Accumulating lines of experimental evidence consistently have indicated that extracts, derivatives and ingredients of Phaseolus vulgaris (Fabaceae) are effective in reducing appetite, body weight, lipid accumulation, hedonic properties of food, carbohydrate absorption and metabolism, and glycaemia in different species of laboratory animals(Reference Obiro, Zhang and Jiang1–Reference Carai, Fantini and Loi3). With regard to appetite, food intake and body weight, acute and repeated administration of P. vulgaris extracts has been reported to reduce intake of food, body weight and feed efficiency (i.e. an index of the quantity of energy converted from food into body weight) in rats and mice(Reference Tormo, Gil-Exojo and Romero de Tejada4–Reference Maccioni, Colombo and Riva7). Furthermore, addition of P. vulgaris extracts or ingredients to chow results in the reduction of food intake and body weight in rats(Reference Carai, Fantini and Loi3, Reference Donatucci, Liener and Gross8–Reference Pusztai, Grant and Buchan12). Moreover, acute and repeated administration of P. vulgaris extracts reduces postprandial glycaemia in several experimental settings(Reference Carai, Fantini and Loi3–Reference Fantini, Cabras and Lobina6, Reference Donatucci, Liener and Gross8, Reference Bardocz, Grant and Pusztai11, Reference Pusztai, Grant and Buchan12).
This laboratory has recently investigated different aspects of the pharmacological profile of a P. vulgaris dry extract (named Beanblock®; Indena SpA, Milan, Italy) prepared as to exert the dual action attributed to P. vulgaris preparations: inhibition of α-amylase and phytohaemagglutinin-induced anorectic effects(Reference Obiro, Zhang and Jiang1–Reference Carai, Fantini and Loi3). Specifically, intragastric administration of this P. vulgaris dry extract dose dependently reduces intake of food (including highly palatable foods and fluids), body weight and glycaemia in unselected, non-obese Wistar rats(Reference Carai, Fantini and Loi3, Reference Fantini, Cabras and Lobina6, Reference Maccioni, Colombo and Riva7) and CD1 mice (MAM Carai, unpublished results). When mixed into the chow, it produces an initial suppression of daily food intake and a longer-lasting reduction in body weight in Wistar rats(Reference Carai, Fantini and Loi3). Additionally, treatment with this P. vulgaris dry extract results in a marked reduction in the reinforcing and motivational properties of a highly palatable chocolate-flavoured beverage in Wistar rats(Reference Maccioni, Colombo and Riva7).
In the wake of the above results, the present study was designed to accomplish two aims: (1) to extend the previous results on the anorectic and hypoglycaemic effects of this P. vulgaris dry extract to genetically obese Zucker fa/fa rats (a valid animal model of overeating, obesity and related disorders(Reference Zucker and Zucker13–Reference Beck16)); (2) to investigate the effect of multiple cycles of repeated treatments with P. vulgaris dry extract on food intake and body weight. To our knowledge, none of the previous studies investigating the anorectic effects of P. vulgaris extracts has ever used a multiple cycle schedule. The experimental design adopted in the present study allowed us to assess whether and how potency and efficacy of P. vulgaris extract changed over time, as well as whether tolerance developed to its anorectic effect. This design appears to bear some relevant face validity for those obese patients who alternate periods of drug treatment with others during which drug treatment is discontinued.
Experimental methods
The experimental procedures employed in the present study were in accordance with the European Communities Council Directive (86/609/EEC) and the subsequent Italian Law on the Protection of animals used for experimental and other scientific reasons.
Animals
Adult male Zucker fa/fa rats (Charles River Laboratories, Calco, Italy), weighing approximately 525 g at the start of the study, were used. Rats were individually housed in standard plastic cages with wood chip bedding. The animal facility was under an inverted 12 h light–12 h dark cycle (lights on at 23.00 hours), at a constant temperature of 22 ± 2°C and relative humidity of approximately 60 %. Standard rat chow (60 % (w/w) carbohydrate (46 % starch), 4 % fibres, 16 % protein and 3 % fat (Safe, Augy, France)) and tap water were available ad libitum in the homecage, except as noted below (see Expt 2). Rats were extensively habituated to handling and intragastric infusion.
