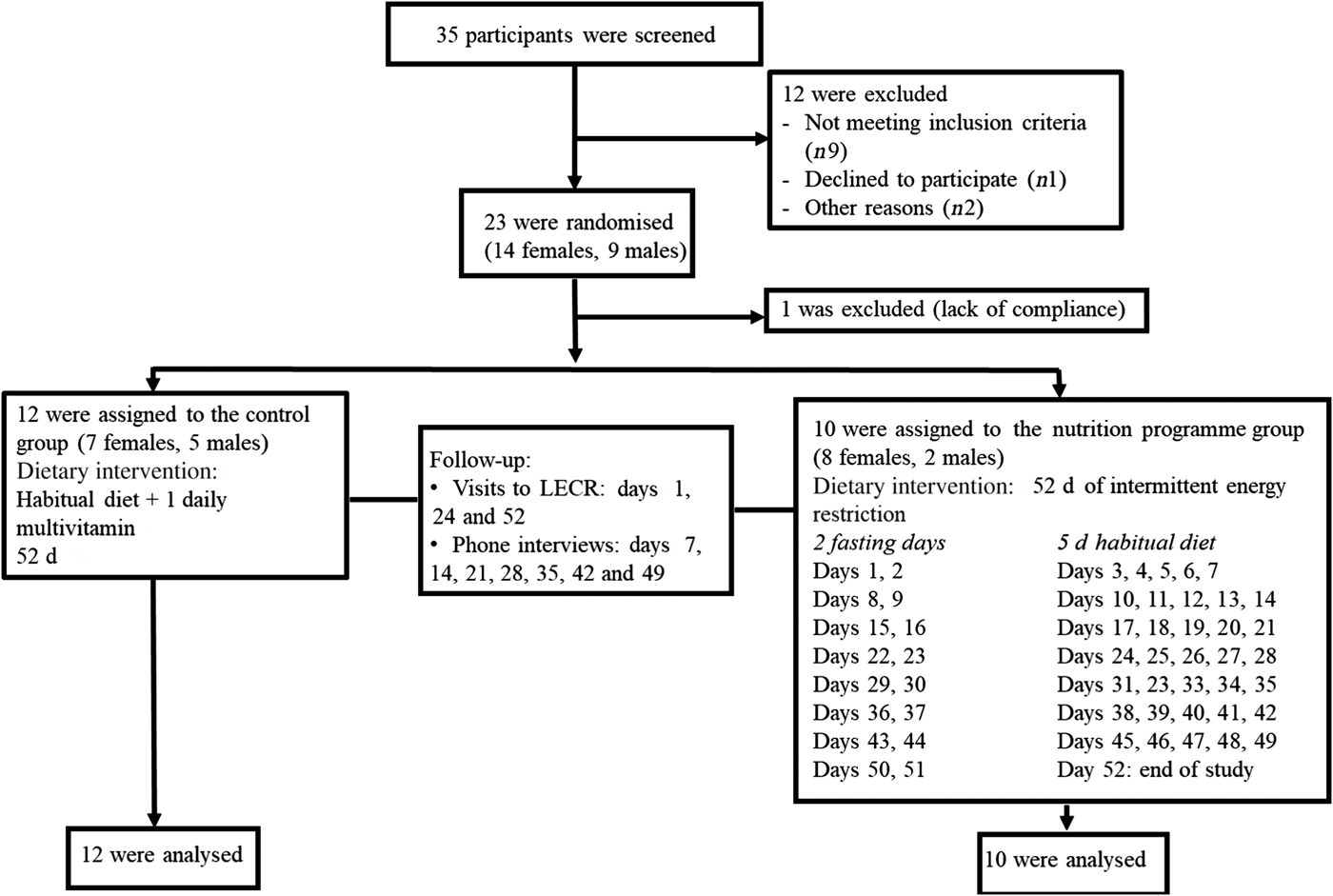
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow diagram for study participants. LECR, Life Extension Clinical Research.
