Coccidiosis, caused by parasites of the genus Eimeria, is a widespread condition which adversely impacts broiler chicken farm’s profitability by reducing growth rate and feed efficiency due to anorexia(Reference Preston-Mafham and Sykes1, Reference Kipper, Andretta and Lehnen2) and impaired nutrient absorption(Reference Preston-Mafham and Sykes1, Reference Su, Miska and Fetterer3, Reference Persia, Young and Utterback4). Malabsorptive coccidiosis, caused by infection with species such as E. maxima and E. acervulina which affect the small intestine, is characterised by inflammation and intestinal epithelium damage, impaired absorption of fat, Ca and P(Reference Turk5, Reference Takhar and Farrell6), and long bone mineralisation(Reference Watson, Matthews and Southern7, Reference Willis and Baker8, Reference Ward, Watkins and Southern9). Our previous study has indicated that E. maxima infection adversely impacts bone development, with effects being more pronounced at later stages of infection, long after birds have recovered and caught up with the performance of their non-infected counterparts (day 13 post-infection (pi))(Reference Sakkas, Oikeh and Blake10).
Dietary vitamin D (vitD) supply plays a critical role in bone mineralisation of broilers(Reference Sakkas, Smith and Hill11). It may be supplied in the form of cholecalciferol (D3) or 25-hydroxycholecalciferol (25D3). D3 is hydroxylated to 25D3, primarily in the liver, and is circulated by the vitD-binding protein(Reference Haussler, Whitfield and Kaneko12). This form is hydroxylated further, primarily in the kidneys, to the hormonally active form 1α,25D3 (1,25D3)(Reference Fleet and Schoch13). 1,25D3 regulates Ca and P metabolism mainly by enhancing intestinal Ca and P absorption and renal reabsorption, while it also stimulates osteoclast differentiation and Ca reabsorption from the bone and promotes mineralisation of the bone matrix(Reference Haussler, Whitfield and Kaneko12). In addition to its skeletal effects, 1,25D3 acts as an immune system modulator(Reference Baeke, Takiishi and Korf14), having beneficial effects in the case of infectious and autoimmune diseases(Reference Dimitrov and White15, Reference Barbachano, Fernandez-Barral and Ferrer-Mayorga16, Reference Gombart, Harder and Schröder17).
To date there have been no studies specifically investigating the effects of coccidiosis on vitD status. D3 is a relatively non-polar molecule; it is solubilised by incorporation into bile salt micellar solutions for movement through the body and repackaged into chylomicrons for transport by the lymphatic route(Reference Borel, Caillaud and Cano18). It has been suggested that absorption of 25D3 is less fat-dependent than D3, as illustrated in patients with cholestatic liver disease(Reference Sitrin and Bengoa19) and in patients with steatorrhoea(Reference Krawitt and Chastenay20). Dietary fat is digested in the small intestine in both avian and mammalian species(Reference Tancharoenrat, Ravindran and Zaefarian21). Although fatty acids are drained directly into the portal blood system instead of the lymph and as portomicrons instead of chylomicrons in birds as opposed to mammals(Reference Bensadoun and Rothfeld22, Reference Zaefarian, Abdollahi and Cowieson23), malabsorptive eimerian infections are accompanied by a pronounced depression of fat digestibility(Reference Sharma and Fernando24, Reference Amerah and Ravindran25) and circulating levels of fat-soluble vitamins A and E(Reference Sakkas, Oikeh and Blake10). VitD has been associated with immunomodulatory roles through the production of antimicrobial peptides, cytokine responses and disease outcomes(Reference Youssef, Miller and El-Abbassi26). A recent study has indicated that increasing dietary vitD supplementation as 25D3 supplementation altered cytokine responses, increasing the transcription of IL-10 and reducing that of interferon-γ (IFN-γ) and IL-1b, in layer chicks infected with a mixed Eimeria sp. infection while increasing their body weight (BW) gain, but had no effect on oocyst production(Reference Morris, Shanmugasundaram and McDonald27).
To the best of the authors’ knowledge, this is the first study which investigated the effects of coccidiosis on vitD status and the consequences of dietary supplementation in the form of D3 or 25D3 in Eimeria-infected broilers. In the present study, we used E. maxima to investigate the hypothesis that circulating levels of 25D3 would be reduced in infected chickens and that dietary supplementation with 25D3 would be more effective than D3 at reducing the effect. As a result, infected birds would benefit from higher circulating levels of 25D3 through increased bone mineralisation, the effects being more pronounced at later points of infection when compensatory nutrient absorption occurs(Reference Turk5). In addition, we investigated whether vitD supply influences parasite replication and cytokine transcription in the jejunum, the primary site of E. maxima colonisation and replication, at the peak of parasite replication (i.e. day 6 pi(Reference Blake, Hesketh and Archer28)), and on intestinal histomorphometric features which are indicative of gastrointestinal tract (GIT) damage.
Materials and methods
Birds, husbandry and feeds
All procedures were conducted under the UK Animals (Scientific Procedures) Act 1986 and EU Directive 2010/63/EU for animal experiments, carried out under Home Office authorisation (P441ADF04). A total of 336 male, 308-d-old Ross chicks were housed in a windowless, thermostatically controlled building in forty-eight pens of 0·85 m2. Pens were equipped with tube feeders and bell-drinkers, and wood shavings served as litter. Birds had ad libitum access to feed and water. Pen temperature was maintained according to Aviagen recommendations(29) and a lighting schedule of 23 h light–1 h darkness was applied for the first 7 d of age, switched to 18 h light–6 h darkness for the remainder of the trial. Basal starter (days 0–10) and grower (days 11–25) diets were manufactured according to Aviagen nutrition specifications(30) (Table 1), to which different sources and levels of vitD were added in order to formulate four dietary treatments (Table 2): LD3 (low level of D3; 25 μg/kg), L25D3 (low level of 25D3; 25 μg/kg), MD3 (commercial level of D3; 100 μg/kg) and M25D3 (commercial level of 25D3; 100 μg/kg). The medium vitD levels (M) were selected to reflect commercial practice and breeder recommendations, whereas low levels (L) have been previously shown to reduce bone mineralisation(Reference Sakkas, Smith and Hill11). Diets were analysed for vitD3 and 25D3 contents at the DSM Laboratory (Basel, Switzerland) according to previously published methodology(Reference Jakobsen, Maribo and Bysted31) (Table 2). The starter diet was offered in crumbled form and the grower diet in pelleted form. Birds were assessed daily for potential adverse effects of our LD treatments on their locomotion capacity. No birds were euthanised due to health-related disorders, and coccidiosis caused anorexia and reduced weight gain according to expectations.
Table 1. Ingredients and chemical composition of the starter (days 0–10) and grower (days 11–25) basal diets offered to chickens
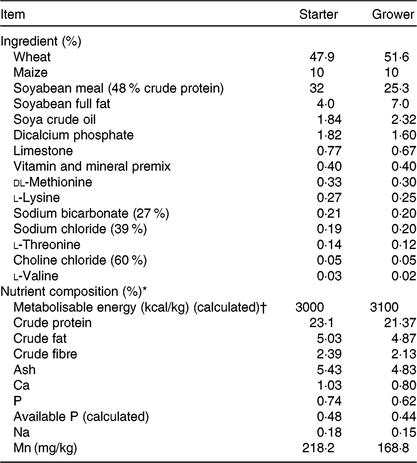
* Nutrient composition was in accordance with Aviagen nutrient specifications(30) apart from vitamin D source and level of supply.
