INTRODUCTION
Advanced age is now recognized as a risk factor for sepsis [Reference Angus1–Reference Girard and Ely4]. This may be explained by several predisposing factors for infection encountered in the elderly, including immunosenescence [Reference Marik and Zaloga5, Reference Opal, Girard and Ely6], denutrition [Reference Lesourd7], anatomical modifications favouring bacterial colonization [Reference Nicolle8] and frequent comorbidities, such as diabetes mellitus, chronic obstructive pulmonary disease, heart failure and renal insufficiency [Reference Borrego and Gleckman9]. Age also represents an independent predictor of mortality [Reference Angus1, Reference Brun-Buisson, Doyon and Carlet10, Reference Laupland11], as a consequence of host fragility combined with a less specific clinical presentation of infection [Reference Chassagne12, Reference Leibovici13], leading to delayed diagnosis and management. Important age-related differences exist in the species distribution of pathogens causing sepsis. The risk of infection with Gram-negative bacilli is increased in the elderly, and Escherichia coli represents the primary cause of community-acquired bacteraemia in patients aged > 65 years [Reference Martin, Mannino and Moss2, Reference Crane, Uslan and Baddour14, Reference Diekema, Pfaller and Jones15]. Through the French prospective multicentre COLIBAFI study analysing 1051 E. coli bacteraemia episodes in adults, we identified advanced age as an independent risk factor for death [odds ratio (OR) 1·25, 95% confidence interval (CI) 1·09–1·43 for each 10-year increment] [Reference Lefort16]. A better knowledge of risk factors for death from E. coli bacteraemia in the geriatric population may help to improve the management of the disease. The objectives of this ancillary study of COLIBAFI are to specifically describe age-related epidemiological, clinical, microbiological characteristics and outcome of E. coli bacteraemia in the elderly, and to determine the independent risk factors for death according to age.
METHODS
The materials and methods of the prospective multicentre COLIBAFI study have been reported previously [Reference Lefort16]. Briefly, 1051 adults with E. coli bacteraemia hospitalized in 15 French hospitals (one general and 14 university hospitals) were prospectively and consecutively included between January and December 2005. E. coli bacteraemia was defined as the isolation of E. coli from at least one set of aseptically inoculated blood culture bottles. The primary endpoint was in-hospital overall mortality, up to 28 days after the first positive blood culture. Follow-up ended at hospital discharge or 28 days after the first E. coli-positive blood culture for patients still hospitalized. The study was approved by the institutional Ethics Committee (Comité de Protection des Personnes, Hôpital Saint-Louis, Paris, France; approval no. 2004–06). According to Ethics Committee recommendations, patients were informed by a written information letter and gave their consent orally.
Clinical and microbiological characteristics
The comorbidity score was defined for each patient as the number of comorbidities among the following: current tobacco and alcohol addiction, chronic heart failure, chronic pulmonary and renal insufficiency, diabetes mellitus, cirrhosis, a past history of bacteraemia, and immunosuppression. Immunocompromised patients were those presenting with at least one of the following conditions: human immunodeficiency virus (HIV) infection with CD4 counts of < 200 cells/mm3, underlying progressive solid cancer or malignant haemopathy, prior solid-organ or bone marrow transplantation, neutropenia of <500/mm3, congenital immunodeficiency, current immunosuppressive therapy (>10 mg/day of a prednisone equivalent, immunomodulating treatment, or antineoplastic chemotherapy within the last month).
An infection was considered as healthcare-associated when occurring in a patient living in an institution (nursing home, retirement home, long-term care facility) or in a patient having received anti-neoplastic chemotherapy or on dialysis during the last month, or when the first positive blood culture was obtained 48 h following hospital admission (nosocomial infection).
The portal of entry was established according to compatible clinical and/or radiographic features and the isolation of E. coli from the presumed source of infection. When E. coli isolation was not available from the presumed portal of entry (i.e. previous antibiotic treatment or an undesirable examination invasive procedure), the diagnosis was based on a firm clinical suspicion, provided that all other possible sources of infection had been excluded. If the clinical data were ambiguous, the portal of entry was categorized as being ‘undetermined’. A secondary septic focus was defined as a metastatic focus of infection due to bacteraemia that was anatomically distant from the portal of entry, if any. The bacteraemia was polymicrobial when at least one other microorganism was recovered from a set of blood culture bottles positive for E. coli. The antibiotic regimen was considered to be adequate when the E. coli isolate was susceptible in vitro to at least one of the antibiotics given.