Dietary interventions
The nutrition programme consisted of 52 d of intermittent energy restriction. On two consecutive days (Monday and Tuesday) of each week, participants decreased their energy intake (730 kcal/d; 3050 kJ/d) by consuming a commercially available shake (170 kcal (710 kJ/d); 12, 6, 24 and 24 % of total energy from fat, carbohydrate, fibre and protein, respectively) (Life Extension) four times per d (in the morning, at lunch, in the afternoon, and at dinner). On the remaining 5 d (Wednesday to Sunday) participants were instructed to eat their habitual diet with no specific dietary recommendations. The nutrition programme also included the daily consumption of a set of commercially available dietary supplements (Life Extension) selected not only for their potential overall health benefits, but to ensure adequate intake of key essential nutrients, including vitamins and minerals, and support weight management efforts. Ingredients in the supplements included long-chain n-3 fatty acids (EPA and DHA)(Reference Howe and Buckley30, Reference Filipovic, Aeschbacher and Reiner31) found in fish oil (this supplement provided an additional 50 kcal/d (210 kJ/d)), sesame lignans(Reference Kamal-Eldin, Moazzami and Washi32), olive (Olea europaea) fruit and leaf extract(Reference Poudyal, Campbell and Brown33), plant-based polyphenols, saturated fats, nuts and olive extract to mimic the Mediterranean diet(Reference Rosato, Temple and La Vecchia34), soluble fibres (xylooligosaccharides)(Reference Lyon and Kacinik35), Italian Borlotto variety of white kidney bean (Phaseolus vulgaris)(Reference Celleno, Tolaini and D'Amore36, Reference Wu, Xu and Shen37), saffron(Reference Hausenblas, Heekin and Mutchie38), clove bud (Syzygium aromaticum) and maqui berry (Aristotelia chilensis) extracts(Reference Hidalgo, Flores and Hidalgo39–Reference Kato, Tani and Terahara42), curcumin(Reference Marin, Wall and Wang43–Reference Noorafshan and Ashkani-Esfahani45), coenzyme Q10–ubiquinol and shilajit(Reference Bhattacharyya, Pal and Banerjee46), Gynostemma pentaphyllum extract(Reference Mishra and Joshi47, Reference Park, Huh and Kim48) and hesperidin(Reference Rizza, Muniyappa and Iantorno49). Participants in the control group were required to follow their habitual diet without any restriction and take one tablet of a commercially available multivitamin daily for 52 d. All subjects were instructed to drink at least eight cups of water/d and engage in their typical physical activity.
Study design and procedures
This 52-d, single centre, parallel-group, randomised and controlled trial (13 November–3 January) was conducted at Life Extension Clinical Research (LECR) (Fort Lauderdale, FL). Subjects were randomly assigned by blocks of four to either the nutrition programme or control group. Three visits were scheduled at LECR throughout the holiday season. The initial pre-holiday visit took place on 13 November (day 1; baseline), 10 d before Thanksgiving. Subjects were brought back for a second mid-holiday visit (day 24) on 6 December (between Thanksgiving and Christmas) and a third and final visit (day 52) on 3 January (post-holiday). Assessments were conducted in the morning following a 12 h fast on days 24 and 52 in the control group, and in the morning after the two fasting days in the nutrition programme group. Regular telephone interviews and email communications were conducted to discuss any change in medical history or concomitant medications, monitor adverse events, reinforce compliance, and remind subjects of the date and time of their next visit. For both groups, the allowable window was +1 d for visits 3 (day 24) and 4 (day 52), and −3 d for scheduled telephone interviews. Body weight was measured using a DIGI DS-160 calibrated scale (Rice Lake Weighing Systems) with no shoes on. Blood pressure and heart rate were obtained with a Digital Blood Pressure Monitor Model UA-767 Plus device (LifeSource). Blood pressure was measured in duplicate after 10 min at rest while subjects were in a seated position, and the mean value was calculated. A venous blood sample was also drawn. Immediately following collection, tubes containing K2 EDTA were gently inverted up to eight times to ensure proper mixing before centrifugation (10 min at 2500 rpm). Serum was promptly collected, and specimens were refrigerated until shipped to LabCorp for processing. Insulin was measured using a two-site electrochemiluminescent immunoassay on the Elecsys 1010/2010 and MODULAR ANALYTICS E170 automated platform (Roche Diagnostics Corporation). Enzymic methods (Roche Diagnostics Corporation) were used to assess glucose (assay no. 05168791), total cholesterol (assay no. 05168538), LDL (assay no. 07005768), HDL (assay no. 07528582), TAG (assay no. 05171407), aspartate aminotransferase (AST) (assay no. 05850819) and alanine aminotransferase (ALT) (assay no. 05850797) levels on a Roche/Hitachi Cobas c 701/702 automated analyser (Roche Diagnostics Corporation).
Primary outcome assessment
Within- and between-group absolute changes from baseline in body weight were assessed at days 24 and 52.