† To convert energy in kcal to kJ, multiply by 4·184.
Table 2. Analysed cholecalciferol (D3) and 25-hydroxycholecalciferol (25D3) content (μg/kg of feed) of the four dietary treatments: LD3 (low level of D3; 25 μg/kg), L25D3 (low level of 25D3; 25 μg/kg), MD3 (commercial level of D3; 100 μg/kg) and M25D3 (commercial level of 25D3; 100 μg/kg)
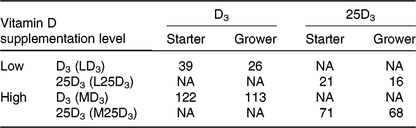
NA, not applicable.
Experimental design and inoculations
The experiment followed a 2 × 2 × 2 factorial design with vitD level, source and infection status as independent variables. Upon arrival, chicks were randomly assigned to dietary treatment groups at one of two vitD levels (M v. L) and one of two sources of vitD activity (D3 v. 25D3). At 11 d of age (day 0 pi) they were further allocated to two levels of infection (non-infected control group (C) v. infected group (I)) and were orally inoculated with a single 0·5 ml oral dose of water (C) or 7·0 × 103 (I) of sporulated E. maxima oocysts of the Weybridge strain. Each treatment group consisted of six replicate pens and the initial stocking density was seven birds per pen. Pen BW was measured at placement (day 0 of age), while individual bird’s BW and pen feed intake were measured at days 0, 6, 10 and 14 pi (days 11, 17, 21 and 25 of age, respectively). One bird per pen with a BW close to the pen average was selected at weighing on days 6, 10 and 14 pi for sampling.
Sampling
The selected birds were individually weighed before blood sampling via the wing vein and were subsequently euthanised with a lethal injection of sodium 135–137 pentobarbitone (Euthatal®; Merial). Blood was placed in 5 ml sodium heparin plasma tubes (BD Vacutainer, SST II Advance Plus Blood Collection Tubes; BD). Collected samples were immediately placed on ice and centrifuged for 600 s at 1500 g at 4°C within 1·5 h of collection. Aliquoted plasma samples were stored at −80°C pending analyses. Following blood sampling of the selected birds at day 6 pi, 6 cm of intestinal tissue were excised from the immediate region of Meckel’s diverticulum, which is the midpoint of the intestinal area infected by E. maxima (Reference Long, Millard and Joyner32), opened longitudinally and digesta contents were removed. Following this, 5 cm of tissue was submerged in 7 ml bijous and 1 cm proximal to the jejunum in 1·5 ml screw cap microtubes (ThermoScientific) filled with RNAlater® (Life Technologies). Samples were immediately stored at −80°C pending analyses. Additionally, three segments of 1 cm, one from the duodenal loop, one from the mid-jejunum (midway between Meckel’s diverticulum and the end of the duodenal loop) and one from the mid-ileum (midway between Meckel’s diverticulum and the ileocaecal junction), were sampled from all dissected birds on days 6, 10 and 14 pi, and were fixed in 10 % buffered formalin for histomorphometrical assessment. Following intestinal tissue sampling, the right tibia and femur were dissected, defleshed and stored at −20°C pending analysis in airtight sealable polyethylene bags.
Bone analysis
Bones were thawed at 4°C and tibia and femur length were measured with digital callipers. Subsequently, bone weight was recorded. Robusticity(Reference Riesenfeld33) and Seedor(Reference Seedor, Quartuccio and Thompson34) indices were calculated using the following formulae:
Bones were subjected to a three-point break test using an Instron testing machine (Instron 3340 Series Single Column-Bluehill 3) using previously employed methodology(Reference Sakkas, Oikeh and Blake10, Reference Sakkas, Smith and Hill11). Broken tibias were boiled for 300 s in deionised water at 100°C to facilitate removal of cartilage caps, and bones were split in half for manual removal of the bone marrow. Following this, bones were placed in vessels containing 10 ml acetone and 10 ml petroleum ether (VWR) and were subjected to fat extraction in a Mars 6 Microwave-Assisted Reaction System 6 (CEM) with a set temperature of 180°C for 4800 s. Fat-extracted tibias were then placed in an oven at 105°C for 18 h and were weighed to obtain dry defatted bone weight. Subsequently these were ashed in a Phoenix CEM ashing microwave furnace (CEM) at 850°C for 1·5 h to obtain ash weight (g).
Plasma levels of calcium, phosphorus and 25-hydroxycholecalciferol
Plasma concentration of 25D3 (ng/ml) was analysed using the 25-Hydroxy Vitamin D Direct EIA kit (IDS Diagnostics), and plasma concentrations of Ca and P (mmol/l) were determined with an ABX Horiba Pentra 400 automatic analyser (Horiba Medical) in duplicate, according to manufacturer’s instructions.
Histology
Excised, formalin-fixed intestinal sections were processed according to previously used methodology and stained with haematoxylin/eosin(Reference Sakkas, Oikeh and Blake10). Mounted slides were scanned (Leica SCN400; Leica Microsystems), and images were captured using the Leica Image Viewer Software (SlidePath Gateway Client Viewer 2.0). Captured images were assessed for the determination of villus length (VL) and crypt depth (CD) using ImageScope® software (Aperio Technologies). Ten villi with their corresponding crypts were measured per section to obtain an estimated length, expressed in micrometres.
Eimeria maxima genome copy number
To assess parasite replication, we used quantitative real-time PCR to measure parasite genome copy number (GC) in tissues surrounding Meckel’s diverticulum. This method supports higher throughput analysis and minimises the impact of variation related to the temporal manner of oocyst excretion(Reference Blake, Hesketh and Archer28). The methodology was used as described previously in studies of parasite replication in chicken lines differing in growth rate(Reference Sakkas, Oikeh and Blake10).
RNA isolation, reverse transcription and real-time quantitative PCR
RNA was extracted from intestinal tissue using the Isolate II RNA Mini Kit (Bioline Reagents) following the manufacturer’s protocol. RNA concentration and quality was confirmed using a NanoDrop spectrophotometer (NanoDrop™ 2000; NanoDrop Products). Isolated RNA extracts were reverse-transcribed using a Transcriptor First Strand cDNA Synthesis Kit (Roche) following the manufacturer’s protocol and stored at −20°C until use. Oligonucleotide primers for cytokine and reference gene transcripts were adopted from the published literature (Table 3). Standard PCR was carried out on a cDNA sample with each primer pair using MyFi Mix polymerase (Bioline) as described by the manufacturer to provide template for serial dilution standard curves. Tenfold serial dilution was performed in molecular-grade water to generate standard curves (1010–101) for three reference genes (glyceraldehyde 3-phosphate dehydrogenase (GAPDH), ribosomal protein L13 (RL13) and TATA-binding protein (TBP)) and for the cytokine genes of interest, IL-10 and IFN-γ. Real-time quantitative PCR (qPCR) was performed with amplification and detection carried out using Roche 96 LightCycler detection system (Roche). qPCR was performed in a 20-μl reaction containing 2 μl cDNA from the RT reaction, 10 μl SYBR Green PCR Master Mix (Roche), 0·75 μl primer (at 10 μM concentration) and 6·5 μl RNase-free water, using the following cycle: pre-incubation: 95°C for 600 s; three-step amplification: forty cycles at 95°C/10 s, 60°C/10 s, 72°C/20 s; melting: 95°C/10 s, 65°C/60 s, 97°C/1 s (continuous) conditions. Each RT-PCR experiment contained triplicate no-template controls, test samples and a log7–log1 dilution series of standard cDNA. Calculation of copy number of each qPCR target was performed according to the slope and intercept of the corresponding dilution series. Absolute gene transcription was quantified for each target test gene, followed by normalisation of their expression ratio using the geometric mean of the three reference genes.