Extract preparation
The procedure applied in the preparation of P. vulgaris dry extract has been described in detail elsewhere(Reference Fantini, Cabras and Lobina6). Briefly, P. vulgaris dry extract was prepared by means of aqueous extraction and alcoholic precipitation from the common kidney bean (P. vulgaris). Bean extract was obtained by extraction with citrate buffer and precipitation with ethanol. The extract obtained was characterised by a standardised composition: (1) 8·5 % (w/w) α-amylase inhibitor, with an inhibiting activity of 1400 U/mg, calculated according to the United States Pharmacopoeia by the Marshall and Landa test (see Fantini et al.(Reference Fantini, Cabras and Lobina6) for details); (2) phytohaemagglutinin (haemagglutinating activity equal to 16 haemagglutinating units/mg).
Experimental procedures
Expt 1: effect of three 5 d treatments with Phaseolus vulgaris dry extract on food intake and body weight
Rats were divided into three groups of six to seven rats each, matched for body weight and food intake over the 3 d preceding the start of the first treatment with P. vulgaris dry extract. Rats underwent three different treatments with P. vulgaris dry extract, each lasting five consecutive days (treatments 1–3). Each treatment was followed by a post-treatment phase of twenty consecutive days with no drug administration (off-treatments 1–3). On each treatment day, rats were treated with 0, 50 and 500 mg/kg P. vulgaris dry extract. Administration of P. vulgaris dry extract occurred 60 min before lights off. P. vulgaris dry extract was suspended in distilled water +0·5 % methylcellulose and administered orally at an infusion volume of 2 ml/kg. Doses of P. vulgaris dry extract were chosen on the basis of the results of previous experiments(Reference Carai, Fantini and Loi3, Reference Fantini, Cabras and Lobina6, Reference Maccioni, Colombo and Riva7).
On each day of the treatment and off-treatment phases, daily food and water intake was recorded once a day (approximately 60 min before lights off) by weighing food pellets and water bottles with a 0·1 g accuracy. Rat body weight was recorded once a day (approximately 60 min before lights off) with a 0·5 g accuracy.
Expt 2: effect of acute treatment with Phaseolus vulgaris dry extract on postprandial glycaemia
Over the 4 weeks preceding the experiment with P. vulgaris dry extract, rats were exposed to weekly sessions of restricted feeding. Specifically, rats were fasted for 23 h and then exposed to a restricted amount of food for 1 h/d (the first hour of the dark phase of the light–dark cycle). Water was available 24 h/d. This regimen was introduced to accustom the rats to the testing procedure. In these sessions, starch-enriched food pellets (63 % (w/w) carbohydrate (100 % starch), 4 % fibres, 20 % protein and 5 % fat (Rieper, Vandoies, Italy)) were used. The experiment with P. vulgaris dry extract was performed once stable amounts of food were consumed in each session. On the test day, rats were divided into three groups of five rats each and treated with 0, 50 and 500 mg/kg P. vulgaris dry extract, suspended as described previously. P. vulgaris dry extract was administered orally by 60 min before food presentation at the infusion volume of 2 ml/kg. At lights off, rats were given a restricted amount of food (9 g/kg); this amount, chosen on the basis of previous observations, corresponded to the quantity consumed fully when Zucker rats were treated with 500 mg/kg P. vulgaris dry extract under this specific experimental procedure. Glycaemia was determined 0, 60, 120 and 360 min after the start of the 60 min feeding session. Blood samples (0·05 ml) were collected from the tip of the tail of each rat and analysed enzymatically by means of GL5 Analox (Analox Limited, Hammersmith, London, UK).
Statistical analysis
Expt 1
Data on (1) daily food intake (expressed as g/kg), (2) daily water intake (ml/kg) and (3) daily changes in body weight (percentage of change in comparison with baseline) from each treatment and off-treatment phase were analysed by separate two-way (treatment, time) ANOVA with repeated measures on the factor time.
Expt 2
Data on the effect of P. vulgaris dry extract on glycaemia over time were analysed by a two-way (treatment, time) ANOVA with repeated measures on the factor time. Data on the effect of P. vulgaris dry extract on the area under the curve of the time course of glycaemia were analysed by a one-way ANOVA.