Secondary outcome assessments
Serum metabolic markers
Within- and between-group absolute changes from baseline in fasting insulin, LDL-cholesterol, HDL-cholesterol, total cholesterol and TAG levels were evaluated at day 52. Changes in insulin sensitivity were estimated at day 52 using the updated homoeostasis model assessment (HOMA2)(Reference Levy, Matthews and Hermans50–Reference Song, Hwang and Ahn52). Lower HOMA2 values indicated higher insulin sensitivity, and higher HOMA2 values indicated lower insulin sensitivity, and therefore suggested greater insulin resistance.
Compliance
At each visit at days 24 and 52, subjects in the nutrition programme were asked to return containers to evaluate remaining study product, discuss adherence, and calculate compliance. Subjects were counselled when compliance was less than 85 %. Participants were asked to not transfer the study product from the original container to another container.
Safety
Within- and between-group absolute changes from baseline in vital signs (systolic blood pressure, diastolic blood pressure and heart rate) and liver blood chemistry (AST and ALT) were assessed at day 52. Participants were asked to contact LECR immediately if experiencing adverse effects. Symptoms and signs of adverse events were documented throughout the study.
Statistical analysis
Statistical analysis was performed using GraphPad Prism software, version 5.01 (Abacus Concepts GraphPad Software) and SAS statistical software, version 9.4 (SAS Institute Inc.). Results in tables are presented as means and standard deviations. The Shapiro–Wilk test was used to assess normal distribution of the data sets. For the primary outcome, within-group absolute changes were analysed with one-way repeated-measures ANOVA and between-group absolute changes with one-way independent-variables ANOVA, with Bonferroni's multiple comparison post-test. For secondary outcomes, paired Student's t test or Wilcoxon matched pairs test were used to analyse within-group changes, and unpaired Student's t test or Mann–Whitney U test were used to analyse between-group changes. Unpaired Student's t test was used to evaluate baseline differences between groups. Pearson correlation coefficients (r) were calculated to evaluate the correlation between baseline body weight and changes in body weight at day 52 in both groups. The level of significance was set at P < 0·05.
Results
Clinical characteristics at baseline
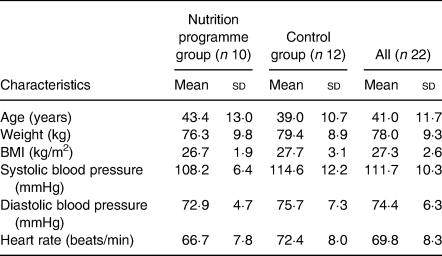
Results are shown in Table 1. In all, twenty-two subjects completed the study (nutrition programme group, n 10 (eight females, two males) and control cohort, n 12 (five females, seven males)) and were included in the final analyses. Mean age of all participants was 41·0 years, mean body weight was 78·0 kg, and mean BMI was 27·3 kg/m2. There was no statistically significant difference in clinical pre-holiday baseline (before Thanksgiving) characteristics between groups.
Table 1. Clinical characteristics of study participants at baseline (pre-holiday on 13 November, 10 d before Thanksgiving)
(Mean values and standard deviations)
Primary outcome
Results are shown in Table 2. After 24 d, subjects in the nutrition programme group started losing weight (75·3 (sd 9·5) v. 76·3 (sd 9·8) kg), although this was not statistically significant. After 52 d, participants lost a total of 1·3 kg (1·7 %) from baseline (75·0 (sd 9·8) v. 76·3 (sd 9·8) kg; P < 0·05). Subjects in the control group lost 0·3 kg from baseline at day 24 and 0·4 kg at day 52, which was not statistically significant. There was no significant between-group difference in weight loss at day 24 or day 52. When classified based on sex, males in the nutrition programme group (n 2) lost significantly more weight at day 24 than males in the control group (n 7) (−1·9 (sd 0·7) v. −0·5 (sd 0·7) kg; P = 0·0481), while no difference was found in females. No difference was found at day 52 for either males or females. No significant correlation was found between baseline body weight and changes in body weight at day 52 for subjects in the control group (r −0·37; P = 0·2365) or nutrition programme group (r −0·09; P = 0·8001).
Table 2. Body weight in the nutrition programme group (n 10) and control group (n 12) at baseline (pre-holiday on 13 November, 10 d before Thanksgiving), day 24 (mid-holiday on 6 December, between Thanksgiving and Christmas) and day 52 (post-holiday, 3 January)
(Mean values and standard deviations)

* Mean value was significantly different from that at baseline (P < 0·05; within-group with one-way repeated-measures ANOVA and Bonferroni multiple comparison post-test).