All E. coli isolates were centralized at a single research laboratory (INSERM, UMR 1137), which performed molecular epidemiology studies. For each strain, a bacterial resistance score was defined as the number of antibiotics to which it was resistant to from the five following drugs: amoxicillin, cefotaxime, gentamicin, ofloxacin and cotrimoxazole. Antimicrobial susceptibility was determined by the disk diffusion method as recommended by the Comité de l'Antibiogramme de la Société Française de Microbiologie (www.sfm.asso.fr). Multidrug resistance was defined as resistance to at least amoxicillin, ofloxacin and cotrimoxazole. Determination of the phylogenetic group (A, B1, B2, D) of each strain was performed according to Clermont et al. [Reference Clermont, Bonacorsi and Bingen17]. Sequence type (ST) 131, which belongs to the B2 phylogroup, was identified by a pabB allele-specific polymerase chain reaction (PCR) in all B2 isolates according Clermont et al. [Reference Clermont18] and confirmed as belonging to the O25b type by rfb PCR [Reference Clermont19]. The presence of integrons (classes I, II, III), which are molecular markers of resistance, was detected by triplex real-time PCR [Reference Skurnik20].
For each isolate, a virulence score was defined as the number of virulence factors present over the 18 tested: adhesins (papC, papG including papG alleles, sfa/foc, iha, hra, ibeA), toxins (hlyC, cnf1, sat), iron-capture systems (fyuA, irp2, iroN, iucC, ireA), protectins (neuC, chromosomal ompT, traT) as well as a gene encoding the uropathogenic-specific protein, usp were tested by PCR. As it is well known that numerous virulence genes are clustered on genomic islands called pathogenicity-associated islands (PAIs) [Reference Hacker21], we deduced the presence of six PAIs from the presence of the individual virulence genes [Reference Bingen-Bidois22–Reference Kurazono24]: PAI ICFT073 (papGII, hly, iucC positive), PAI IIJ96 (presence of at least three of the four following genes: papGIII, hly, cnf1, hra), PAI III536 (sfa/foc and iroN positive), PAI IV536, a high-pathogenicity island (HPI) (irp2 and fyuA positive), GimA (ibeA positive), and PAIUSP (usp positive). For each isolate, a PAI score, defined as the number of PAIs present over the six tested, was calculated.
Statistical methods
The database containing all variables of the 1051 patients included in the COLIBAFI study was used for the present study. Although there are limitations in using chronological age as a marker for senescence, we decided to analyse data according to the three age groups: ‘young’ (18–64 years), ‘old’ (65–79 years) and ‘very old’ (⩾80 years) patients. These three age groups were chosen because of their frequent use in the literature [Reference Leibovici13, Reference Sogaard25] and because they correspond to the segmentation established by the MeSH thesaurus (‘aged’ and ‘aged and over’) and also the World Health Organization's definition (www.who.int)
Characteristics were described as means±standard deviations (s.d.) or medians and ranges for continuous variables and as frequencies and percentages for categorical variables. Differences in the means in the three age groups were initially evaluated using one-way analysis of variance (ANOVA). If a significant difference was found in the age groups, pairwise differences between groups were assessed using Tukey's Honestly Significant Difference test. Differences in proportions in groups were analysed using the χ2 test or Fisher's exact test, as appropriate. Statistical significance was set at P < 0·05.