Results
Expt 1: effect of three 5 d treatments with Phaseolus vulgaris dry extract on food intake and body weight
ANOVA revealed a significant effect of treatment with P. vulgaris dry extract on daily food intake during treatment 1 (F treatment(2,16) = 8·41, P < 0·005; F time(4,64) = 1·93, P>0·05; F interaction(8,64) = 1·75, P>0·05). Specifically, treatment with P. vulgaris dry extract resulted in a dose-dependent reduction in daily food intake (Fig. 1(a)). In comparison with vehicle-treated rats, reduction in food intake during treatment 1 averaged approximately 20 and 35 % in rat groups treated with 50 and 500 mg P. vulgaris dry extract per kg, respectively.
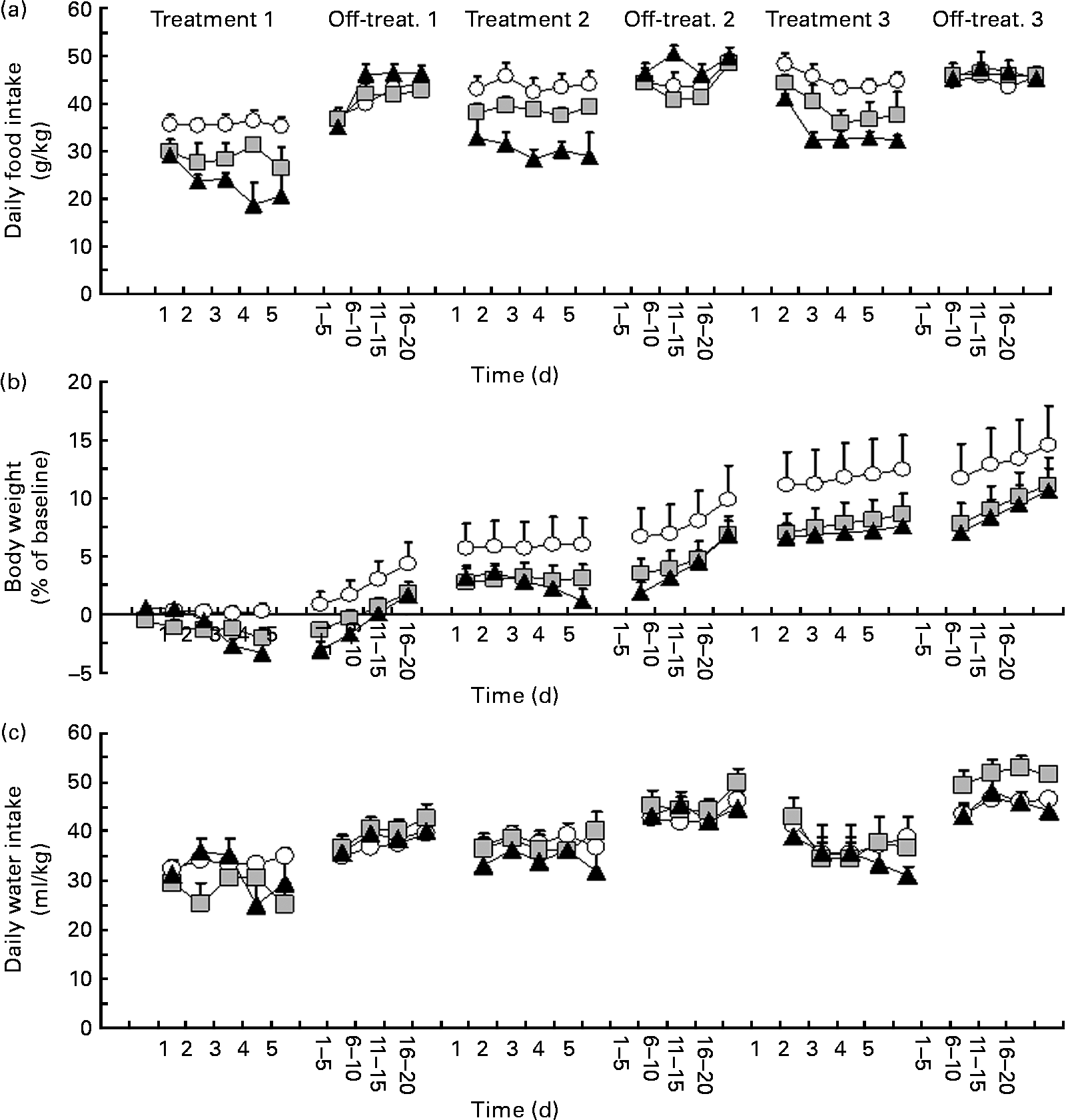
Fig. 1 Reducing effect of a Phaseolus vulgaris dry extract on (a) daily food intake (g/kg), (b) changes in body weight (% of baseline values) and (c) water intake (ml/kg) in obese Zucker fa/fa rats. Rats were given unlimited access to a standard rat chow and water throughout the study. P. vulgaris dry extract was administered intragastrically, once a day, in three different 5 d periods (treatments 1–3) followed by three 20 d no-treatment periods (off-treatments 1–3). Each point of the treatment periods is means, with their standard errors represented by vertical bars (n 6–7). Data from the off-treatment periods are collapsed in groups of 5 d. ![]() , 0 mg/kg P. vulgaris;
, 0 mg/kg P. vulgaris; ![]() , 50 mg/kg P. vulgaris;
, 50 mg/kg P. vulgaris; ![]() , 500 mg/kg P. vulgaris. Off-treat., off-treatment.
