The Women's Health Initiative as well as the Million Women Study indicated that oestrogen or hormone replacement therapy increased the risk of postmenopausal women to develop breast cancer, stroke, thrombosis and CVD(Reference Gambacciani, Ciaponi and Genazzani1–3). These findings have led to the advice that hormone replacement therapy should not be considered first-line therapy for the prevention of osteoporosis. As existing therapeutic agents, such as bisphosphonates, selective oestrogen receptor modulators, teriparatide and calcitonin(Reference Papaioannou, Kennedy and Dolovich4), are either of high cost or associated with different side effects, alternative approaches for the prevention or treatment of osteoporosis are worth exploring.
Herba epimedii (HEP) is commonly used in traditional Chinese medicine for ‘strengthening the kidney’(Reference An, Li and Li5, Reference Wang, Xu and Jin6) and nourishes the bone. HEP is one of the most frequently prescribed herbs in traditional Chinese medicine formula for the management of osteoporosis in China. Studies have shown that HEP extract could reduce bone loss in an ovariectomised (OVX) rat model(Reference Yu, Chen and Li7–Reference Sun, Lee and Wong11) as well as promote cell proliferation and increase alkaline phosphatase activity in primary rat calvarial osteoblasts(Reference Qian, Zhang and Lu12–Reference Zhang, Cheng and Wang18). A recent study also demonstrated that it can promote the osteogenic differentiation of human bone marrow-derived mesenchymal stem cells(Reference Zhang, Li and Chan19). Our previous study indicated that HEP total extract could increase trabecular bone mineral density (BMD) in ovariectomised rats and stimulate osteoblastic cell proliferation and differentiation in UMR 106 cells. HEP total extract could also induce the expression of osteoprotegerin (OPG, a soluble, decoy receptor that binds receptor activator of NF-κB ligand (RANKL) mRNA expression) and the ratio of OPG to RANKL (a membrane-bound TNF ligand family) expression in UMR 106 cells, suggesting that it could modulate the process of osteoclastogenesis(Reference Xie, Wu and Lai20).
A recent study by Zhang et al. (Reference Zhang, Qin and Shi21) demonstrated that a preparation containing 60 mg icariin (the major flavonoid compound in HEP), 15 mg daidzein and 3 mg genistein could reduce bone loss in late postmenopausal women in a 24-month randomised, double-blind and placebo-controlled trial. This study provides evidence to support that the flavonoid compounds in HEP might account for the bone-protective effects of HEP in vivo. Furthermore, numerous in vitro (Reference Huang, Yuan and Wang22–Reference Mok, Chen and Lai24) and in vivo (Reference Nian, Ma and Nian25) studies showed that icariin could stimulate osteoblastic cell functions, suppress osteoclastic activities and improve bone mineral density and bone strength in ovariectomised rats. Our recent study further suggests that the anabolic effects of icariin on bone might be mediated through ligand-independent activation of oestrogen receptor(Reference Mok, Chen and Lai24). These studies further indicate that flavonoid compounds are the active components in HEP extract, which account for its bone-protective effects in vivo. However, the optimal dosage and the mechanism of actions by which flavonoid compounds in HEP exert bone-protective effects in vivo are not clear.
In the present study, we aim to characterise the dose-dependent effects of the total flavonoid (TF) fraction of HEP extract on bone and mineral metabolism in ovariectomised mice as well as to elucidate the mechanism of actions involved in mediating its bone-protective effects in vivo.
Materials and methods
Preparation of total flavonoids fraction from Herba epimedii total extract
HEP (E. Koreanum Nakai) was collected in June 2003–July 2003 in a valley in Xinbin, Liaoning Province, and authenticated by Q.S. Sun, Professor of Pharmacognosy, Shenyang Pharmaceutical University, China. A voucher specimen (no. 19980816) has been deposited in the Institute of Traditional Chinese Medicine and Natural Products of Jinan University, China. The total extract was subjected to water extraction and column chromatography to yield four fractions with different polarities, namely water, 30 %, 50 % and 95 % ethanol. The fractions of 50 and 95 % ethanol were pooled to give the TF fraction. The preparation was filtered and concentrated under vacuum to produce a powder at a yield of 9·5 %. The TF fraction was stored at room temperature before use. Fig. 1 shows a typical chromatographic profile of the TF fraction of the HEP total extract. HPLC analysis has been performed with standard compounds using the same elution procedure as that used with the TF extract of HEP. Peaks in the profile of TF with the same retention time with authentic markers (epimedin B, icariin, caohuoside E and baohuoside I) were identified.