The risk factors associated with death during follow-up were analysed for each age group. First, univariate logistic regression analyses were performed for clinical and bacteriological factors. The studied clinical factors were age; sex; weight; body mass index; hospitalization or institutionalization before bacteraemia; antibiotic therapy during the 2 weeks preceding bacteraemia; presence of a urinary catheter; comorbidities [including a history of bacteraemia, chronic alcoholism, tobacco addiction (current smoker), congestive heart failure, chronic respiratory insufficiency, chronic renal insufficiency, diabetes mellitus, immunocompromised, cirrhosis]; nosocomial or healthcare-associated infection; a portal of entry (including urinary tract, digestive tract, pulmonary, cutaneous, and venous catheter). The bacteriological determinants of strains were: a phylogenetic group B2 (known to be associated with high extraintestinal virulence) [Reference Picard26], the presence of any of the 18 virulence factors; virulence score; polymicrobial infection; resistance to amoxicillin, cefotaxime, gentamicin, ofloxacin, or cotrimoxazole; multidrug resistance; resistance score, PAI score and the presence of ST131. All categorical variables were defined by presence or absence. For all continuous variables entered in the multivariate logistic regression model, the linearity assumption was assessed by plotting the logarithm of the odds of mortality against each explanatory variable. The clinical and bacteriological risk factors achieving a P value of <0·10 were entered into the multivariate logistic regression model. A backward selection method was used to obtain a model in which all clinical risk factors had a P value <0·05. All data collected were processed with SPSS version 16·0 (SPSS Inc., USA).
RESULTS
Of the 1051 patients, 395 (37·6%) were ‘young’ (18–64 years), 372 (35·4%) were ‘old’ (65–79 years) and 284 (27·0%) were ‘very old’ (⩾80 years).
Clinical characteristics
The characteristics of the patients with E. coli bacteraemia according to age are shown in Table 1. The proportion of males was lower in the very old population. The rate of patients already hospitalized at the time of bacteraemia was higher in the young and old groups than in the very old group; very old patients were more likely to come from institutions.
Table 1. Demographic, epidemiological and clinical characteristics of 1051 patients with Escherichia coli bacteraemia according to age group

s.d., Standard deviation.
* Because of missing values, percentages are calculated based on available data.
† Some patients had more than one host-predisposing condition.
‡ Other immunocompromised conditions included human immunodeficiency virus (HIV) infection with CD4 counts of <200 cells/mm3, prior solid-organ or bone marrow transplantation, congenital immunodeficiency, current immunomodulating treatment.
§ Other portals of entry included surgical site infection and female genital tract infection.
The comorbidity score was higher in young and old patients than in very old patients. Some differences in comorbidities were observed according to age: young patients were more likely to be chronic alcoholics and smokers; the highest prevalence of diabetes mellitus was observed in old patients; congestive heart failure was more frequently encountered in old and very old patients than in young patients; young and old patients were more often immunocompromised than very old patients. Infections were less frequently nosocomially acquired in the very old patient group than in the two other groups. The portal of entry was unknown for 26·8% of patients and in each age group the most frequent source of infection was the urinary tract. Of the latter group of patients, 87 (14·5%) had a urinary catheter and the proportion of patients with symptomatic urinary tract infections decreased with age; there were 128 (68·1%) in the young group, 105 (58·3%) in the old group and 60 (44·1%) in the very old group (P < 0·001). Very old patients were more prone to develop bacteraemia from a pulmonary portal of entry than others. The proportion of patients given adequate treatment on the first day and the delay in starting this treatment were not significantly different between age groups.
Bacterial determinants
The microbiological characteristics of the 1051 patients according to age group are shown in Table 2. The phylogenetic groups’ distribution and the frequency of the O25b-ST131 emerging clone were not different between age groups. No age-related significant difference was also observed in the frequency of 18 virulence factors tested, except for iroN, which was more frequent in E. coli isolates from infections in young patients. The PAI score was not significantly different between age groups. Regarding antibiotic resistance, young and old patients were infected with more resistant strains, as shown by a higher resistance score. Indeed, higher resistance rates to amoxicillin, cotrimoxazole and gentamicin were observed for these two former groups and the cefotaxime resistance rate was similar between age groups. The presence of integron I was higher in the young and old groups than in the very old group.
Table 2. Microbiological characteristics of 1051 patients with Escherichia coli bacteraemia according to age group

s.d., Standard deviation; PAI, pathogenicity-associated island.
* Indicates papGII and papGIII alleles are individualized.