, 500 mg/kg P. vulgaris. Off-treat., off-treatment.
ANOVA revealed a significant interaction between treatment and time on daily food intake during off-treatment 1 (F treatment(2,16) = 0·74, P>0·05; F time(19,304) = 21·14, P < 0·0001; F interaction(38,304) = 2·86, P < 0·0001). Specifically, on several days of this first off-treatment phase, daily food intake was higher in 500 mg/kg P. vulgaris dry extract-treated rats than in vehicle-treated rats; at the end of this off-treatment phase, daily food intake was virtually identical throughout the three rat groups (Fig. 1(a)).
Rats were then exposed to treatment 2. ANOVA revealed a significant effect of treatment with P. vulgaris dry extract on daily food intake also during this treatment phase (F treatment(2,16) = 10·33, P < 0·005; F time(4,64) = 0·79, P>0·05; F interaction(8,64) = 0·45, P>0·05). As in treatment 1, this second treatment with P. vulgaris dry extract resulted in a dose-dependent reduction in daily food intake (Fig. 1(a)). In comparison with vehicle-treated rats, reduction in food intake during Treatment 2 averaged approximately 10 and 30 % in rat groups treated with 50 and 500 mg P. vulgaris dry extract per kg, respectively.
ANOVA revealed a significant interaction between treatment and time on daily food intake during off-treatment 2 (F treatment(2,16) = 1·68, P>0·05; F time(19,304) = 10·52, P < 0·0001; F interaction(38,304) = 2·51, P < 0·0001). Specifically, several days of overeating were observed in the rat group treated with 500 mg P. vulgaris dry extract per kg; at the end of this off-treatment phase, daily food intake was again virtually identical in the three rat groups (Fig. 1(a)).
Finally, rats underwent treatment 3. ANOVA revealed a significant effect of treatment with P. vulgaris dry extract on daily food intake also during this last treatment phase (F treatment(2,16) = 13·92, P < 0·0005; F time(4,64) = 6·22, P < 0·0005; F interaction(8,64) = 0·45, P>0·05). As observed in the two previous treatment phases, this third treatment with P. vulgaris dry extract resulted in a dose-dependent reduction in daily food intake (Fig. 1(a)). In comparison with vehicle-treated rats, reduction in food intake during Treatment 3 averaged approximately 10 and 30 % in rat groups treated with 50 and 500 mg P. vulgaris dry extract per kg, respectively.
ANOVA failed to reveal significant effects of treatment and interaction between treatment and time on daily food intake during off-treatment 3 (F treatment(2,16) = 0·32, P>0·05; F time(19,304) = 5·12, P < 0·0001; F interaction(38,304) = 0·90, P>0·05). At variance with the two previous off-treatment phases, overeating in the rat group treated with 500 mg P. vulgaris dry extract per kg was sparse and of relatively modest magnitude (Fig. 1(a)).
Data on daily water intake in each experimental phase are depicted in Fig. 1(c). Data from ANOVA are given in Table 1.
Table 1 Results of two-way (treatment, time) ANOVA for daily water intake in obese Zucker fa/fa rats*
(Degrees of freedom and F ratios)

* Rats were given unlimited access to a standard rat chow and water throughout the study. Phaseolus vulgaris dry extract was administered intragastrically, once a day, in three different 5 d periods (treatments 1–3) followed by three 20 d no-treatment periods (off-treatments 1–3).