Fig. 1 Reverse-phase HPLC for the qualitative analysis of the total flavonoid extract of Herbal epimedii (HEP). Icariin, epimedin B, caohuoside E and baohuoside I are the main active compounds in HEP according to the Chinese Pharmacopoeia. HPLC analysis has been performed with standard compounds using the same elution procedure as that used with the total flavonoid (TF) extract of HEP. The peaks in the profile of TF with the same retention time with authentic markers were identified and used for confirmation of the identity of the TF extract HEP.
Animals
Experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of The Hong Kong Polytechnic University. Female C57BL/6J mice were purchased from the Laboratory Animal Services Centre (the Chinese University of Hong Kong, HK).
Expt 1: dose-dependent effects of total flavonoids fraction of Herba epimedii extract in ovariectomised mice
Experimental groupings
One-month-old C57BL/6J mice were randomly separated into seven groups including Sham+vehicle (Sham, n 8), OVX+vehicle (OVX, n 8), OVX+17β-oestradiol (E2, 4 μg/g per d, n 8), OVX+TF (TF50, 50 μg/g per d, n 8), OVX+TF (TF100, 100 μg/g per d, n 8), OVX+TF (TF200, 200 μg/g per d, n 8) and OVX+TF (TF400, 400 μg/g per d, n 8). The animals were either ovariectomised or sham operated. After recovery from surgery for 18 d, they were orally administered with vehicle, E2 or TF for 6 weeks. The animals were fed with diet containing 0·6 % Ca and 0·65 % P (TD 98 005; Teklad, Madison, WI, USA) throughout the course of the studies. During the study, mice were pair-fed and body weight was recorded weekly. All the mice had free access to distilled water and were fed 3 g/d per mice of the respective diet, the minimum average food intake of the mice during the acclimatisation period. Before killing, the mice were housed individually in a metabolism cage for the collection of urine. On the day of killing, blood was collected from the orbital venous sinus of the mice. The uterus was collected, and the uterine index or uterus/body weight ratio was calculated by normalising the weight of the uterus with the final body weight of the mice. Kidneys were collected for mRNA expression detection. The bone specimens were obtained for peripheral computed tomography analysis.
Biochemical assays of serum and urine samples
Ca and inorganic P concentrations in serum and urine were determined by an o-cresolphthalein complexone method and a p-methylaminophenol reduction method, respectively, using commercial kits (Wako Pure Chemical Industries Limited, Osaka, Japan). Urinary Ca and P concentrations were normalised with creatinine concentration and were expressed as urinary Ca to creatinine ratio and urinary P to creatinine ratio, respectively.
Bone mineral density analysis by peripheral quantitative computed tomography
Peripheral quantitative computed tomography scanning was performed using XCT-2000 (StraTec Medizintechnik GmbH, Durlacher, Germany). Femurs were placed on a plastic holder and oriented at the centre of the scanning area. The long axis of diaphysis was adjusted parallel to the scanning direction. At a distance of 1·5 mm away from the apex of femur, proximal and distal ends were scanned at a voxel size of 0·3 mm2. BMD (in mg/cm3) and polar stress–strain index (polar-SSI) (in mm3) of femur were measured. SSI is an index for the estimation of torsional bone strength(Reference Lind, Lind and Larsson26).