Table 3. Oligonucleotides used for quantitative RT-PCR
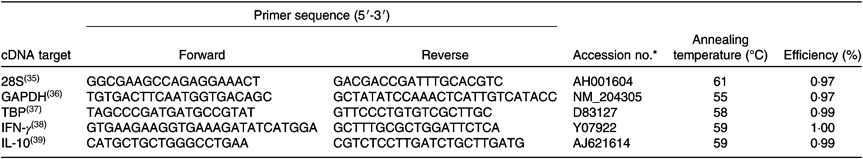
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; TBP, TATA-binding protein; IFN-γ, interferon-γ.
* Genomic DNA sequence (NCBI GenBank).
Calculations and statistics
The calculation of sample size was performed using G*power software (version 3.1). Based on the results of previous studies(Reference Sakkas, Oikeh and Blake10, Reference Sakkas, Smith and Hill11), we determined that we needed 10 replicates for the interaction between level of vitD supply and infection status to achieve 80 % power at a significance level of 0·05 for tibia ash% at the end of grower period. Since we lacked experimental data on the effect of source of dietary vitD supply or on its interactive effects with vitD level and infection, it was not possible to estimate the required sample size to investigate these two- and three-way interactions. We employed a greater sample size than the one indicated by the power analysis (twelve instead of ten replicate pens) to investigate the two-way interaction between level and infection. Under the hypothesis that in the presence of infection, circulating levels of vitD would be severely depressed and that source would be a critical factor, we estimated that the currently employed sample size of six replicate pens would suffice to investigate the three-way interactions among the main factors. All statistical analyses were conducted in SAS 9.4 (SAS Institute). For all statistical assessments, pen was considered the experimental unit, and all variables were analysed with vitD level, source and infection status as main effects and their interactions using PROC GLM. Pen data included average BW pre-infection (day 11 of age) and at the end of the experiment (day 25 of age), daily feed intake (ADFI; g/d), average daily gain (ADG; g/d) and feed conversion ratio (FCR) calculated over the pre-infection period (days 0–11 of age) and over the early (days 0–6 pi), acute (days 7–10 pi), recovery (days 11–14 pi) periods, and overall period pi (days 0–14 pi). Tibia and femur bone breaking strength (BBS; N) as well as ash (g) were calculated as a proportion of the bird’s BW (kg) prior to dissection. Single time point data deriving from one bird per pen dissected on days 6, 10 or 14 pi included circulating plasma levels of 25D3, Ca and P, bone parameters and histological measurements, as well as parasite GC and mRNA transcription levels of IFN-γ and IL-10 at day 6 pi. Uninfected birds were excluded from the model for E. maxima GC and IL-10 expression levels since both were below the level of detection. For all statistical procedures, the normality of residuals was assessed with the Shapiro–Wilk test. Predicted E. maxima GC, cytokine transcription levels and plasma levels of 25D3 were log-transformed to normalise residual distribution. When significant differences were detected, treatment means were separated and compared by the Tukey’s multiple comparison test. Significance was determined at P < 0·05. All values are expressed as model-predicted least square means along with their pooled standard errors.
Results
Performance
No significant difference was detected in chick BW at placement between treatment groups (average 43·5 g; sem 0·41; P > 0·1). The main effects of vitD level, vitD source and infection on performance variables over the periods pre- and post-infection are presented in Table 4. VitD level significantly interacted with infection for FCR (P < 0·05) over the overall period pi (days 0–14 pi), being the highest in infected birds on LD diets (Fig. 1). There were no other two- or three-way interactions between vitD level, vitD source and infection status on broiler growth performance parameters. At day 0 pi (day 11 of age), bird BW, ADG and ADFI were significantly higher for birds on M diets (P < 0·05) than for those on L diets. Infection significantly reduced ADFI and ADG, and increased FCR over the early, acute and overall periods pi (P < 0·0001), while performance of C and I birds was similar (P > 0·1) over the recovery period. Birds on M diets had significantly higher final BW (day 25 of age) and ADG over the early and acute periods (P < 0·05), and lower FCR (P < 0·05) over the early, acute and overall periods pi than birds on L diets. Birds on 25D3 diets had significantly higher final BW and ADG over the acute and overall period pi, and lower FCR over the overall period pi (P < 0·05) than birds on D3 diets.
Table 4. Main effects of level, source of vitamin D supply and Eimeria infection status on chicken performance pre-infection (days 0–11 of age) and over the early (days 0–6), acute (days 6–10), recovery (days 10–14) and overall periods (days 0–14) post-infection (pi)*
(Mean values and pooled standard errors)
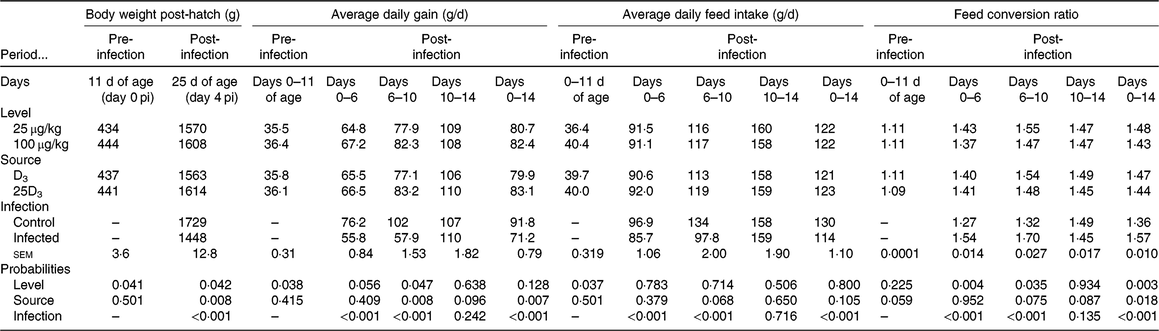
D3, cholecalciferol; 25D3, 25-hydroxycholecalciferol.
* Chickens orally inoculated with 0 (control) or 7 × 103 sporulated E. maxima oocysts (infected) at day 11 post-hatch (day 0 pi).
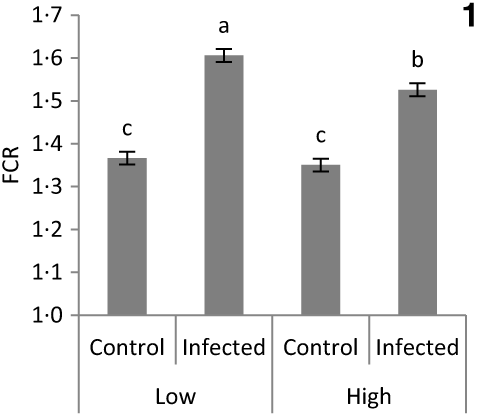
Fig. 1. Significant interaction between vitamin D level (25 or 100 μg/kg) and infection status (control or infected with 7 × 103 sporulated oocysts of Eimeria maxima at day 11 post-hatch) on the feed conversion ratio (FCR) of broiler chickens over the course of infection (days 1–14 post-infection) (P = 0·039). a,b,c Least square mean values with unlike letters were significantly different (P < 0·05; Tukey’s honestly significant difference test).
