Introduction
Based on the report in the British Medical Journal (BMJ), medical error is the third leading cause of death in the USA, after heart disease and cancer. As such, medical errors should be a top priority for research and resources [Reference Makary and Daniel1]. Globally, it is estimated that 142,000 people died in 2013 from adverse effects of medical treatment; this is an increase from 94,000 in 1990 [Reference Abubakar2]. The problem of medical errors affects many countries, especially developing countries. Much of the evidence on the burden of harm from medical care is from developed nations, although enough evidence exists from developing countries and countries with economies in transition to suggest that unsafe medical care is a major problem in those nations with major implications for health policy, planning and resource allocation as well [Reference Wilson3].
An important part of medical errors is related to errors in diagnosis. Errors related to delayed or missed diagnoses are a frequent and underappreciated cause of patient injury [Reference Schiff4]. It is difficult to discern exactly how a given diagnosis was reached. In other word, the root cause of the diagnostic error is difficult to study as errors tend to be defined only in hindsight and the ‘microscope’ that can enable detection of mental processes in live time has yet to be invented [Reference Norman and Eva5]. Generally, physicians begin the diagnosis generation very quickly in dealing with the patient. The dual-process theory describes two systems used by physicians for diagnostic decisions: intuitive (mental perception) and analytical approaches. The experienced physicians are well aware of how to manoeuvre between these two approaches and when it is appropriate to slow down and devote more time to analyse existing data [Reference Vick6]. However, no physician is immune to diagnostic errors, no matter how experienced or knowledgeable he or she is [Reference Kirch and Schafii7]. Although the study of physicians’ diagnostic thinking process is a complicated issue, it is estimated that 75% of diagnostic errors can be attributed to a failure in physician thinking [Reference Schiff4].
In every study of clinical vs. autopsy diagnoses, a significant incidence of discrepancies has been found. Not all errors or discrepancies carry equal weight: some are relatively inconsequential, but others have considerable impact and might have influenced patient survival if recognised during life [Reference Burton and Rutty8]. As an example of the latter, one can cite several numbers of serious, life-threatening but curable infectious diseases.
One of the diseases that usually is subject to diagnostic errors (delayed or misdiagnosis) is infective endocarditis (IE). Despite the major advances in diagnostic technology and improvements in antimicrobial selection and monitoring, accompanied by parallel advances in surgical techniques, IE continues to be characterised by increased morbidity and mortality and is now the third or fourth most common life-threatening infectious disease [Reference Baddour9]: one in five patients dies during the initial hospital admission [Reference Wallace10]. It has been shown that globally, in 2010, IE was associated with 1.58 million disability-adjusted life-years or years of healthy life lost as a result of death and non-fatal illness or impairment [Reference Murray11]. Although it is reported relatively rare, the high morbidity and mortality rates in the absence of appropriate care necessitate a thorough understanding of the obstacles towards the early diagnosis and management of IE. Furthermore, IE incidence has increased over the past decade in some area [Reference Pant12], thus increases its importance; besides its epidemiology has been changed worldwide over the last half-century to be more prevalent among the elderly, injection drug users (IDUs), and those who had healthcare contact which further increases the incidence of atypical and confusing presentation of IE. IE is one of the diseases that is usually subject to diagnostic errors [Reference Pant12, Reference Slipczuk13]. Although there is limited information about the epidemiology and characteristics of IE in Iran, few studies reported an increasing trend of hospitalisation due to IE and increasing trend of the proportion of IDUs with IE in Iran [Reference Hajihossainlou, Heidarnia and Kashani14–Reference Heydari16]. The mortality associated with IE in Iran has been reported to range from 7% to 25% [Reference Hajihossainlou, Heidarnia and Kashani14, Reference Heydari16].
The aim of this study is to evaluate the frequency of discrepant diagnosis on admission and associated factors in patients with IE. We defined discrepant diagnosis as the discrepancy between reason for admission and discharge (final) diagnosis [Reference Shenvi and El-Kareh17]. Because of the variability in the clinical presentation, IE could be a tough diagnosis that requires a diagnostic strategy. Therefore, we also considered early diagnostic and therapeutic approach of patients (in the first days of hospitalisation) important to label a diagnosis as discrepant.
Materials and methods
The study conducted in a 1000-bed academic general hospital in Mashhad, Iran during the period from March 2007 to February 2015. It was a retrospective review of hospital records of all hospitalised adult patients (⩾18 years) with the discharge diagnosis of IE.