Secondary outcomes
Serum metabolic markers
Results are shown in Table 4. There was no significant difference at baseline between groups. After a 12 h fast in the control group, there was a significant 49·2 % increase compared with baseline in insulin (10·0 (sd 6·5) v. 7·0 (sd 3·2) μIU/ml (69·5 (sd 45·1) v. 48·6 (sd 22·2) pmol/l); P = 0·0256), 8·4 % increase in LDL-cholesterol (121·1 (sd 35·7) v. 111·7 (sd 29·9) mg/dl (3·1 (sd 0·9) v. 2·9 (sd 0·8) mmol/l); P = 0·0426), and 7·1 % increase in total cholesterol (196·8 (sd 48·1) v. 183·6 (sd 41·0) mg/dl (5·1 (sd 1·2) v. 4·8 (sd 1·1) mmol/l); P = 0·0154) levels after 52 d. Between-group difference in changes from baseline in insulin levels and total cholesterol:HDL-cholesterol ratio was significant (P = 0·0227 and P = 0·0419, respectively). In the nutrition programme group, there was a significant 13 % increase in HDL-cholesterol (70·0 (sd 21·0) v. 62·0 (sd 18·0) mg/dl (1·8 (sd 0·5) v. 1·6 (sd 0·5) mmol/l); P = 0·0245) and a significant 22·8 % reduction in TAG levels (70·5 (sd 34·8) v. 91·9 (sd 46·6) mg/dl (0·8 (sd 0·4) v. 1·0 (sd 0·5) mmol/l); P = 0·0416) compared with baseline after the two fasting days. In addition, there was a significant 43 % increase in HOMA2 in the control group after 52 d compared with baseline (1·33 (sd 0·86) v. 0·93 (sd 0·43); P = 0·025) v. no significant change in the nutrition programme group; however, there was a trend towards a decline (0·73 (sd 0·49) v. 0·91 (sd 0·45)) seen in the nutrition programme group. The between-group difference in change from baseline in HOMA2 was statistically significant (−0·18 (sd 0·27) in the nutrition programme group v. 0·40 (sd 0·53) in the control group (P = 0·0041).
Compliance
Compliance with the nutrition programme group was very good throughout the study (98·0 (sd 7·3) %), with similar rates of adherence at day 24 (97·9 (sd 10·3) %) and day 52 (98·0 (sd 6·5) %).
Safety: vital signs
There was no significant change in systolic blood pressure, diastolic blood pressure and heart rate in either the nutrition programme or control group throughout the study (Table 3).
Table 3. Vital signs in the nutrition programme group (n 10) and control group (n 12) at baseline (pre-holiday on 13 November, 10 d before Thanksgiving) and day 52 (post-holiday, 3 January)
(Mean values and standard deviations)

Safety: liver blood chemistry
Results are shown in Table 4. At baseline, all measured serum parameters were within normal range in both groups. However, ALT levels were significantly higher in the control group than the nutrition programme group (25·5 (sd 14·0) v. 15·5 (sd 4·5) IU/l; P = 0·0428). In addition, although ALT levels significantly increased in the nutrition programme group after 52 d compared with baseline, they were still within normal limits (21·2 (sd 8·2) v. 15·5 (sd 4·5) IU/l; P = 0·0278). Of note, ALT levels also increased in the control group from 25·5 (sd 14·0) IU/l at baseline to 29·8 (sd 20·2) IU/l at day 52, although not significantly. AST levels did not significantly change.
Table 4. Liver blood chemistry and fasting serum metabolic markers in the nutrition programme group (n 10) and control group (n 12) at baseline (pre-holiday on 13 November, 10 d before Thanksgiving) and day 52 (post-holiday, 3 January)
(Mean values and standard deviations)

ALT, alanine aminotransferase; AST, aspartate aminotransferase; HOMA2, updated homoeostasis model assessment.
* Mean value was significantly different from that at baseline (P < 0·05; within group; paired Student's t test).
† Mean value was significantly different from that at baseline (P < 0·05; within group; Wilcoxon matched-pairs test).
‡ Mean value was significantly different from that of the control group (P < 0·05; between groups; unpaired Student's t test)
§ Mean value was significantly different from that of the control group (P < 0·05; between groups; Mann–Whitney U test).