RNA isolation from kidney and quantitative real-time RT-PCR
Whole kidney was harvested and immediately frozen in liquid N2 and stored at − 80°C. Frozen kidney was thawed in Trizol reagent and homogenised. Total RNA was isolated from kidney using Trizol according to the manufacturer's protocol. Total RNA was reverse transcribed using the High-Capacity complementary DNA Reverse Transcription Kit (Applied Biosystems, Inc., Foster City, CA, USA) at 25°C for 10 min, 37°C for 2 h and 85°C for 5 s. The sequences of the PCR primers were 5′-TCCACGATGGATCTGAATGA-3′ (vitamin D receptor (VDR) forward) and 5′-GCAGCACATGRRCTTCCTCA-3′ (VDR reverse) for VDR, 5′-ATGCCAGCAACTGAAGTCCT-3′ (CaBP-28K forward) and 5′-AGCAAAGCATCCAGCTCATT-3′ (CaBP-28K reverse) for CaBP-28K, and 5′-GACCACAGTCCATGCCATCAC-3′ (glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward) and 5′-GCTGTTGAAGTCGCAGGAGAC-3′ (GAPDH reverse) for the housekeeping gene GAPDH. PCR was carried out in 20 μl reaction mixture containing 10 μl iQ™ SYBR Green Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and 0·5 μl of complementary DNA template using an ABI 7900HT Fast Real-Time PCR system (Applied Biosystems, Inc.). The following cycle parameters were used: one cycle of 95°C for 1 min and forty cycles of 95°C for 20 s; different melting temperature for 20 s and 72°C for 18 s. Upon completion, a melting curve was examined. Standard curves were generated using serially diluted solutions of complementary DNA derived from control sample. The target gene transcripts in each sample were normalised on the basis of its GAPDH.
Expt 2: bone anabolic effect of total flavonoid at optimal dosage in ovariectomised mice
Experimental groupings
Based on the results of Expt 1, the optimised dosage of TF (100 μg/g per d) for increasing total BMD and polar SSI at distal femur as well as renal CaBP-28K and VDR mRNA expression was chosen for the second study. One-month-old C57BL/6J mice were randomly separated into four groups including Sham+vehicle (Sham, n 8), OVX+vehicle (n 8), OVX+E2 (n 8) and OVX+TF (TF100, 100 μg/g per d, n 8). After recovery from surgery for 18 d, they were orally administered with vehicle, E2 (4 μg/g per d) and TF (100 μg/g per d) for 6 weeks.
Bone microarchitecture analysis by microCT
Right femurs from mice were scanned using the microCT system (viva-CT40; Scanco Medical, Bassersdorf, Switzerland) to evaluate trabecular bone volume fraction and microarchitecture in the epiphysis region of the femur end. The scanning position of distal femur was initiated at the distal end of the femur. All scans were done in 21 μm sections to a total of 100 slices per scan. Raw data generated were reconstructed to the image files. The two-dimensional and three-dimensional evaluation can be generated by the Scanco compiler in the Virtual Memory System. Different threshold values were chosen for binarising the trabecular and cortical bones, and the volume of interest was contoured in twenty serial slices in 100 scanned slices for evaluation. The trabecular and the cortical parts of the distal femur were separated with semi-automatically drawing contours. Only trabecular bones were contoured, and the threshold value is set as 210 mg hydroxyapatite/cm3. After evaluations, five microstructural parameters were used in the data analysis such as bone volume/tissue volume, trabecular number, trabecular thickness, trabecular separation and structural model index.