This study was approved by the Research Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Outcome measurements and statistical analysis
The primary outcome was the frequency of discrepancy between the admitting and discharge diagnosis. Secondary outcomes included clinical and demographic features and clinical outcome of patients and factors associated with diagnosis non-discrepancy.
Statistical analysis was completed using SPSS version 12.5. Data were expressed as means ± standard deviation (s.d.). Histograms were used to determine the distribution of data and appropriate non-parametric or parametric tests were selected. A chi-square and Fisher's exact test of association were used to compare nominal data. Univariate analyses were used to assess the association between each variable and discrepancy in diagnosis. We used multiple logistic regression analysis to identify independent clinical predictors of non-discrepant diagnosis on admission. All test results were considered significant with a P-value of less than or equal to 0.05.
Information could not be identified for disease-related variables for all patients, therefore, denominators sometimes varied for the variables.
Case definitions
IE was defined according to Duke criteria [Reference Li18].
IE was defined as healthcare associated according to the following criteria: (1) onset of symptoms >48 h after hospitalisation with no evidence of IE at the time of hospital admission, or (2) onset of symptoms in the first year after heart valve replacement, or (3) prior antibiotic use in the last 6 months diagnostic or therapeutic manipulations in the ambulatory setting within 3 months before symptom onset, or (4) prior antibiotic use or hospital admission for more than 48 h in the last 3 months, or (5) immunosuppression.
Elderly was defined as those ⩾60 years of age at diagnosis.
Pleuropulmonary complications were defined as radiographic evidence of new or increasing pulmonary infiltrate(s).
Major embolic events defined as arterial embolic events that were diagnosed by imaging.
Results
Demographic and clinical information
From March 2007 to February 2015, 118 episodes of IE were identified in 114 individuals. The characteristics of episodes of IE are summarised in Table 1. Based on predisposing conditions, three groups of IE patients were defined: (1) IDUs 37 (32.2%); (2) healthcare-associated subgroup 36 (30.5%); and (3) elderly 16 (13.5%).
Table 1. Characteristics of episodes of infective endocarditis
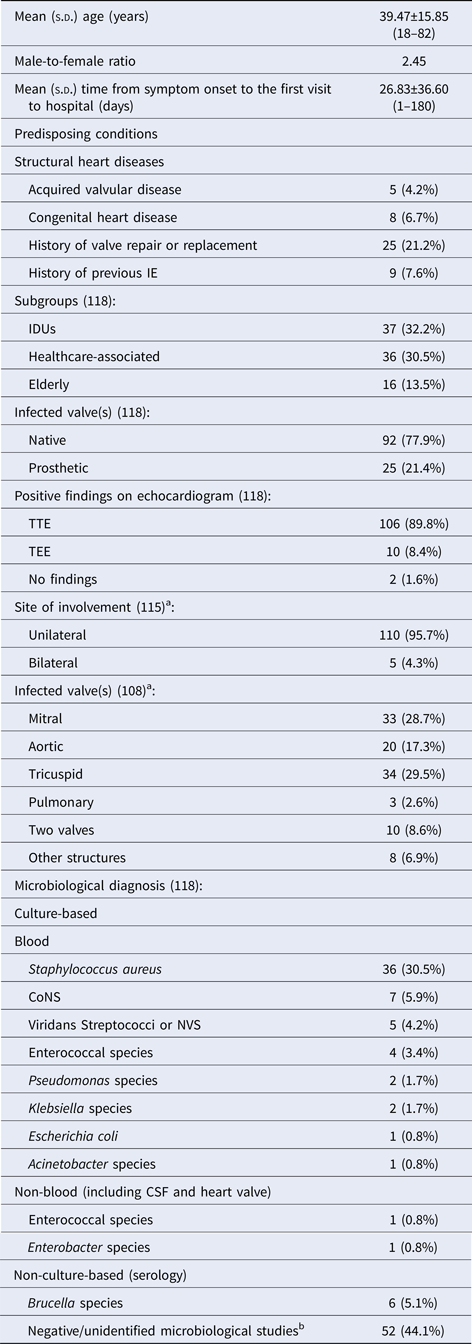
a Information could not be identified for disease-related variables for all patients; therefore, denominators sometimes varied for the variables.
b Blood culture tests were performed in 104 patients.
s.d., standard deviation; IE, infective endocarditis; IDU, intravenous drug user; TTE, trans-thoracic echocardiography; TEE, trans-oesophageal echocardiography; CoNS, coagulase-negative staphylococci; NVS, nutritionally variant streptococci; CSF, cerebrospinal fluid.