‖ To convert insulin in μIU/ml to pmol/l, multiply by 6·945. To convert cholesterol in mg/dl to mmol/l, multiply by 0·0259. To convert TAG in mg/dl to mmol/l, multiply by 0·0113.
Adverse events
Although a total of sixty-nine adverse events were reported by all participants in the nutrition programme group, only ten (14·5 %) were possibly related to the dietary intervention. Those ten events, intermittent and gastrointestinal in nature, and of mild or moderate severity, were reported by two (20 %) subjects on fasting days. One subject reported flatulence on eight separate occasions over a total of 18 d. The other subject reported two separate episodes of nausea, the first lasting 13 d and the second lasting 2 d. Those events did not require any treatment and were ultimately resolved. No severe adverse events were reported. On the basis of these results, the nutrition programme appears overall safe and well tolerated.
Discussion
This pilot study showed that a modified 5:2 nutrient-supported intermittent energy restriction nutrition programme might be a promising strategy to manage and prevent weight gain and support metabolic health during the winter holiday season in healthy overweight individuals. The compliance rate was very high, and no severe adverse events were reported, suggesting that this dietary intervention appears to be safe and well tolerated.
Subjects who followed the nutrition programme for 52 d during the winter holiday season not only did not gain weight, but also lost a modest yet significant 1·3 kg compared with their baseline pre-holiday weight. The results are in line with studies reporting the beneficial effects of various intermittent energy restriction interventions on body weight(Reference Varady, Bhutani and Klempel21, Reference Bhutani, Klempel and Kroeger53–Reference Williams, Mullen and Kelley63). However, no between-group difference in weight loss after 52 d was observed, indicating that the study was underpowered. In contrast to prior studies conducted in the USA(Reference Yanovski, Yanovski and Sovik1–Reference Helander, Wansink and Chieh5), subjects in the control group who consumed their habitual holiday diet did not gain any weight. Although surprising, this could in part be explained by subjects being inclined to slightly change their food choices, and therefore their energy intake, knowing they were participating in a weight management study. Similar results were reported by others in adults(Reference Wagner, Larson and Wengreen64) and college students(Reference Hull, Hester and Fields65) between Thanksgiving and New Year. Results also indicated the beneficial effects of 2-d energy restriction on key metabolic markers in subjects enrolled in the nutrition programme. In participants in the control group who consumed their holiday diet between Thanksgiving and New Year, fasting insulin and total and LDL-cholesterol levels significantly increased compared with normal range values at baseline. HOMA2 also increased, indicative of greater insulin resistance. Overindulgence of festive foods, often rich in carbohydrates and fat, may have negatively impacted metabolic health, probably increasing insulin requirements and lipid levels(Reference Rees, Holman and Turner6). Those effects are detrimental, as insulin resistance is associated with a cluster of metabolic disorders, including dyslipidaemia, and is a risk factor for CVD(Reference Ormazabal, Nair and Elfeky66). In contrast, while still consuming their holiday festive food ad libitum 5 d a week, subjects who followed the nutrition programme experienced a significant reduction in fasting TAG and an increase in HDL-cholesterol levels compared with the pre-holiday baseline. Worthy of attention is the fact that although overweight, these subjects had a lipid profile within normal range at the beginning of the study and therefore were unlikely to benefit from substantial improvements in serum metabolic parameters. However, these predictable acute changes are potentially due to the 2-d energy restriction rather than the nutrition programme itself(Reference Harvie, Pegington and Mattson22, Reference Horne, Muhlestein and Lappe67), and might normalise within a few days of resuming habitual energy intake. Although likely to be transient, these positive changes from fasting remain clinically meaningful, as maintaining an optimal lipid profile is known to be critical to promote cardiovascular health(Reference Ference, Graham and Tokgozoglu68). Long-term consequences of these changes are yet to be investigated, but, if repeated, these effects might help maintain healthy TAG and HDL-cholesterol levels already within normal range in healthy individuals. We did not observe any reduction in fasting insulin level resulting from 2-d intermittent energy restriction in subjects in the nutrition programme group(Reference Harvie, Pegington and Mattson22). However, we observed a significant between-group difference in changes from baseline in fasting insulin levels, although it was mainly driven by the aforementioned increase in insulin levels in the control group. Between-group differences were also significant for total cholesterol:HDL-cholesterol ratio and HOMA2, suggesting beneficial effects of the intermittent energy restriction intervention on known risk factors for CVD.