Bone variables
The main effects of vitD level, vitD source and infection on bone variables over the time points pi are presented in Tables 5 and 6, and all significant interactions are presented in Figs. 2 and 3. VitD level and infection interacted for femur BBS at day 10 pi (P < 0·005) as I birds at the low level of supplementation had reduced BBS in comparison to all other treatment groups (Fig. 2). In addition, vitD level, source and infection significantly interacted (P < 0·01) for ash weight at day 14 pi with I birds on LD3 treatment displaying the lowest values (Fig. 3). There were no other two- or three-way interactions between factors for any of the bone variables. Femur and tibia seedor indices were significantly decreased (P < 0·05) at all time points pi, and robusticity index (P < 0·05) was significantly increased at days 6 and 14 pi, in response to infection. Infection significantly reduced tibia ash (%) at all time points (P < 0·01, <0·0001, <0·0001 at days 6, 10 and 14 pi, respectively). On the other hand, tibia ash weight was significantly reduced only at day 14 pi (P < 0·0001). Femur BBS was affected on day 6 pi (P < 0·0001) and on day 14 pi (P < 0·001), while tibia BBS was affected on day 10 (P < 0·05) and day 14 pi (P < 0·0001). Offering commercial levels of vitD (M) supply significantly improved both seedor and robusticity indices of the femur on day 10 pi (P < 0·05), but did not affect the tibia. At the same time, it increased femur BBS on day 10 (P < 0·05) and tibia BBS on day 6 pi (P < 0·01). Although tibia ash weight was not affected by the level of vitD supply, tibia ash% was significantly (P < 0·05) increased at days 10 and 14 pi. The source of vitD supply significantly affected tibia seedor index at day 6 pi and robusticity index at day 14 pi (P < 0·05). There was no significant effect of source of vitD supply on BBS. However, birds receiving 25D3 achieved significantly higher tibia ash values at days 6 and 10 pi (P < 0·05) than birds receiving D3. Finally, 25D3 significantly increased tibia ash % at days 10 and 14 pi (P < 0·05).
Table 5. Main effects of level, source of vitamin D supply and Eimeria infection status on chicken femur and tibia seedor and robusticity indices at days 6, 10 and 14 post-infection (pi)*
(Mean values and pooled standard errors)
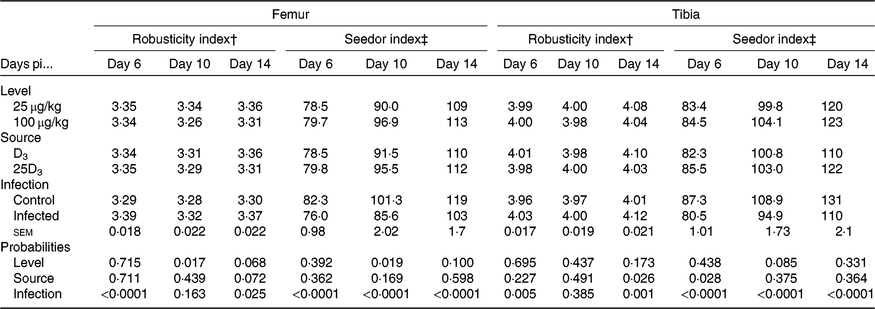
D3, cholecalciferol; 25D3, 25-hydroxycholecalciferol.
* Chickens orally inoculated with 0 (control) or 7 × 103 sporulated E. maxima oocysts (infected) at day 11 post-hatch (day 0 pi).
† Robusticity index = (bone length (mm))/(bone weight (mg)1/3).
‡ Seedor index = (bone weight (mg))/(bone length (mm)).
Table 6. Main effects of level, source of vitamin D supply and Eimeria infection status on chicken femur and tibia bone breaking strength (BBS, N) and tibia ash (g) expressed as a proportion of body weight (BW, kg) and on tibia ash percentage (%) at days 6, 10 and 14 post-infection (pi)*
(Mean values and pooled standard errors)
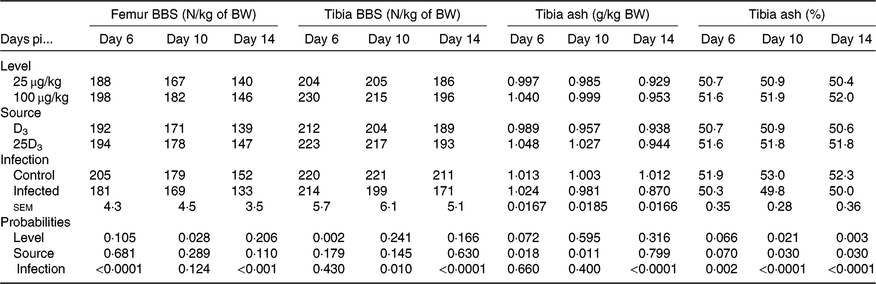
D3, cholecalciferol; 25D3, 25-hydroxycholecalciferol.
* Chickens orally inoculated with 0 (control) or 7 × 103 sporulated E. maxima oocysts (infected) at day 11 post-hatch (day 0 pi).
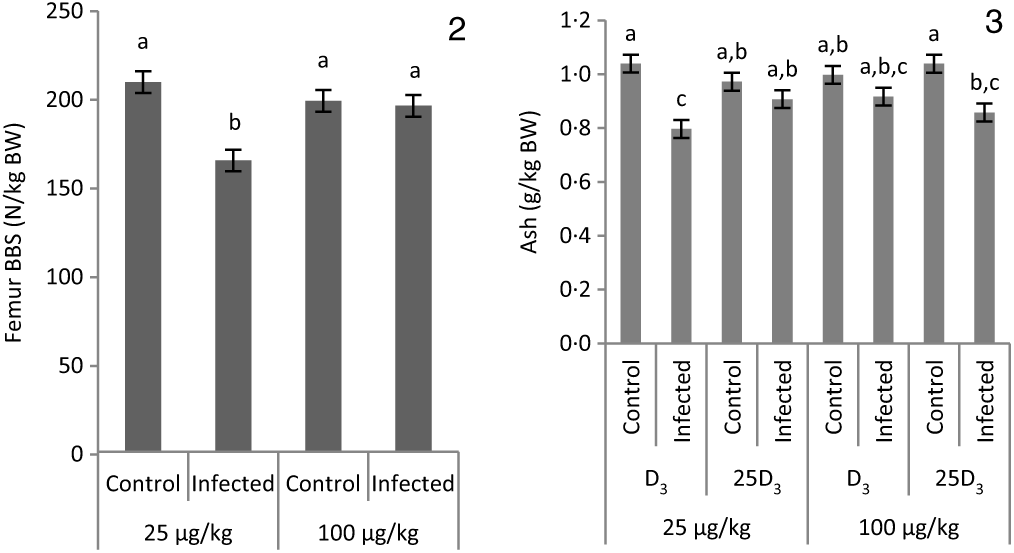
Figs. 2 and 3. Interactive effects of main factors – vitamin D (vitD) level (25 or 100 μg/kg), source of vitD supply (25-hydroxycholecalciferol (25D3) or cholecalciferol (D3)) and infection status (control or infected with 7 × 103 sporulated oocysts of Eimeria maxima at day 11 post-hatch) – on bone variables of broiler chickens. Significant interactions between vitD level and infection on femur bone breaking strength (BBS) (P = 0·002) at day 10 post-infection (pi) (2) and between vitD level, source of vitD supply and infection on ash weight (g) expressed as a proportion of body weight at dissection (g/kg BW) (P = 0·005) at day 14 pi (3). a,b,c Least square mean values with unlike letters were significantly different (P < 0·05; Tukey’s honestly significant difference test).