Overall 61/104 (58.7%) patients had positive blood culture results. In ten others, aetiologic diagnosis established based on culture results obtained from other sterile sites (3.7%) or serologic test results (5.5%). No pathogenic organism was identified in 37/108 (34.2%) other episodes (Table 1).
Discrepancy between the reason for admission (primary diagnosis) and discharge (final) diagnosis
The data about primary diagnosis were available for 118 episodes. Discrepancy between the reason for admission or primary diagnosis and discharge diagnosis was observed in 64 (54.2%) episodes of IE.
Cases with discrepant diagnoses were grouped into three categories: (1) a complication of endocarditis that was not considered as a complication of IE on admission (70.3%); (2) inconsistent infectious disease unrelated to the discharge diagnosis (14%); and (3) inconsistent non-infectious disease (15.6%) (Table 2).
Table 2. The frequency of primary diagnoses on admission
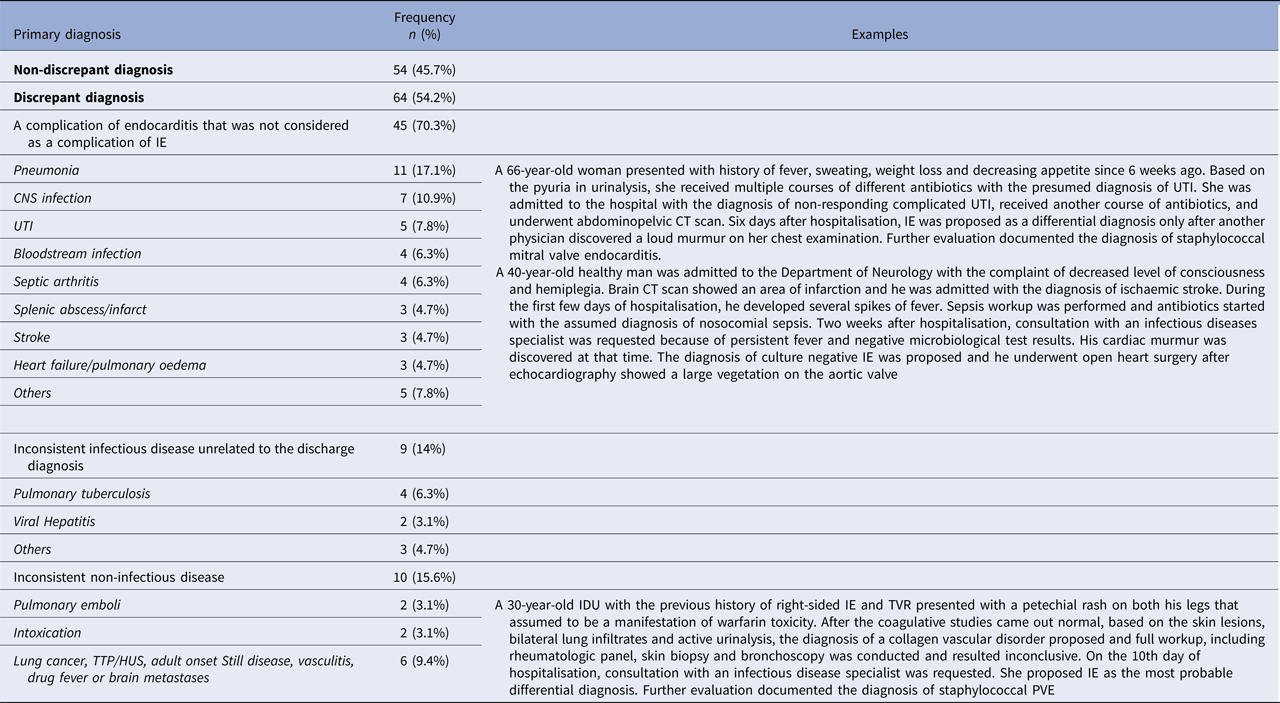
IE, infective endocarditis; CNS, central nervous system; UTI, urinary tract infection; IDU, IV drug user; PVR, prosthetic valve replacement; TTP/HUS, thrombotic thrombocytopenic purpura/haemolytic uremic syndrome.
Clinical outcome
Overall, 53.9% of patients developed pleuropulmonary complications. Major embolic events were noted in 14.4% of patients. Among those with emboli, the following were reported: pulmonary emboli (48.3%), brain emboli (10.1%), splenic infarcts (3.3%) and arterial emboli (0.8%).
Sixty-seven (56.8%) patients recovered and were discharged from hospital, 20 (16.9%) died, three (5.2%) transferred to another hospital for neurosurgical intervention, and 26 (22%) left the hospital against medical advices (AMAs) and their outcome remained unknown. By omitting the latter from the analysis, the all-cause in-hospital mortality rate was 22.2%.