ANOVA revealed a significant interaction between treatment and time on daily changes in rat body weight during treatment 1 (F treatment(2,16) = 2·42, P>0·05; F time(4,64) = 14·50, P < 0·0001; F interaction(8,64) = 8·87, P < 0·0001). Specifically, body weight tended to be progressively reduced in rat groups treated with P. vulgaris dry extract (Fig. 1(b)). On day 5, the percentage of change in comparison with baseline was 0·2, − 2·0 and − 3·4 % in 0, 50 and 500 mg/kg P. vulgaris dry extract-treated rats, respectively.
ANOVA failed to reveal significant effects of treatment and interaction between treatment and time on daily changes in body weight during off-treatment 1 (F treatment(2,16) = 2·67, P>0·05; F time(4,64) = 58·74, P < 0·0001; F interaction(8,64) = 1·10, P>0·05). Body weight in all rat groups tended to increase at a comparable trend (Fig. 1(b)).
ANOVA revealed a significant interaction between treatment and time on daily changes in body weight during treatment 2 (F treatment(2,16) = 1·27, P>0·05; F time(4,64) = 2·73, P < 0·05; F interaction(8,64) = 4·87, P < 0·0005). Specifically, body weight tended to be steadily lower in rat groups treated with P. vulgaris dry extract than in vehicle-treated rats (Fig. 1(b)). On day 5, the percentage of change in comparison with baseline was 6·0, 3·1 and 1·2 % in 0, 50 and 500 mg/kg P. vulgaris dry extract-treated rats, respectively.
ANOVA revealed a significant interaction between treatment and time on daily changes in body weight during off-treatment 2 (F treatment(2,16) = 1·17, P>0·05; F time(4,64) = 65·89, P < 0·0001; F interaction(8,64) = 1·59, P < 0·05). Body weight in the two rat groups treated with P. vulgaris dry extract tended to increase more rapidly than in vehicle-treated rats (Fig. 1(b)).
ANOVA failed to reveal significant effects of treatment and interaction between treatment and time on daily changes in body weight during treatment 3 (F treatment(2,16) = 1·27, P>0·05; F time(4,64) = 2·73, P < 0·05; F interaction(8,64) = 4·87, P < 0·0005). This lack of statistical significance was probably due to the large variability recorded in the vehicle-treated rat group. On day 5, the percentage of change in comparison with baseline was 10·9, 6·9 and 6·8 % in 0, 50 and 500 mg/kg P. vulgaris dry extract-treated rats, respectively.
ANOVA failed to reveal significant effects of treatment and interaction between treatment and time on daily changes in body weight during off-treatment 3 (F treatment(2,16) = 0·79, P>0·05; F time(4,64) = 5·74, P < 0·005; F interaction(8,64) = 0·69, P>0·05). Body weight in all rat groups tended to increase at a comparable trend (Fig. 1(b)).
Expt 2: effect of acute treatment with Phaseolus vulgaris dry extract on postprandial glycaemia
Glycaemia in fasted Zucker rats averaged approximately 1000 mg/l in all rat groups (Fig. 2). In vehicle-treated rats, consumption of the starch-enriched meal resulted in an increase in glycaemia at all three recording times; it rose to more than 1300 mg/l at the 60 and 120 min recording times, declining slowly to approximately 1150 mg/l at the 360 min recording time (Fig. 2).

Fig. 2 Reducing effect of a Phaseolus vulgaris dry extract on the time course of glycaemia in Zucker fa/fa rats given a 1 h (corresponding to the 0–60 min time interval) access to a fixed amount of regular rodent chow and water. Each point is means, with their standard errors represented by vertical bars (n 5). ![]() , 0 mg P. vulgaris per kg;
, 0 mg P. vulgaris per kg; ![]() , 50 mg P. vulgaris per kg;
, 50 mg P. vulgaris per kg; ![]() , 500 mg P. vulgaris per kg.
, 500 mg P. vulgaris per kg.