RNA isolation from bone and quantitative real-time RT-PCR
Whole left femur was harvested and immediately frozen in liquid N2 and stored at − 80°C. Frozen femur was put in an RNase-free mortar and pestle which contained liquid N2, and then grinded into a fine pieces immersed in liquid N2. Then, the frozen powders were transferred into a tube containing Trizol. Total RNA was extracted, and the gene expression of OPG, RANKL, type I collagen, osteocalcin, IL-6 and GAPDH was assessed as described in Expt 1. The sequences of the PCR primers were 5′-TGAGTGTGAGGAAGGGCGTTA-3′ (OPG forward) and 5′-CCATCTGGACATTTTTTGCAAA-3′ (OPG reverse), 5′-GCACACCTCACCATCAATGCT-3′ (RANKL forward) and 5′-GGTACCAAGAGGACAGAGTGACTTTA-3′ (RANKL reverse), 5′-CTTGGTGGTTTTGTATTCGATGAC-3′ (type I collagen forward) and 5′-GCGAAGGCAACAGTCGCT-3′ (type I collagen reverse), 5′-CTCACAGATGCCAAGCCCA-3′ (osteocalcin forward) and 5′-CCAAGGTAGCGCCGGAGTCT-3′ (osteocalcin reverse), 5′-TGGGAAATCGTGGAAATGAGA-3′ (IL-6 forward) and 5′-CAAGTGCATCATCGTTGTTCATAC-3′ (IL-6 reverse), and 5′-GACCACAGTCCATGCCATCAC-3′ (GAPDH forward) and 5′-GCTGTTGAAGTCGCAGGAGAC-3′ (GAPDH reverse), respectively.
Statistical analysis
The data were analysed by one-way ANOVA, and followed by Tukey's multiple comparison test as a post test to compare the group means if overall P < 0·05. GraphPad Prism (El Camino Real, CA, USA) version 4.4 software was used. The results were reported as means with their standard errors. A P-value < 0·05 was considered statistically significant.
Results
Dose-dependent effects of total flavonoid on body weight, uterine index and biochemical parameters in ovariectomised mice
Body weight increased significantly in the OVX group and 50 μg/g TF group. Oestradiol, but not TF, prevented the OVX-induced increase in weight gain in mice (Table 1). The uterine index was significantly reduced in OVX mice, suggesting that the surgery was successful. In contrast to E2, TF did not increase the uterus index in OVX mice (Table 1). Serum Ca levels were not significantly altered by ovariectomy or treatment with E2, or 50 and 100 μg/g TF; while 200 and 400 μg/g TF decreased serum Ca levels in OVX mice (v. OVX, P < 0·05). Urinary Ca excretion was suppressed in OVX mice treated with E2 and TF at 50 μg/g (v. OVX, P < 0·05 and 0·01, respectively). The suppression of urinary Ca excretion by TF in OVX mice was found to be dose dependent. There were no statistically significant differences in serum P level and urinary P excretion between each group.
Table 1 Effects of 17β-oestradiol (E2) and total flavonoids (TF) on body weight, uterine index and biochemical parameters in ovariectomised mice*
(Mean values with their standard errors, n 8 animals)
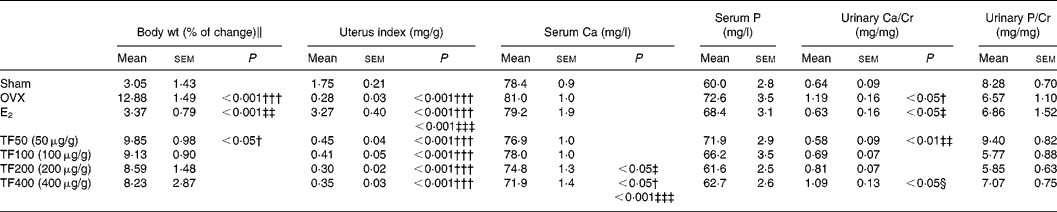
OVX, ovariectomy; Urinary Ca/Cr, urinary Ca to creatinine ratio; urinary P/Cr, urinary P to creatinine ratio.
Mean values were significantly different when compared with that of the Sham group: †P < 0·05, †††P < 0·001.
Mean values were significantly different when compared with that of the OVX group: ‡P < 0·05, ‡‡P < 0·01, ‡‡‡P < 0·001.
Mean values were significantly different when compared with that of TF50 (50 μg/g): §P < 0·05.
* The data were analysed by one-way ANOVA and followed by Tukey's multiple comparison test.
∥ Body weight (% of change) from baseline to 6 weeks.