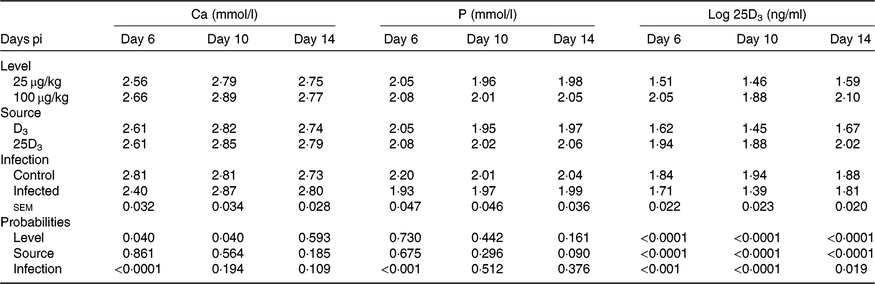
Plasma levels of calcium, phosphorus and 25-hydroxycholecalciferol
The main effects of vitD level, vitD source and infection on plasma levels of Ca, P and 25D3 over the time points pi are presented in Table 7, and all significant interactions are presented in Figs. 4 and 5. There were no significant three-way interactions between factors on plasma levels of Ca, P and 25D3. VitD level and infection interacted (P < 0·05) for P level at day 10 pi with I birds on L diets having significantly lower values compared with C birds on L and M diets (Fig. 4(A)). VitD source and infection interacted (P < 0·05) for Ca levels at day 10 pi with I birds on 25D3 treatment, achieving significantly higher values compared with C birds on the same dietary treatment (Fig. 4(B)). VitD level interacted with vitD source for 25D3 levels (P < 0·0001) at day 10 pi; these were similar for MD3 and L25D3 diets and significantly higher (P < 0·0001) than LD3 and lower (P < 0·0001) than M25D3 diets (Fig. 5(A)). Furthermore, vitD level and infection interacted for 25D3 levels on day 10 pi (P < 0·05), being similar for LD3 uninfected and MD3 infected birds and significantly higher (P < 0·0001) than LD3 infected birds and significantly lower (P < 0·0001) than MD3 uninfected birds (Fig. 5(B)). There were no other two-way interactions between factors for any of the plasma variables. Infection significantly reduced levels of Ca and P only at day 6 pi (both P < 0·0001). The level of vitD supply significantly affected Ca levels (P < 0·05) on days 6 and 10 pi, with birds on L diets having lower values. On the other hand, vitD level did not affect P at any of the time points. Source did not affect the level of Ca or P, at any of the three time points. Plasma levels of 25D3 were significantly affected at days 6, 10 and 14 pi by vitD level (P < 0·0001), source of vitD supply (P < 0·0001) and infection status (P < 0·0001); being significantly higher at all time points in birds on 25D3 treatments than birds on D3 treatments, at high levels than low levels of vitD supply and in C than I birds.
Table 7. Main effects of level, source of vitamin D supply and Eimeria infection status on chicken plasma calcium and phosphorus concentrations (mmol/l) and log-transformed plasma levels of 25-hydroxyvitamin D3 (25D3) (ng/ml) at days 6, 10 and 14 post-infection (pi)*
(Mean values and pooled standard errors)
D3, cholecalciferol; 25D3, 25-hydroxycholecalciferol.
* Chickens orally inoculated with 0 (control) or 7 × 103 sporulated E. maxima oocysts (infected) at day 11 post-hatch (day 0 pi).
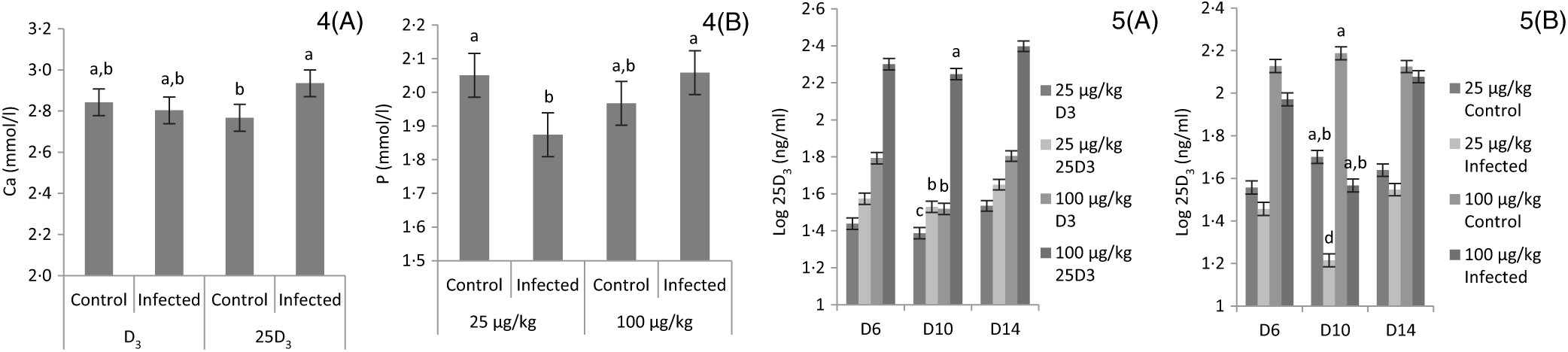
Figs. 4 and 5. Interactive effects of main factors – vitamin D (vitD) level (25 or 100 μg/kg), source of vitD supply (25-hydroxycholecalciferol (25D3) or cholecalciferol (D3)) and infection status (control or infected with 7 × 103 sporulated oocysts of Eimeria maxima at day 11 post-hatch) – on plasma parameters of broiler chickens. Significant interactions between source of vitD supply and infection status on plasma calcium concentration (mmol/l) (P = 0·040) (4(A)) and between vitD level (25 or 100 μg/kg) and infection on plasma P concentration (mmol/l) (P = 0·046) (4(B)) at day 10 post-infection (pi). Significant interactions between vitD level and source (P < 0·0001) (5(A)) and between vitD level and infection (P = 0·033) on log-transformed circulating levels of 25D3 (ng/ml; 25D3) (5(B)) at day 10 pi. a,b,c,d Least square mean values with unlike letters were significantly different (P < 0·05; Tukey’s honestly significant difference test).