To our knowledge, there is little research on 5:2 dietary interventions, and none conducted during the winter holiday period. In a 6-month randomised study, Harvie et al.(Reference Harvie, Pegington and Mattson22) described the benefits of a 2-d/week energy restriction intervention (about 650 kcal/d; 2720 kJ/d) on body weight, total cholesterol, LDL-cholesterol, TAG, fasting insulin and insulin sensitivity in young overweight or obese women. In another study, obese male adults who consumed a 5:2 diet with 2 d of 600 kcal/d (2510 kJ/d) and 5 d of habitual eating per week for 6 months experienced significant weight loss(Reference Conley, Le Fevre and Haywood24). And, in a third study, Sundfør et al.(Reference Sundfør, Svendsen and Tonstad29) reported improvements in TAG and HDL-cholesterol and weight loss in obese adults engaged in intermittent energy restriction with 400–600 kcal/d (1670–2510 kJ/d) on 2 d per week for 6 months. Although limited, these data highlight the significance of 5:2 dietary strategies to support weight loss and metabolic health.
Strengths and limitations of the study
One strength of the present study is that this is the first randomised clinical trial to investigate the efficacy of a modified 5:2 nutrition plan combined with dietary supplements for weight management during the winter holiday season. Another strength is the very high compliance rate. Adherence to conventional weight-loss programmes such as energy restriction interventions in human subjects is notoriously low(Reference Dansinger, Gleason and Griffith69), especially during the holiday season. The very high compliance rate reported in this study indicates that participants did not have any difficulty adhering to this intermittent energy restriction nutrition programme in a real-life situation during the holiday season. One reason might be that this nutrition programme has the advantage over other dietary interventions for weight management of being achievable, simple and easy to follow, with no ‘calorie counting’ or food restrictions 5 d per week. In addition, participants consumed a balanced shake combined with dietary supplements to support weight management efforts, which were likely to help with compliance. This plan was tested among healthy overweight individuals who were most likely to benefit from a real-life dietary intervention and motivated to lose weight.
This pilot study had several limitations. First, this single-centre study used a small convenience sample, mainly from Life Extension employees, and not a population-based sample. Therefore, subjects who participated in this trial might have been more health conscious and more engaged in weight-loss efforts knowing they were part of a weight management study. For this reason, the results may lack external validity and may not be applicable to the general population. Future studies on a larger cohort of participants recruited from the whole population are needed to confirm the study findings. The second limitation was an imbalance in the number of participants between both groups, which may be due to the block size. Third, serum metabolic markers were measured in the morning after the two fasting days in the nutrition programme group. In future studies, 12-h fasting blood samples should be collected after 5 d of ad libitum energy intake to ascertain effects of the nutrition programme on serum metabolic markers. Fourth, the absence of a between-group difference in body weight changes after 52 d indicated that the nutrition programme did not have an overall effect compared with the control group, and that the study was underpowered. After conducting a post hoc power analysis, it was determined that enrolment of twenty subjects per group would provide 80 % power to detect a between-group difference of 1·36 kg at a two-sided 0·05 level of significance with an estimated standard deviation of 1·5 kg. Fifth, participants were not required to self-report their food intake describing their dietary pattern on fed days during the holiday season. They did not have to log their physical activity either. Self-reported data can be inherently biased and imprecise and needs to be interpreted with caution(Reference Subar, Freedman and Tooze70). However, validated self-reporting tools can provide a valuable piece of information about energy intake and expenditure during non-fasting days. Sixth, the study did not include a survey to determine how participants felt about the nutrition programme, if they would recommend it to others, and if they would be likely to use it again. Finally, the study lacked validated questionnaires to evaluate the impact of the nutrition programme on various aspects of participants’ lives, including sleep, mood and quality of life.
Taken together, results from this pilot study suggest this modified 5:2 intermittent energy restriction nutrition programme is a promising dietary strategy to support weight management in healthy overweight adults during the winter holiday season. Additional research in a larger sample is warranted to assess long-term adherence and effectiveness of this nutrition plan and confirm these encouraging findings.
Acknowledgements
We are grateful to Richard Stein, MD, PhD, Blake Gossard, ELS, MWC, Shayna Sandhaus, PhD and Lori Feldman, RD, CCRC for reviewing this paper.
This study was sponsored by Supplement Formulators, Inc.
S. P. H. conducted and managed the study. M. P. analysed data and wrote the manuscript. S. V. J. designed the study. A. G. S. designed the study and revised the manuscript.
All the authors were, at the time of preparing the manuscript, employed at Life Extension.