Dose-dependent effects of total flavonoid on bone mineral density and bone strength in femur of ovariectomised mice
The effects of OVX, E2 and TF on bone mass and SSI at distal femur are presented in Table 2. Ovariectomy decreased total BMD ( − 11 %), trabecular BMD ( − 13 %) and polar SSI ( − 53 %) at distal femur in mice. Treatment of OVX mice with E2 significantly increased total BMD, trabecular BMD as well as polar SSI at distal femur by 40 % (P < 0·001), 56 % (P < 0·001) and 228 % (P < 0·001), respectively, when compared with OVX mice. TF increased total BMD, and the most effective dosages were 50 and 100 μg/g (v. OVX), but the increase did not reach statistical significance. TF also increased the trabecular BMD with the most effective dosage at 50 μg/g (v. OVX, P < 0·05). Treatment of OVX mice with TF (50 and 100 μg/g) dramatically increased polar SSI at the distal femur by 119 % (P < 0·001) and 131 % (P < 0·001), respectively.
Table 2 Effects of 6-week treatment of 17β-oestradiol (E2) and total flavonoids (TF) on bone mineral density and bone strength at distal femur in ovariectomised mice analysed by peripheral quantitative computed tomography*
(Mean values with their standard errors, n 6–8 animals)
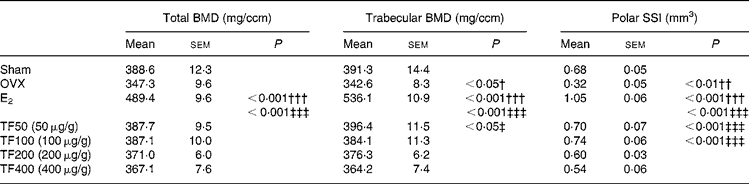
BMD, bone mineral density; SSI, stress–strain index; OVX, ovariectomy.
Mean values were significantly different when compared with that of the Sham group: †P < 0·05, ††P < 0·01, †††P < 0·001.
Mean values were significantly different when compared with that of the OVX group: ‡P < 0·05, ‡‡‡P < 0·001.
* The data were analysed by one-way ANOVA and followed by Tukey's multiple comparison test.
Dose-dependent effects of total flavonoid on vitamin D receptor and calcium transport protein mRNA expression in kidney
1,25-Dihydroxyvitamin D3 regulates Ca absorption through acting on the epithelial Ca-transporting proteins primarily via a genomic action after binding with its receptor (VDR). CaBP-28K is the major vitamin D-dependent CaBP-28K expressed in the kidney(Reference Lambers, Mahieu and Oancea27). To determine whether the suppression of urinary Ca excretion by TF was associated with the changes in renal expression of major proteins involved in renal Ca transport, renal expression of CaBP-28K and VDR mRNA was determined. As shown in Fig. 2, ovariectomy did not alter renal expression of CaBP-28K and VDR mRNA in mice. E2 significantly increased renal VDR mRNA, but not CaBP-28K expression, in OVX mice (v. OVX, P < 0·05). TF increased CaBP-28K and VDR mRNA expression in OVX mice, and the maximal effect was at 100 and 50 μg/g, respectively.

Fig. 2 Effects of 17β-oestradiol (E2) and total flavonoid (TF) on Ca transport protein (CaBP-28K) and vitamin D receptor (VDR) mRNA expressions in kidney. Ovariectomy (OVX) mice were treated with vehicle (Sham or OVX), E2 (4 μg/g per d) or four doses of TF (TF50, 50 μg/g per d; TF100, 100 μg/g per d; TF200, 200 μg/g per d and TF400, 400 μg/g per d) for 6 weeks. At killing, kidney was collected and total RNA was isolated. Real-time RT-PCR was performed to determine the mRNA expressions of (a) CaBP-28K and (b) VDR, which were normalised with that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The data were obtained at 6 weeks and were expressed as a percentage of the control mice treated with vehicle. Results were expressed as means with their standard errors. Mean values were significantly different when compared with that of the sham group: *P < 0·05, **P < 0·01, ***P < 0·001. Mean values were significantly different when compared with that of the OVX group (n 5–6): †P < 0·05, ††P < 0·01.