Risk factors for death
Overall, 136 (12·9%) patients died. Death rates were significantly different between age groups (P = 0·039): 10·4% (n = 41) in the young patients group, 16·4% (n = 61) in the old patients group and 12·0% (n = 34) in the very old patients group (Table 3). Regarding nosocomial infections (n = 202), the mortality rate was also higher for old patients than for young and very old patients [25 (59·5%) vs. 13 (31%) and 4 (9·5%); P = 0·024]. Of patients with healthcare-associated infection (n = 378), a higher mortality rate was also observed in old patients than in young and very old patients [38 (50·0%) vs. 25 (32·9%) and 13 (17·1%); P = 0·035]. Overall, 100 (9·5%) patients required transfer to an intensive care unit (ICU) because of the severity of bacteraemia; the percentage of patients who were transferred did not differ according to age (Table 3).
Table 3. Mortality and severity of Escherichia coli bacteraemia by age group

ICU, Intensive care unit; s.d., standard deviation.
* From blood culture positivity to death.
The linearity assumption of all the continuous variables entered in the multivariate logistic regression model was graphically checked; the corresponding plots were considered satisfactory for all selected continuous explanatory variables.
The bacteriological risk factors for death identified by multivariate logistic regression analysis according to age are shown in Table 4. Each age group presented specific bacteriological risk factors. Four different virulent genes (papGII for young, ireA and papC for old, and hra for very old) had a protective role in each age group. The genes ireA and hra are known to be associated with the urinary portal of entry, but they remained independently associated with death when this portal of entry was forced into an additional multivariate regression model (data not shown). Cefotaxime resistance of the strains was a risk factor for death only in the young group.
Table 4. Bacteriological risk factors for death from Escherichia coli bacteraemia identified by multivariate logistic regression analysis according to age group
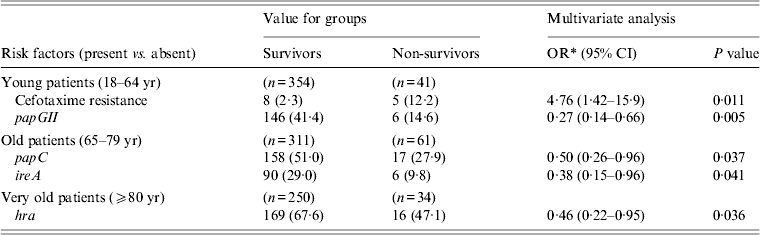
CI, confidence interval; OR, odd ratio; s.d., standard deviation.
* Adjusted odds ratios taking into account the remaining risk factors of the model.
By multivariate analysis, the host and bacteriological risk factors associated with death (Table 5) in the young patients group were: cirrhosis, being immunocompromised, a polymicrobial bacteraemia and cefotaxime resistance; a urinary tract portal of entry was negatively correlated with death. For old patients, the risk factors identified were cirrhosis, being immunocompromised and nosocomial infections. A lower death rate was observed for patients infected with an isolate having the ireA virulence gene. In the very old patients group, the sole risk factor for death was renal insufficiency while the presence of the hra virulence gene was associated with lower mortality.
Table 5. Risk factors for death from Escherichia coli bacteraemia identified by multivariate logistic regression analysis according to age group

OR, Odd ratio; CI, confidence interval.
* Adjusted odds ratios taking into account the remaining risk factors of the model.
DISCUSSION
Through the original COLIBAFI cohort study regarding all the population without age stratification [Reference Lefort16], we found, as did others [Reference Kennedy, Roberts and Collignon27, Reference McCue28], that the majority of patients hospitalized for E. coli bacteraemia were aged >65 years, and that an advanced age was an independent risk factor for death from E. coli bacteraemia.
We thus conducted this ancillary study focusing on the elderly to better describe the demographic, clinical, microbiological and prognostic characteristics of E. coli bacteraemia in a large cohort of patients, and to compare them with younger patients. Host and bacterial determinants were studied jointly, for a proper understanding of the role of host or bacteria in the severity of the disease.
We were surprised to observe that the highest mortality rate was in the old (65–79 years) group rather than in the very old (⩾80 years) group. Previous studies on bacteraemia in the elderly have reported a higher mortality rate for old patients than for younger ones [Reference Sogaard25, Reference Ismail29], but most of these studies did not find any differences between old and very old patients [Reference Leibovici13, Reference McCue28, Reference Gavazzi30–Reference Lee32] and did not analyse specifically E. coli bloodstream infections. The highest mortality rate we observed in old patients may be explained by the highest comorbidity score in this age group, probably reflecting a more frail population than our very old population. The fact that the ICU transfer rates did not differ with the age might indirectly reflect the relative good general condition of the very old group. While virulence scores did not differ between age groups, strains were more resistant in the young and old patients groups than in the very old group. This may be due to the fact that young and old patients had more comorbidities, were more often already hospitalized at the time of bacteraemia and had more often received antibiotics within the 2 weeks preceding bacteraemia than older patients, which may increase the selection of resistant strains.