Histology
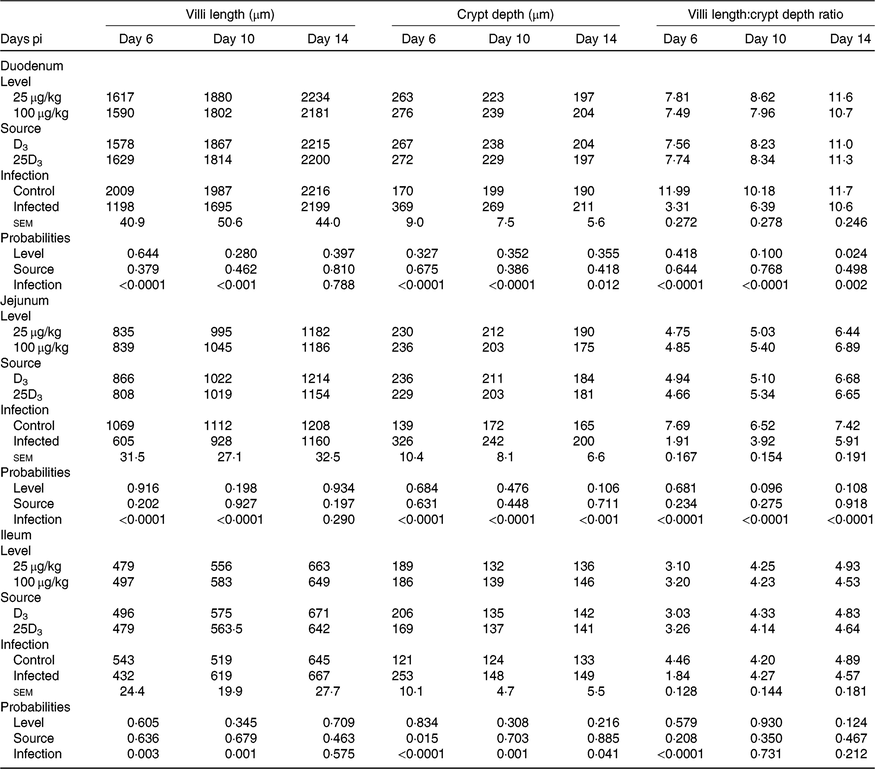
The main effects of vitD level, vitD source and infection on histomorphometric measurements pi are presented in Table 8, and all significant interactions are presented in Figs. 6–8. There were no significant three-way interactions between factors on histological measurements. VitD level and source interacted on jejunal VL at day 10 pi (P < 0·01), being significantly higher in birds on LD3 treatments than birds on MD3 treatments (Fig. 6(A)). Furthermore, jejunal VL:CD ratio was significantly higher in LD3 birds than MD3 birds at day 14 pi (P < 0·05; Fig. 6(B)). VitD level and infection interacted for jejunal VL at day 10 pi, with I birds on high vitD treatments having significantly lower values than all other treatment groups (P < 0·01; Fig. 7). Source and infection interacted for ileal VL (P < 0·05) and VL:CD ratio (P < 0·01), at day 6 pi being significantly higher for uninfected 25D3 birds (P < 0·05) than infected birds receiving either D3 or 25D3 (Fig. 8(A) and (B), respectively). There were no other two- or three-way interactions between factors for any of the histomorphometric measurements. At both days 6 and 10 pi, infection significantly decreased duodenal VL (P < 0·0001 and <0·001, respectively), increased CD (P < 0·0001) and reduced VL:CD ratio (P < 0·0001). At day 14 pi, effects persisted only on CD (P < 0·05) and VL:CD ratio (P < 0·01). The same direction of effects, on the same days, was observed for histomorphometric measurements of the jejunum and ileum, albeit the ileal VL:CD ratio was significantly affected only at day 6 pi (P < 0·0001) (Table 8). VitD level significantly affected duodenal length to villi crypt depth ratio (VCR) at day 14 pi, with birds on LD treatments having higher values (P < 0·05). On the other hand, 25D3 treatments had significantly higher CD at day 6 pi in comparison to D3 treatments (P < 0·05).
Table 8. Main effects of level, source of vitamin D supply and Eimeria infection status on chicken intestinal morphology at days 6, 10 and 14 post-infection (pi)*
(Mean values and pooled standard errors)
D3, cholecalciferol; 25D3, 25-hydroxycholecalciferol.
* Chickens orally inoculated with 0 (control) or 7 × 103 sporulated E. maxima oocysts (infected) at day 11 post-hatch (day 0 pi).
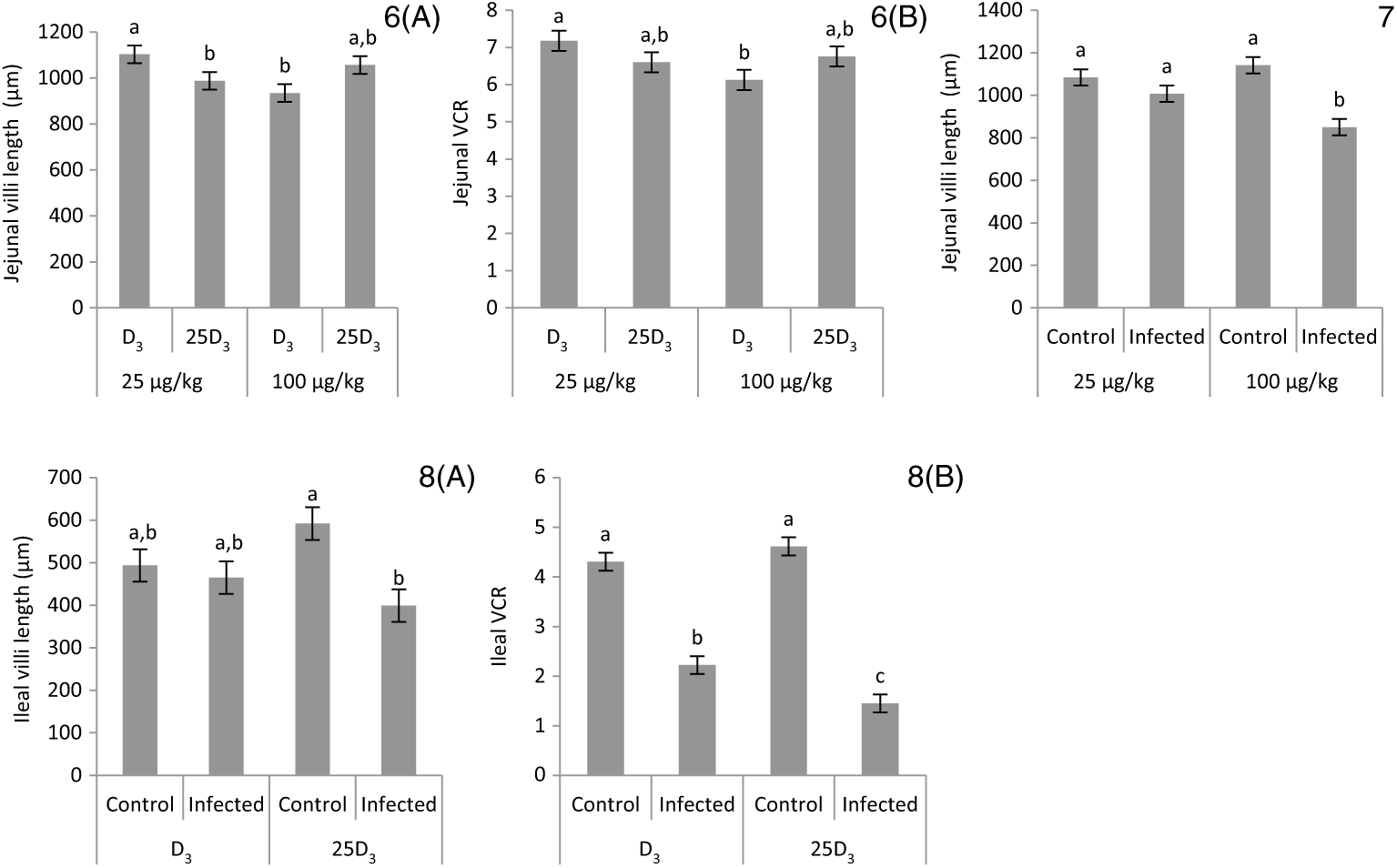
Figs. 6–8. Interactive effects of main factors – vitamin D (vitD) level (25 or 100 μg/kg), source of vitD supply (25-hydroxycholecalciferol (25D3) or cholecalciferol (D3)) and infection status (control or infected with 7 × 103 sporulated oocysts of Eimeria maxima at day 11 post-hatch) – on histological parameters of broiler chickens. Significant interactions between vitD level and source of vitD supply on jejunal villi length at day 10 post-infection (pi) (P = 0·004) (6(A)) and on jejunal villus length:crypt depth ratio (VCR) at day 14 post-infection (pi) (P = 0·008) (6(B)). Significant interactions between vitD level and infection on jejunal villus length at day 10 pi (P = 0·008) (7). Significant interactions between vitD source and infection status on ileal villi length (P = 0·022) (8(A)) and ileal VCR (P = 0·005) (8(B)) at day 6 pi. a,b,c Least square mean values with unlike letters were significantly different (P < 0·05; Tukey’s honestly significant difference test).