Effects of total flavonoid on bone microarchitecture at distal femur in ovariectomised mice analysed by microCT
The present results showed that the maximal bone-protective effects on total BMD, trabecular BMD as well as polar SSI were between 50 and 100 μg/g of TF of HEP, and the optimal stimulating effect on renal CaBP-28K and VDR mRNA expression was also between 50 and 100 μg/g of TF. Based on these results, 100 μg/g of TF was chosen for subsequent experiments. The bone-protective effects of E2 and TF on bone microarchitecture at distal femur of OVX mice were clearly illustrated by their three-dimensional images obtained from microCT analysis as shown in Fig. 3(a). Ovariectomy significantly decreased bone volume/tissue volume, trabecular number, and trabecular thickness, and increased trabecular separation and structural model index when compared to the sham group. Treatment with E2 and TF significantly prevented the reduction in bone volume/tissue volume, trabecular number, and trabecular thickness as well as the increase in trabecular separation and structural model index in OVX mice (Fig. 3(b)–(f)).

Fig. 3 Effects of 17β-oestradiol (E2) and total flavonoid (TF) on bone microarchitecture at distal femur in ovariectomised mice analysed by microCT. Ovariectomy (OVX) mice were treated with vehicle (Sham or OVX), E2 (4 μg/g per d) or TF (100 μg/g per d) for 6 weeks. (a) Representative 3D microCT images of distal femur. Graphical measurement of bone volume/tissue volume (BV/TV) (b), trabecular number (Tb.N) (c), trabecular thickness (Tb.Th) (d), separation (Tb.Sp) (e) and structural model index (SMI) (f) as determined from the microCT. Results were expressed as means with their standard errors. Mean values were significantly different when compared with that of the Sham group: *P < 0·05, **P < 0·01, ***P < 0·001. Mean values were significantly different when compared with that of the OVX group (n 8): †P < 0·05, †††P < 0·001.
Effects of total flavonoid on type I collagen, osteocalcin, osteoprotegerin, receptor activator of NF-κB ligand and IL-6 gene expressions in femur
OVX appeared to decrease the expression of type I collagen (Fig. 4(a)) and osteocalcin (Fig. 4(b)) mRNA in the femur of mice, but the decrease was NS. E2 and TF significantly increased type I collagen and osteocalcin mRNA expressions in the femur of OVX mice (Fig. 4(a) and (b)). Treatment of OVX mice with E2 significantly decreased RANKL mRNA expression (Fig. 4(d)), but did not significantly affect OPG mRNA expression (Fig. 4(c)) in femur. Both E2 and TF significantly increased the ratio of OPG/RANKL in the femur of OVX mice (Fig. 4(e)), suggesting that they might modulate the process of osteoclastogenesis via their actions on OPG and RANKL expression in bone cells. OVX significantly increased the gene expression of IL-6 (P < 0·01). TF, but not E2, treatment significantly decreased the IL-6 gene expression in the femur of OVX mice (P < 0·05).

Fig. 4 Effects of 17β-oestradiol (E2) and total flavonoid (TF) on type I collagen, osteocalcin, osteoprotegerin (OPG), receptor activator of NF-κB ligand (RANKL) and IL-6 mRNA expressions in femur. Ovariectomy (OVX) mice were treated with vehicle (Sham or OVX), E2 (4 μg/g per d) or TF (100 μg/g per d) for 6 weeks. At killing, femurs were collected and total RNA was isolated. Real-time RT-PCR was performed to determine the mRNA expressions of type I collagen (a), osteocalcin (b), OPG (c), RANKL (d), OPG/RANKL (e) and IL-6 (f), which were normalised with that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The data were obtained at 6 weeks and were expressed as a percentage of the control mice treated with vehicle. Results were expressed as means with their standard errors. Mean values were significantly different when compared with that of the Sham group: **P < 0·01. Mean values were significantly different when compared with that of the OVX group (n 5–8): †P < 0·05, ††P < 0·01, †††P < 0·001.
