Published online by Cambridge University Press: 22 November 2024
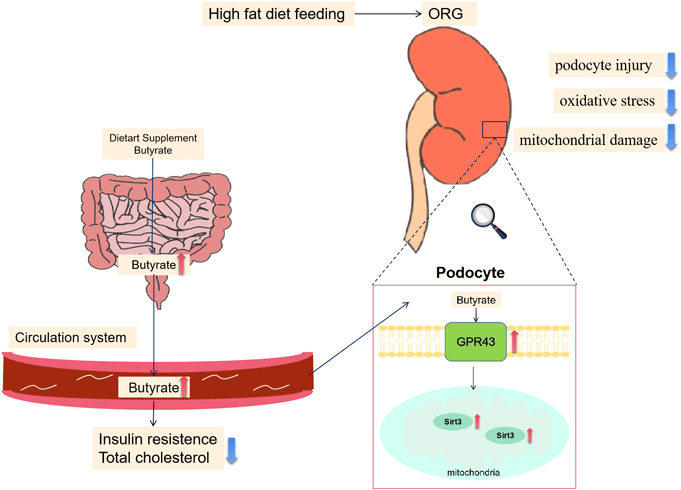
The incidence of obesity-related glomerulopathy (ORG) is rising worldwide with very limited treatment methods. Paralleled with the gut–kidney axis theory, the beneficial effects of butyrate, one of the short-chain fatty acids (SCFA) produced by gut microbiota, on metabolism and certain kidney diseases have gained growing attention. However, the effects of butyrate on ORG and its underlying mechanism are largely unexplored. In this study, a mice model of ORG was established with a high-fat diet feeding for 16 weeks, and sodium butyrate treatment was initiated at the 8th week. Podocyte injury, oxidative stress and mitochondria function were evaluated in mice kidney and validated in vitro in palmitic acid-treated-mouse podocyte cell lines. Further, the molecular mechanisms of butyrate on podocytes were explored. Compared with controls, sodium butyrate treatment alleviated kidney injuries and renal oxidative stress in high-fat diet-fed mice. In mouse podocyte cell lines, butyrate ameliorated palmitic acid-induced podocyte damage and helped maintain the structure and function of the mitochondria. Moreover, the effects of butyrate on podocytes were mediated via the GPR43-Sirt3 signal pathway, as evidenced by the diminished effects of butyrate with the intervention of GPR43 or Sirt3 inhibitors. In summary, we conclude that butyrate has therapeutic potential for the treatment of ORG. It attenuates high-fat diet-induced ORG and podocyte injuries through the activation of the GPR43-Sirt3 signalling pathway.