Parasite replication
E. maxima GC were not affected by the interaction between vitD level and source. However, these were significantly affected by both vitD level (P < 0. 0007) and vitD source (P < 0·0001); birds on MD3 had higher parasite burdens than birds on LD diets (11·5 v. 11·1; sem 0·08), and birds receiving 25D3 had higher parasite burdens than birds receiving D3 (11·6 v. 11·0; sem 0·08).
Interferon-γ and IL-10 mRNA levels
Both IFN-γ and IL-10 were not affected by vitD level (P = 0·800 and 0·721, respectively), vitD source (P = 0·998 and 0·488, respectively) or their two-way interaction (P = 0·737 and 0·488, respectively). Gene expression of IFN-γ was significantly up-regulated by infection (P < 0·0001), and it was not affected by the two-way interaction with level (P = 0·726) and source (P = 0·904), or their three-way interaction (P = 0·940).
Discussion
In a previous study using the same host–parasite model, E. maxima infection reduced bone mineralisation both in fast- and slow-growing broiler lines(Reference Sakkas, Oikeh and Blake10). In the present study, we assessed whether offering differing dietary levels of vitD (100 v. 25 μg/kg), and/or different forms (25D3 instead of D3), would alleviate the effects of infection on performance and bone mineralisation in fast-growing broilers. We also assessed parasite-related aspects of the infection through cytokine expression and parasite GC at peak parasite replication. The basis of the hypothesis was that fat-soluble vitamin status is impaired during coccidiosis, which in turn may further aggravate a marginal vitD deficiency and that 25D3 may be absorbed in a more fat-independent manner, being more potent in mediating vitD activity.
Consistent with previous findings(Reference Sakkas, Oikeh and Blake10), infection penalised the performance of infected chickens during early and acute periods of infection, but it was identical to that of uninfected birds during the recovery period. Gastrointestinal damage occurred across all segments of the small intestine around peak parasite replication(Reference Blake, Hesketh and Archer28), the effects being more pronounced and persisted longer in the proximal and mid-intestine, which is the predilection site for E. maxima (Reference Williams40–Reference Conway, McKenzie, Conway and McKenzie42). Compensatory ileal villi development took place as described previously in similar studies with the same parasite(Reference Idris, Bounous and Goodwin43), but not at the acute stage of infection (day 6 pi). In terms of bone mineralisation, the effects of infection were present throughout the pi period for both femur and tibia with both showing inferior robusticity and seedor indices. Femur BBS responded to infection earlier than tibia BBS, which could be attributed to the faster mineralisation rate of the former in comparison to the latter at initial stages of broiler growth(Reference Applegate and Lilburn44). Despite the fact that the proportion of tibia ash to BW at dissection was constant for uninfected birds throughout days 17–25 post-hatch(Reference Bar, Shinder and Yosefi45), this was not the case for infected birds where a progressive decrease was noted. By day 14 pi, infected birds matched the growth rates of their non-infected counterparts, but their tibias carried 14 % less ash (g). Moreover, tibia ash% was severely depressed at all time points, being more pronounced at day 10 pi but persisted at day 14 pi. These results bear significance considering that although ADG was comparable between infected and uninfected birds over the recovery period, the BW of infected birds was significantly lower, indicating that proportionally more stress was applied to their long bones.
Consistent with our hypothesis, vitD status was impaired in response to infection with E. maxima. Infection reduced the levels of 25D3 across the pi period, reaching the lowest levels on day 10 pi. Studies in mammalian species suggest that some storage occurs in the liver, adipose and muscle tissues(Reference Heaney, Horst and Cullen46–Reference Burild, Lauridsen and Faqir48). Furthermore, stores can be released slowly in periods of vitD deficiency, raising plasma 25D3 levels, the rate of release being higher when subjected to a negative energy balance(Reference Heaney, Horst and Cullen46, Reference Brouwer, van Beek and Ferwerda47, Reference Burild, Frandsen and Poulsen49). Although there is no information on vitD storage and kinetics in avian species, these reserves are depleted within a week in the absence of dietary supply in minipigs(Reference Heaney, Horst and Cullen46). Our results suggest that within a few days of coccidian challenge, systemic circulating 25D3 levels become severely depressed. At day 6 pi, levels of plasma Ca and P, and bone mineralisation, were penalised, likely due to their reduced absorption as a result of GIT damage. However, homeostasis of both Ca and P was attained later during infection, while penalties on vitD concentration and bone mineralisation persisted throughout.
The results of feed analysis suggested that the amount of dietary 25D3 was consistently lower than D3, in both the starter and grower diets. The reason for this discrepancy is likely analytical in nature, that is, related with the methodology of estimating 25D3 contents rather than associated with feed mixing. Ultimately, 25D3 status was significantly higher for birds receiving 25D3 than D3 diets. Therefore, results presented in the current study can be interpreted with confidence. Overall, plasma 25D3 levels were significantly increased by higher vitD supplementation and by offering 25D3 as the source of vitD activity in both uninfected and infected birds. The interaction between level and source indicates that offering 25D3 is more efficient than D3 in raising its concentration and is consistent with previous reports on chickens where serum or plasma concentrations of the metabolite were assessed(Reference Sakkas, Smith and Hill11, Reference Yarger, Saunders and McNaughton50–Reference Hutton, Vaughn and Litta52). Although there was no formal interaction between level, source and infection on circulating levels of 25D3, at day 10 pi when the effects of infection were maximised, 25D3 levels were similar between MD3 and L25D3 birds, suggesting a better absorption efficiency for dietary 25D3 (Fig. 5(A)). On the other hand, vitD supply interacted with infection status for levels of 25D3 at day 10 pi, being significantly depressed in L infected birds but maintained in M infected birds to similar levels as L uninfected birds. Infected birds on low vitD diets also showed inferior FCR across the pi period and had the lowest femur BBS and circulating P levels on the same day pi. The effect of vitD on phosphate absorption is thought to be mediated via the saturable transcellular mechanism as increased levels of NaPiIIb in the brush border membrane have been measured in response to 1,25D3 treatment of patients with renal failure and in vitD-deficient rats(Reference Davis, Zerwekh and Parker53, Reference Kurnik and Hruska54). The only formal interaction between level, source and infection was detected at day 14 pi for ash (g) where LD3 infected birds showed the lowest ash (g) overall. Collectively, these results indicate that a low vitD supply penalised bone development in infected chickens, with the greatest impact at later stages of infection when offered in the form of D3. On the other hand, although dietary 25D3 was more efficient for maintaining vitD status, it did not offer additional benefits in the presence of infection. Previous studies involving increased dietary supply of Ca(Reference Watkins, Vagnoni and Southern55) and P(Reference Willis and Baker8) have been unsuccessful in improving bone mineralisation in coccidiosis-infected birds, while phytase supplementation had limited efficacy(Reference Watson, Matthews and Southern7, Reference Shaw, van Ginkel and Macklin56). It is apparent that there are limitations in the capacity of infected birds to compensate for penalties imposed on their bone development, at least within the time period studied.