Discussion
The present study clearly demonstrated that the TF of HEP could suppress OVX-induced increase in urinary Ca excretion as well as loss in bone mass and bone strength in mice in a dose-dependent manner. In addition, renal expressions of CaBP-28K and VDR mRNA were dose dependently induced by treatment with TF in OVX mice. Furthermore, TF could improve trabecular microarchitecture in OVX mice, and significantly increased the mRNA expression of type I collagen, osteocalcin and OPG/RANKL ratio and suppressed IL-6 mRNA expression in the femur of OVX mice.
The present study demonstrated that the effects of TF on bone mass in OVX mice were dose dependent. It increased total BMD and trabecular BMD of the distal femur in OVX mice with the most effective dosages between 50 and 100 μg/g. Moreover, the present study demonstrated that the effects of TF on torsional bone strength (polar SSI) at distal femur in OVX mice were also dose dependent, and the most effective doses were also between 50 and 100 μg/g. It was of interest to note that higher concentration of TF (200 and 400 μg/g) did not result in further increase in bone mass and torsional bone strength at the distal femur in OVX mice.
Oestrogen plays an important role in Ca2+ homoeostasis, and oestrogen deficiency results in a negative Ca2+ balance and bone loss in postmenopausal women(Reference Hoenderop, Nilius and Bindels28, Reference Young and Nordin29). Ovariectomy induced a significant increase in urinary Ca level, and this effect could be reversed by TF. The present results indicated that the suppression of urinary Ca excretion by TF was negatively associated with the increase in renal expressions of CaBP-28K and VDR mRNA in OVX mice. The most effective dosages for their expressions in kidney were 100 and 50 μg/g, respectively. As CaBP-28K is a vitamin D-dependent CaBP-28K in the kidney, it is possible that the decrease in urinary Ca loss by TF in OVX mice might be in part mediated through an increase in renal Ca transport via the induction of CaBP-28K expression. In addition, the induction of VDR expression in kidney by TF in OVX mice might increase renal responsiveness to vitamin D, thereby increasing vitamin D-dependent expression of CaBP-28K in kidneys. Further study will be needed to confirm whether TF will increase renal Ca transport in OVX mice. Nonetheless, the present study indicated that TF not only exerts protective effects in bone tissues but also exerts additional effects on the mRNA expression of CaBP-28K in kidney, which lead to the conservation of bone mass in OVX mice.
Ovariectomy produced apparent deterioration of trabecular three-dimensional microarchitecture in mice. The present study showed that TF treatment prevented OVX-induced deterioration of microstructural parameters at the distal femur in mice. Specifically, TF at 100 μg/g per d could restore the loss of bone volume/tissue volume, trabecular number and trabecular thickness while suppressing the increase in trabecular separation and structural modulus index at distal femur in OVX mice. These results indicated that TF was effective not only in preserving bone mass but also in preventing the deterioration of bone microarchitecture associated with oestrogen deficiency in mice.
The study of the changes in femur gene expression in response to TF provides insights to understand its mechanism of actions involved in improvement of bone quality in OVX mice. Type I collagen and osteocalcin are the most abundant extracellular proteins produced by osteoblasts in bone and are essential for maintaining bone strength(Reference Mahonen, Jukkola and Risteli30, Reference Power, Iwaniec and Wronski31). TF could significantly increase type I collagen and osteocalcin mRNA expression in femur of OVX mice to a level comparable with those treated with oestradiol. These in vivo results are in agreement with previous reports by us(Reference Xie, Wu and Lai20) and others(Reference Han, Liu and Xu15–Reference Zhang, Li and Chan19) that HEP could enhance bone formation through its action on cells in the osteoblastic lineage. The equilibrium of OPG and RANKL expression plays an important role in controlling bone remodelling(Reference Bord, Ireland and Beavan32). The secretion of OPG by osteoblastic cells could block the interaction of RANKL with its functional receptor RANK expressed on the osteoclastic cell surface, thereby inhibiting osteoclastogenesis. The present results revealed that both oestradiol and TF increase the ratio of OPG/RANKL mRNA expression in the femur of OVX mice, suggesting that both agents can inhibit the process of osteoclastogenesis in vivo. However, the mechanism by which TF modulates osteoclastogenesis might be different from that of oestradiol. The results indicated that the increase in the OPG/RANKL ratio by TF was due to its inductive effects on OPG mRNA expression, while the increase by oestradiol was due to its suppressive effect on RANKL mRNA expression in vivo.