Observing the risk factor for death according to age, it is interesting to note that, as in the COLIBAFI study [Reference Lefort16], and whatever the age group studied, host factors outweighed bacterial determinants in predicting mortality. Risk factors for death were age group-specific. Young and old patients shared several host risk factors, such as immunodepression and cirrhosis. In the very old group, chronic renal insufficiency was associated with a higher mortality, in accord with other studies focusing on this population [Reference Leibovici13, Reference Greenberg31, Reference Lark33, Reference Shmuely34]. Chronic renal insufficiency may be associated with a drug misuse (over or under dosage), which is frequent and has heavy consequences in this population.
Whereas cefotaxime resistance had a negative impact on prognosis in the young patients group, no antibiotic resistance characteristic was associated with death in the elderly. Moreover, there was no age-related significant difference in the phylogenetic groups’ distribution or in the frequency of the O25b-ST131 emerging clone [Reference Clermont19, Reference Nicolas-Chanoine35], in contrast to previous studies which indicated an increased prevalence of this clone with age [Reference Banerjee36]. These results may be explained by the small number (3%) of these strains, which were not as prevalent as now.
Two virulence factors (ireA and hra, encoding an iron capture system and an adhesin, respectively) were negatively correlated with death, in the old and very old patient groups, respectively. The ireA gene was also found to be negatively correlated with death considering the whole cohort [Reference Lefort16]. Both virulence factors are known to be implicated in infection of the urinary tract [Reference Russo, Carlino and Johnson37, Reference Srinivasan, Foxman and Marrs38]. We failed to find evidence of a link between a urinary portal of entry and better outcome in the elderly population, contrary to that observed in younger patients, in the whole cohort [Reference Lefort16] and in previous studies [Reference Ismail29, Reference Jaureguy39–Reference Whitelaw, Rayner and Willcox43]. Nevertheless, we first hypothesized that the protective role of these two virulence factors may reflect a more favourable evolution of urinary tract-related bacteraemia. However, a multivariate analysis where the urinary portal of entry was forced into the model showed that these virulence factors remained independently associated with a lower mortality
The selection of different virulence factors in young, old and very old patients could be related to a possible specific host–pathogen relationship evolving with age, that is suggested by variations of the link between virulence characteristics and prognosis according to age group. These results could also be explained by chance related to the complexity of the model-building procedure in each age group.
Our study has several limitations. First, COLIBAFI was not designed to study specifically the elderly population, so that specific geriatric comorbidities, such as Parkinson's disease, or a past history of stroke or dementia were not available. Moreover, functional, nutritional and cognitive status were lacking; the term ‘institution’ may be considered as an indirect marker of loss of autonomy and did not impact on old and very old patients’ prognosis. Second, data were collected in 2005 and cephalosporin resistance in E. coli has increased since then (8·2%; European Centre for Disease Prevention and Control, Antimicrobial Resistance Surveillance in Europe 2011, www.ecdc.europa.eu). In the present study decreased susceptibility to third-generation cephalosporins was observed in 41 (3·8%) strains [Reference Courpon-Claudinon44]. Third, the phylogenetic relationships between the strains were studied at the phylogroup level and not at the clonal level except for the ST131 clone. Further study of the clonal distribution in our collection could reveal age-specific patterns as suggested by a recent work on 300 consecutive non-duplicate extraintestinal E. coli isolates [Reference Banerjee45]. Fourth, it is important to note that the mortality rate we considered was the overall mortality at day 28, and not the mortality attributable to E. coli infection. This choice was based on the fact that mortality attributable to E. coli sepsis is very difficult to assess, especially in the elderly population, due to the numerous comorbidities of the patients, and to the possible indirect non-infectious consequences of the sepsis. As an example, numerous non-infectious complications such as myocardial infarcts, stroke or pneumonia that may lead to death were recently recognized as indirect consequences of flu [Reference Warren-Gash46]. Finally, the impact of E. coli bacteraemia on the long-term evolution of the patients has not been studied. It may be hypothesized that complications of such a septic episode on the nutritional and functional status would decrease life expectancy of the elderly population, as already observed for other acute events, such as hip fracture [Reference Robbins, Biggs and Cauley47] or acute cardiac failure [Reference Mahjoub48]. Despite these limitations, our multicentre analysis of more than 600 patients aged ⩾65 years allowed us to draw some informative conclusions.