Final BW was improved by both vitD level and source, but vitD level affected ADG only over the pre-infection period, while vitD level only during the pi period and FCR was affected only during the pi period by both vitD level and source. Although performance responses to vitD supply were typically present when offering suboptimal levels of Ca and P supply, our results are consistent with previously published studies(Reference Yarger, Saunders and McNaughton50, Reference Whitehead, McCormack and McTeir57) and suggest that vitD requirements of broilers for growth functions may remain high throughout the grower period. On the other hand, increased vitD supplementation, or 25D3, resulting in improved markers of bone mineralisation was not consistent across the sampling points. Nonetheless, tibia ash%, which is the most important marker of bone mineralisation, was significantly increased by days 10 and 14 pi when offering commercial levels of vitD or in the form of 25D3. These results showed that the benefits of increased vitD supply on bone mineralisation extend beyond the starter period and are in agreement with a recently published study evaluating the effects of vitD supply in fast-growing broiler lines(Reference Sakkas, Smith and Hill11). A higher level of vitD supply also increased plasma concentration of Ca but not of P. Although this could have occurred due to increased bone resorption or enhanced Ca and/or P absorption, ultimately, bones were more mineralised, promoting the mineralisation of the bone matrix(Reference Haussler, Whitfield and Kaneko12, Reference Bikle58, Reference St-Arnaud59). The efficiency of Ca absorption is low in vitD-deficient animals(Reference Pansu, Bellaton and Roche60) and has been related to transcellular and paracellular absorption mechanisms(Reference James, Munro, Nordin, Morris and Anderson61, Reference Christakos62).
In the present study, offering a higher level of vitD, or replacing with 25D3, was associated with a higher degree of parasite replication. Likewise, a higher degree of GIT damage was observed with higher levels of vitD activity. With the presence of an infection, offering MD3 diets evoked greater jejunal VL than LD3 diets at day 10 pi, and 25D3 diets resulted in smaller ileal VL and VCR at day 6 pi than D3 birds. Regardless, intestinal transcription of IFN-γ and IL-10 was not differentially affected by dietary vitD supply. E. maxima evokes a complex cytokine response characterised by increased production of Th1 proinflammatory cytokines such as IL-1b, IL-6, IL-8, IL-17 and IFN-γ in the small intestine, as well as Th2 anti-inflammatory cytokines such as IL-4 and IL-10(Reference Williams40, Reference Min, Kim and Lillehoj63, Reference Hong, Lillehoj and Lillehoj64). In particular, increased IFN-γ mRNA levels are thought to be associated with antigen-specific resistance to coccidiosis, promoting Th1 cell production while preventing Th2 cell production(Reference Cornelissen, Swinkels and Boersma41, Reference Laurent, Mancassola and Lacroix39), balanced by IL-10(Reference Rothwell, Young and Zoorob65). Elevated IL-10 mRNA levels have been described in susceptible compared to resistant broiler chicken lines(Reference Rothwell, Young and Zoorob65), while dietary fed antibody to chicken IL-10 prevents growth depression due to a mixed Eimeria spp. infection(Reference Sand, Arendt and Repasy66). On the other hand, 1,25D3 may support conversion of naïve T cells into T regulatory cells, which produce IL-10 and transforming growth factor-β that inhibit the expression of proinflammatory cytokines such as IFN-γ and IL-17(Reference Jeffery, Burke and Mura67) and to up-regulate IL-10 production in the macrophages(Reference Sharma and Fernando24, Reference Korf, Wenes and Stijlemans68). Previous research has shown that increased supplementation of 25D3, >50 μg per kg of feed, in white Leghorn chicks infected with a mixed Eimeria spp. resulted in smaller penalties on their ADG similar to the present study(Reference Morris, Shanmugasundaram and McDonald27). However, decreased IL-1β and increased IL-10 transcripts were detected in caecal tonsils. It is possible that in the present study a delayed up-regulation of IFN-γ, or an earlier up-regulation of IL-10, rather than variations in their absolute levels at the peak of infection, may have affected parasitological outcomes and the degree of GIT damage. Further investigation of the immune response at earlier stages of infection is required to elucidate the observed effects. In addition, outcomes may differ according to the parasite species in question; E. maxima, in particular, induces a strong proinflammatory response as opposed to the more balanced Th1/Th2 phenotype which characterises infections with E. acervulina and E. tenella (Reference Williams40). Furthermore, differential effects may be observed in regard to vitD status in single or mixed eimerian species infections, which are known to occur in practice(Reference Adams, Vahl and Veldman69) depending on the species present; E. acervulina and E. maxima significantly decrease fat-soluble vitamin status(Reference Sakkas, Oikeh and Blake10, Reference Haug, Gjevre and Thebo70) as these both affect the regions of the small intestine where fat absorption occurs(Reference Tancharoenrat, Ravindran and Zaefarian21), while species such as E. tenella which affect the caeca have milder effects(Reference Jafari, Kiani and Shahriyari71). Future studies should investigate the magnitude of reduction in bone mineralisation and vitD status over time when infected by different species and under different infection pressures, as it has been previously shown that such effects may be dose-dependent(Reference Fetterer, Miska and Mitchell72).
Interestingly, parasitological and histological findings did not corroborate performance outcomes. It has been previously shown that a higher vitD status resulted in increased fractional rate of synthesis and increased breast muscle yield in broilers(Reference Yarger, Saunders and McNaughton50). Therefore, reduced FCR observed in high vitD-fed infected broilers could be attributed to their increased vitD status and improved ability to accrete body protein in the presence of infection(Reference Yarger, Saunders and McNaughton50). The lack of an interactive effect of source of vitD supply and infection status on performance variables indicates that vitD source is less critical than level of vitD supply under these experimental conditions.
In conclusion, the present study showed that an E. maxima infection penalised broiler chicken performance, bone mineralisation and vitD status, while a low vitD supply seemed to aggravate the adverse effects of infection. In contrast, a higher vitD supply resulted in higher parasite loads and compromised gut architecture in the absence of adverse effects on performance variables. Transcription of IL-10 and IFN-γ was unaffected. Additional studies are needed to elucidate the effects of vitD supply on immune responses over time in different host–pathogen systems.
Acknowledgements
We would like to thank Dr Tom Hill for his guidance in the analysis of 25D3, Mr James McPhail for carrying out quantitative real-time PCR for the measurement of parasite genome copy numbers, and Dr Sarah Macdonald for the training and advice provided for RNA isolation, reverse transcription and real-time qPCR.
This work was conducted under the PROHEALTH project. PROHEALTH received funding from the European Union Seventh Framework Programme for Research, Technological Development and Demonstration under grant agreement no. 613574.
The authors’ responsibilities were as follows: P. S., I. K. designed the research; I. O., P. S., D. P. B. and S. S. conducted the research; D. P. B. provided essential materials; P. S. and I. O. analysed the data; P. S., I. O., I. K. and D. P. B. wrote the manuscript; P. S. and I. K. had primary responsibility for the final content; I. K. was responsible for grant management; all authors have read and approved the final manuscript.
There were no conflicts of interest.