ANOVA revealed a significant effect of treatment with P. vulgaris dry extract on postprandial glycaemia over the 6 h recording period (F dose(2,12) = 6·82, P < 0·05; F time(2,24) = 8·24, P < 0·05; F interaction(4,24) = 1·46, P>0·05). At all recording times, treatment with P. vulgaris dry extract produced a dose-dependent reduction in glycaemia (Fig. 2). In the rat group treated with 500 mg P. vulgaris dry extract per kg, at the 120 min recording time, glycaemia had already decreased to baseline levels (Fig. 2).
In comparison with vehicle-treated rats, the area under the curve of glycaemia time course was approximately 20 and 45 % lower in 50 and 500 mg/kg P. vulgaris dry extract-treated rats, respectively (F(2,12) = 10·11, P < 0·005).
Discussion
The results of the present study indicate that three cycles of repeated (5 d) treatments with P. vulgaris dry extract resulted in substantial and dose-dependent reductions in daily food intake in genetically obese Zucker fa/fa rats. Reduction in daily food intake was associated with significant decrements in rat body weight.
These results support and extend data obtained in previous experimental studies. Specifically, acute and chronic treatment with the same P. vulgaris dry extract utilised in the present study has been found to effectively reduce food intake and body weight in non-obese Wistar rats exposed to different dietary regimens, including starch-enriched food pellets and highly palatable foods(Reference Fantini, Cabras and Lobina6). A marked, concentration-dependent reduction in daily food intake was also observed in Wistar rats when this P. vulgaris dry extract was added to a starch-enriched chow(Reference Carai, Fantini and Loi3). Furthermore, a reduction in body weight was recorded in Zucker fa/fa rats chronically fed on a diet containing kidney beans(Reference Pusztai, Grant and Buchan12) or phytohemagglutinin, a supposedly active ingredient of P. vulgaris (Reference Bardocz, Grant and Pusztai11). Interestingly, a reduction in the body lipid deposit of Zucker fa/fa rats was associated with treatment with kidney beans(Reference Pusztai, Grant and Buchan12) or phytohaemagglutinin(Reference Bardocz, Grant and Pusztai11).
The singular experimental design of the present study (i.e. three different 5 d treatments with P. vulgaris dry extract, each of which interspersed between 20 d periods of no treatment) allowed the effect of P. vulgaris dry extract to be assessed following repeated cycles of treatment. The results obtained indicate that P. vulgaris dry extract-induced reduction in daily food intake was relatively stable and of comparable magnitude at each treatment cycle, averaging approximately 10 and 30 %, in comparison with control rats, in rats treated with 50 and 500 mg P. vulgaris dry extract per kg, respectively. A similar pattern was observed in rat body weight throughout the three different cycles of treatment with P. vulgaris dry extract.
Upon each treatment discontinuation (off-treatment periods), overeating was recorded in rat groups previously treated with P. vulgaris dry extract. This observation is consistent with the results of a previous study in which the P. vulgaris dry extract tested in the present study was repeatedly administered to Wistar rats(Reference Fantini, Cabras and Lobina6), and suggests that treatment with P. vulgaris dry extract is probably devoid of any carry-over effect. The immediate development of overeating after treatment discontinuation is also suggestive of minimal, if any, behavioural toxicity of the tested doses of P. vulgaris dry extract. Accordingly, previous experiments have found that doses up to 500 mg/kg of this P. vulgaris dry extract (i.e. the highest dose tested in the present study) were totally devoid of any effect on spontaneous locomotor activity (a parameter highly sensitive to alterations in the state of well-being of rodents) in Wistar rats(Reference Fantini, Cabras and Lobina6). Additionally, no evident sign of malabsorption, such as diarrhoea or altered number of faecal boluses, has ever been observed in rats treated with this P. vulgaris dry extract(Reference Carai, Fantini and Loi3, Reference Fantini, Cabras and Lobina6, Reference Maccioni, Colombo and Riva7) either in previous studies or in the present study, thus tending to rule out the arrival in the ileum of large amounts of undigested carbohydrates (as predictable due to the administration of a preparation containing an α-amylase inhibitor).