In summary, this study confirms the high prevalence of E. coli bacteraemia in the elderly population and focuses on the impact of age on clinical outcome. The poorest prognosis was that of old patients, which may result from the conjunction of the age itself and several comorbidities. Although clinical characteristics outweighed bacterial determinants in predicting a fatal outcome in all age groups studied, we found that risk factors for death were age group-specific, suggesting a host–pathogen relationship evolving with age. Data on the evolution of gut colonization and mucosal barriers throughout life are scarce and their contribution to the age-related polymorphism in clinical presentation and outcome of E. coli bacteraemia constitute an attractive hypothesis to explore.
APPENDIX. COLIBAFI Study Group
Clinical investigators: Michel Wolff, Loubna Alavoine, Xavier Duval, David Skurnik, Paul-Louis Woerther, Antoine Andremont (CHU Bichat-Claude-Bernard, Paris); Etienne Carbonnelle, Olivier Lortholary, Xavier Nassif (CHU Necker-Enfants Malades, Paris); Sophie Abgrall, Françoise Jaureguy, Bertrand Picard (CHU Avicenne, Bobigny); Véronique Houdouin, Yannick Aujard, Stéphane Bonacorsi, Edouard Bingen (CHU Robert-Debré, Paris); Agnès Meybeck, Guilène Barnaud, Catherine Branger (CHU Louis-Mourier, Colombes); Agnès Lefort, Bruno Fantin, Claire Bellier, Frédéric Bert, Marie-Hélène Nicolas-Chanoine (CHU Beaujon, Clichy); Bernard Page, Julie Cremniter, Jean-Louis Gaillard (CHU Ambroise-Paré, Boulogne-Billancourt); Bernard Garo, Séverine Ansart, Geneviève Herry-Arnaud, Didier Tandé (CHU Brest, Brest); Jean-Claude Renet, René Ze Bekolo, Renaud Verdon, Roland Leclercq (CHU Caen, Caen); Claire de Gialluly, Jean-Marc Besnier, Laurent Mereghetti, Roland Quentin (CHU Tours, Tours); Achille Kouatchet, Alain Mercat, Marie Laure Joly-Guillou (CHU Angers, Angers); Catherine Dalebroux, Pascal Chavanet, Catherine Neuwirth (CHU Dijon, Dijon); Camille Colliard, Martin Dary, Gilles Potel, Jocelyne Caillon (CHU Nantes, Nantes); Françoise Leturdu, Jean-Pierre Sollet, Gaëtan Plantefève (CH Argenteuil, Argenteuil); Agnès de Patureaux, Pierre Tattevin, Pierre-Yves Donnio (CHU Rennes, Rennes).
Responsible for bacterial genotyping: Erick Denamur, Olivier Clermont, Christine Amorin, Jeremy Glodt (INSERM, UMR722, Université Paris-Diderot, Paris, France).
Responsible for methodology: Xavière Panhard, Ludovic Lassel, Quentin Dornic, France Mentré (AP-HP, Hôpital Bichat, Service de Biostatistiques, Paris, France), Estelle Marcault, Florence Tubach (CHU Bichat-Claude-Bernard, Paris, France).
ACKNOWLEDGEMENTS
This work was supported by grants from ‘Réseau de Recherche Clinique’ (INSERM, grant no. RBM-03-58) and ‘Projet Hospitalier de Recherche Clinique’ (Assistance Publique-Hôpitaux de Paris, grant no. AOR 04 053). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Professor Gaetan Gavazzi for his invaluable help and comments regarding infections and the elderly population.
DECLARATION OF INTEREST
None