Analytical results
The frequency of discrepant diagnosis between three subgroups of the study, and the association of discrepant diagnosis with gender, history of congenital heart diseases, previous history of IE, site of cardiac involvement, native vs. prosthetic valve, major septic embolic events, pleuropulmonary complications and clinical outcomes are shown in Table 3.
Table 3. Analysis of the association of demographic and disease-related variables with primary diagnosis

IV, intravenous.
Information could not be identified for all variables because of the limitations of medical record review; therefore, denominators often varied for each of the variables.
The association of discrepancy of diagnosis on admission with clinical outcome was statistically significant (OR: 2.67, 95% CI 0.87–8.16; P-value: 0.029).
Table 4 shows the results of univariate and multivariate analysis on factors significantly associated with the percentage of non-discrepant primary diagnosis (as the dependent variable) for patients with IE.
Table 4. Univariate and multivariate analysis of factors significantly associated with percentage of non-discrepant primary diagnosis (as dependent variable) for patients with infective endocarditis
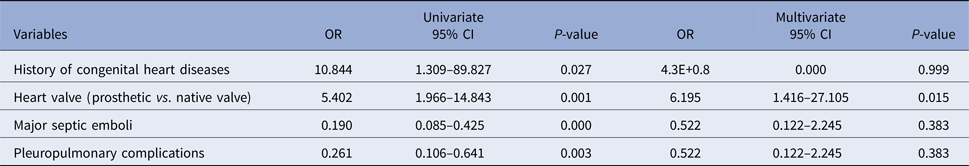
CI, confidence interval; OR, odds ratio.
Discussion
In this study, we showed that discrepancy between primary and discharge diagnosis was associated with more than two times chance of in-hospital mortality, in the patient with IE. Diagnosis discrepancy was evident in more than half (54.2%) of episodes of IE in our study. The discrepancy was more significant in the subgroup of IDUs, compared with the subgroups of the elderly and healthcare-associated IE (62.2% vs. 56.2% and 55.6%, respectively), although it was not statistically significant. The overall discrepancy rates reported herein may actually be underestimated given that our study was not an autopsy-based survey.
Over the last year, epidemiological characteristics of IE have been changing in industrialised countries as a result of advances in medical practice. Therefore, the emerging population at risk for IE consists of patients with healthcare-associated infections, elderly patients with valvular sclerosis, patients with valvular prostheses and haemodialysis patients [Reference Fedeli19, Reference Prendergast20]. The diagnosis of IE is straightforward in the minority of patients who present with a consistent history and classic oslerian manifestations. In most patients, however, the ‘textbook’ history and physical examination findings may be few or absent [Reference Baddour9]. IE is one of the diseases that is usually subject to diagnostic errors [Reference Pant12]. Gruver and Freis found that IE is amongst the four diseases, which accounted for approximately half of the 6% of diagnostic discrepancies discovered in a series of 1106 autopsies [Reference Burton and Rutty8].
Diagnostic discrepancy on admission may be a marker of diagnosis uncertainty or poor patient assessment [Reference Johnson21]. Compared with those with non-discrepant diagnosis on admission, we found that the all-cause in-hospital mortality rate was three times higher among patients with discrepant diagnosis (75% vs. 25%), although how much of that can be attributed to delayed or missed diagnosis remained unknown.
The most frequent category of discrepant diagnosis in our study was the first one (i.e. a complication of endocarditis that was not considered as a complication of IE on admission) probably due to premature closure of diagnosis in early stages. Faulty information synthesis has been shown to be the most frequent cause of cognitive-based diagnostic errors and premature closure the single-most frequent mechanism. Premature closure can occur at any stage of the diagnostic process [Reference Schwanda-Burger22].
Discrepant diagnosis on admission frequently occurred within the typical clinical settings. For example, discrepancy rates were nearly three times higher among patients who developed major embolic events or pleuropulmonary complications in our study. One of the possible reasons for delayed or missed diagnosis in these situations might be lack of paying attention to the predisposing factors and other physical findings. In our study, nearly 40% of patients had predisposing cardiac conditions for IE. Besides, nearly one-third of patients had a history of IV drug use. In another third, IE was healthcare-associated: it was most commonly associated with the central venous catheter. These predisposing conditions are expected to lead to the diagnosis of IE or considering it as a key differential diagnosis in the appropriate clinical setting. However, our study showed different results. Maybe, one of the solutions that would overcome this issue is proposing a comprehensive clinical syndrome that includes predisposing factors instead of a symptom or finding-based diagnosis to help making the differential diagnosis more accurate. In other word, in dealing with such patients with the unusual or complex presentation, there is a need for ‘problem representation’. The problem representation is an abstract one-sentence summary that elaborates the key features of the case. This representation triggers probable diagnostic hypotheses [Reference Keenan23].