Experiments undertaken to evaluate the effect of P. vulgaris dry extract on glycaemia were designed to reproduce the increase in glycaemia following a large, carbohydrate-rich meal. To this end, rats were fasted for 23 h and subsequently exposed to a fixed amount of starch-enriched food pellets, which all rats were expected to consume entirely within an hour. Glycaemia was assayed immediately before food presentation (fasting baseline) and at hourly intervals after the meal. Data collected indicate that acute administration of the same doses of P. vulgaris dry extract to Zucker rats tested in the previous food intake experiment resulted in a dose-dependent decrease in the time course of glycaemia, as well as in the area under the curve of the time course of glycaemia. At the dose of 500 mg P. vulgaris dry extract per kg, the postprandial increase in glycaemia was virtually suppressed at all time intervals. As all rats consumed the same amount of food, the possibility that the reducing effect of P. vulgaris dry extract on glycaemia was merely due to a reduced intake of food can be ruled out. These findings are consistent with the results of several studies indicating the ability of P. vulgaris extracts or preparations to reduce glycaemia in rats exposed to several experimental designs(Reference Carai, Fantini and Loi3–Reference Fantini, Cabras and Lobina6, Reference Donatucci, Liener and Gross8, Reference Bardocz, Grant and Pusztai11, Reference Pusztai, Grant and Buchan12); notably, two of these studies have reported clear tendencies towards a reduction in glycaemia in Zucker rats fed on diets containing kidney bean-derived lectins(Reference Bardocz, Grant and Pusztai11, Reference Pusztai, Grant and Buchan12).
Interestingly, two previous studies have found that P. vulgaris preparations affected plasma levels of insulin in Zucker fa/fa rats. Specifically, Bardocz et al. (Reference Bardocz, Grant and Pusztai11) found that kidney bean-derived phytohaemagglutinin, mixed to the diet, produced a substantial (approximately 40 % with respect to control rats fed on a plain diet) reduction in plasma insulin levels; Pusztai et al. (Reference Pusztai, Grant and Buchan12) reported markedly reduced levels of plasma insulin in rats fed on a diet containing lectins from kidney beans.
The present study did not specifically address the mechanism(s) of the action of P. vulgaris dry extract on food intake, body weight and glycaemia. Nevertheless, some conclusions may be put forward on account of the findings available to date. Specifically, it can be hypothesised that P. vulgaris dry extract exerted two additive effects: (1) inhibition of pancreatic α-amylase, resulting, in turn, in the deceleration of carbohydrate metabolism and absorption, decrease in glycaemia and production of feelings of satiety(Reference Tormo, Gil-Exojo and Romero de Tejada4, Reference Tormo, Gil-Exojo and Romero de Tejada5, Reference Pusztai, Grant and Buchan12, Reference Moreno and Chrispeels17–Reference Jain, Boivin and Zinsmeister23); (2) lectin-induced reduction in appetite and delay in gastrointestinal transit, probably via alterations in the release of cholecystokinin and glucagon-like peptides, i.e. hormones known to play relevant roles in digestive processes and central control of appetite(Reference Pusztai, Bardocz and Ewen2, Reference Donatucci, Liener and Gross8, Reference King, Pusztai and Grant24–Reference Rådberg, Biernatt and Linderoth27).
To conclude, the results of the present study indicate that multiple cycles of treatment with a P. vulgaris dry extract resulted in substantial reductions in food intake and body weight in an animal model of obesity. Additionally, acute treatment with P. vulgaris dry extract suppressed postprandial glycaemia. These data are in close agreement with previous experimental data collected with this P. vulgaris dry extract as well as with other P. vulgaris preparations and derivatives. These data are also in partial agreement with clinical studies suggesting the efficacy of P. vulgaris preparations in reducing postprandial glycaemia, body weight, waist circumference and appetite in human subjects(Reference Layer, Carlson and Di Magno28–Reference Rondanelli, Orsini and Opizzi34).
Acknowledgements
The authors' contributions were as follows: M. A. M. C., G. C. and P. M. conceived the study, designed the experiments, and managed the literature searches and summaries of the previous related work. A. R., E. B. and P. M. prepared and analysed the plant extract. N. F., B. L. and M. A. M. C. conducted the experiments and analysed the data. M. A. M. C. and G. C. drafted the manuscript. G. L. G. supervised the study. All authors contributed to and approved the final draft of the manuscript. M. A. M. C. took responsibility for the paper as a whole. The present study was funded by the Italian National Research Council (Consiglio Nazionale delle Ricerche) and Indena Spa (Milan, Italy). Conflict of interest: A. R. and P. M. are employees at Indena Spa. E. B. is a consultant for Indena Spa.