IL-6 is known to be a potent stimulator of bone resorption, and plays an important role in the induction of osteoclastogenesis and bone loss upon oestrogen depletion(Reference Cuzzocrea, Mazzon and Dugo33, Reference Jilka, Hangoc and Girasole34). The present results indicated that IL-6 mRNA in the femur of mice appeared to be significantly increased with ovariectomy and decreased with TF treatment in OVX mice. These results were in agreement with those reported previously by Wang et al. (Reference Wang, Quan and Guo8) in which HEP increased IL-6 mRNA in bone of OVX rats. Thus, TF might suppress the process of osteoclastogenesis not only via the induction of the OPG/RANKL ratio but also via the inhibition of the OVX-induced increase in levels of IL-6 in bone.
In search for safe and effective alternatives for treatment of postmenopausal osteoporosis, HEP has received much attention in recent years to demonstrate its efficacy as well as mechanism of actions(Reference Yu, Chen and Li7–Reference Zhang, Li and Chan19, Reference Huang, Yuan and Wang22–Reference Mok, Chen and Lai24). In the present study, we have demonstrated that the optimal dosage of TF for improving bone mass and bone strength as well as for decreasing urinary Ca excretion in OVX mice was between 50 and 100 μg/g per d. The present results showed that a further increase in the dosages of TF would compromise its positive effects on bone mass as well as its suppressive effects on urinary Ca excretion. The optimal dosages reported in the present study were in agreement with the dosages used in other reported studies using OVX rats as a model(Reference Qian, Zhang and Lu12–Reference Songlin, Ge and Yixin14). For example, the study by Songlin et al. (Reference Songlin, Ge and Yixin14) showed that treatment of 11-month-old OVX rats with a flavonoid fraction of HEP at 10 mg/kg per d for 12 weeks increased BMD as well as improved bone microarchitecture. The dosage used in their studies is close to the optimal dosages reported in the present study, in which the equivalent dose in rats will be 25–50 mg/kg per d or μg/g per d. Furthermore, the present study was the first to report that TF also exerts additional effects on the mRNA expression of CaBP-28K in kidney, which potentially led to the suppression of urinary Ca excretion and the conservation of bone mass in OVX mice.
The present study clearly demonstrated that the TF of HEP could protect against bone loss and bone deterioration associated with oestrogen deficiency without exerting uterotrophic effects. Gene expression studies indicated that TF treatment achieves its osteoprotective actions in vivo via the modulation of renal Ca transport, osteoblastic functions, the process of osteoclastogenesis as well as osteoclastic functions in OVX mice. The present study showed that TF is the active fraction in HEP and defines the optimal dosages of HEP for achieving bone-protective actions in vivo.
Acknowledgements
The present work was supported by the Areas of Excellence Scheme established under the University Grants Committee of the Hong Kong Special Administrative Region (HKSAR), People's Republic of China (AOE/P-10/01), the Hong Kong Polytechnic University Central Research Fund (GYE-47), the Earmarked Research Grant Council (PolyU 5402/04M, N_PolyU536/04, PolyU 563706), HKSAR and the National Natural Science Foundation of China (30971004). We thank the support of the State Key Laboratory of Chinese Medicine and Molecular Pharmacology, HKSAR. W.-F. C. and S.-K. M. performed the majority of the laboratory work and contributed to the analysis of data and the writing of the manuscript, and contributed equally to the studies. X.-L. W. performed the preparation of the TF fraction from HEP total extract. K.-H. L. and W.-P. L. conducted some of the laboratory work. H.-K. L. performed bone microarchitecture analysis by using microCT. P.-C. L. played a significant role in the design of the study. X.-S. Y. was a co-investigator and grant holder, and played a significant role in the design and performance of the study. M.-S. W. was the principal investigator and grant holder, and played a major role in the design and performance of the study, interpretation of the results and the writing of the paper. The authors have no conflicts of interest to declare.