It seems that physicians tend to treat symptoms without consideration of clinical syndromes and predisposing conditions. When a patient comes to a medical centre with a symptom, it is critical to rapidly differentiate between benign and life-threatening conditions. Performing a detailed and thorough history and physical examination is the first and most important component of the diagnostic evaluation of a patient. However, it is often overlooked and incompletely performed. Incomplete histories, ignored physical findings and failure to correctly interpret existing laboratory data delayed accurate diagnoses in a number of series [Reference Palazzi and Feigin24]. Although the type of diagnostic errors was not thoroughly assessed in our study, failure/delay in eliciting critical piece of history data or physical exam finding was noted in many instances, as can be seen in clinical scenarios presented in Table 2. Another point to be noted is the high rate of negative or unidentified microbiological study results compare with the rate of 21% reported previously for blood-culture negative endocarditis [Reference Fournier25]. One of the possible factors responsible for the high rate of negative results could be not considering the diagnosis of IE that may result in delay or failure in ordering appropriate diagnostic tests, at the right time.
In our study, history of prosthetic valve replacement (PVR) and previous history of IE were significantly associated with higher non-discrepant diagnosis, whereas major embolic events and pleuropulmonary complications were significantly associated with higher discrepant diagnosis on admission. However, multivariate analysis showed that only the association of history of PVR with non-discrepant primary diagnosis among patients with IE was independent of the other covariates.
There are several potential reasons for the lower discrepancy rate among patients with a history of PVR. Perhaps one reason is that the history of PVR in a patient with appropriate clinical setting leads to the intuitive clinical diagnosis. Many, and perhaps most, medical diagnoses are derived intuitively, acknowledging that most conditions are common and present in typical, easily recognised, fashion [Reference Graber26]. It could be assumed that in the face of a previously healthy patient or one with other predisposing factors, hypothetico-deductive reasoning plays the main role.
One of the potential strategies for minimising the frequency and impact of diagnostic errors includes training to improve clinicians’ cognitive skills and their awareness of common biases and disease-specific pitfalls, providing a better infrastructure for learning from diagnostic outcomes and blame-free learning from errors that are identified, and processes to minimise the harmful impacts of diagnostic errors and delays [Reference Organization27]. Although the diagnostic discrepancy between the reason for admission and discharge diagnosis is not necessarily equal to diagnostic error, it can be used as an indicator or clinical criteria for screening diagnostic errors in the lack of prospective or autopsy-based studies [Reference Shenvi and El-Kareh17].
Our study has several limitations. First, this study was a retrospective analysis of patients. Second, we did not evaluate the type of diagnostic errors. Third, the overall discrepancy rates may be underestimated given that our study was not an autopsy-based survey. Fourth, the outcome of those patients who sought discharge AMA (22% of total outcome) remained unknown. While it has been noted that between 1% and 2% of all medical admissions result in an AMA discharge [Reference Alfandre28], it was far more frequently seen in our study. In this regard, several hypotheses can be assumed, including a high proportion of IDUs, prolonged treatment duration in patients with IE, the dissatisfaction of the patients with medical services delivered by the hospital, etc. These factors need to be examined in the future studies.
Conclusion
The diagnostic discrepancy can be used as an indicator or clinical criteria for screening diagnostic errors in the lack of prospective or autopsy-based studies. The most frequent category of discrepant diagnosis in our study was related to a complication of endocarditis that was not considered as a complication of IE on admission probably due to premature closure of diagnosis in early stages. The discrepancy in the diagnosis of IE was associated with higher chance of in-hospital mortality. History of PVR was the single most important factor predicting non-discrepant diagnosis on admission. We suggest that in facing a patient who presented with a complex clinical scenario, proposing a comprehensive clinical syndrome that includes predisposing factors (e.g. multi-organ involvement syndrome in an IDU, or embolic event(s) in a patient with indwelling vascular catheter) instead of symptom or finding-based diagnosis can help making the differential diagnosis more accurate.
Acknowledgements
The authors would like to thank Dr Mona Najaf Najafi, a researcher of the Imam Reza clinical research unit at Mashhad University of Medical Sciences (Mashhad, Iran) for her valuable comments and effort to improve the manuscript.
Declaration of Interest
None.






