Introduction
Malaria is one of the most important infectious diseases and remains a significant global public health problem (Muray et al., Reference Muray, Rosenfeld, Lim, Andrews, Foreman, Haring, Fullman, Naghavi, Lozano and Lopex2012). Only vivax malaria has been indigenous in Democratic People's Republic (DPR) of Korea because other Plasmodium species including Plasmodium falciparum have no capability to survive due to inadequate temperature and duration for their growth (duration of ≥25 °C is shorter than 1 month in the year). Plasmodium vivax accounts for approximately half of all malaria cases outside Africa (Mendis et al., Reference Mendis, Sina, Marchesini and Carter2001; Gething et al., Reference Gething, Elyazar, Moyes, Smith, Battle and Guerra2012). Worldwide, P. vivax accounts for an estimated eight (WHO, 2017) to 80 million cases of malaria each year (Mendis et al., Reference Mendis, Sina, Marchesini and Carter2001). In DPR Korea, malaria was eliminated in 1970s, but it re-emerged in the late 1990s. National malaria control programme has actively done various interventions for early diagnosis and prompt, effective treatment and vector control to eliminate malaria. It resulted in moving forward malaria elimination phase. Indigenous vivax malaria in the country has highly seasonal transmission characteristics, and malaria cases are reported with one peak pattern between July and August.
In the areas where vivax malaria is prevalent, there are two forms based on incubation property, in which one is northern form with long incubation and another southern form with short incubation (Sun-Yong, Reference Sun-Yong1980, Reference Sun-Yong1982). Frequent relapse is a very important characteristic of vivax malaria (Sun-Yong, Reference Sun-Yong1980). Of the Plasmodium species that affect humans, only P. vivax and P. ovale form hypnozoites, which are dormant parasite stages in the liver that cause relapse weeks to years after the primary infection (Sutherland et al., Reference Sutherland, Tanomsing, Nolder, Oguike, Jennison and Pukrittayakamee2010). To be well aware of the characteristics associated with incubation and relapse of vivax malaria that is prevalent in the country is a key problem in implementing malaria control activities on planned and reasonable basis.
Fight against malaria is mainly conducted by taking measures for control of infectious source and malaria mosquitoes. As a part of fighting against malaria infectious source, the world has extensively used mass antimalarial drug administration in various forms over the past 80 years. The objective is to provide therapeutic concentrations of antimalarial drugs to as large a proportion of the population as possible in order to cure any asymptomatic infections and also to prevent reinfection during the period of post-treatment prophylaxis (WHO, 2015).
Primaquine is currently the only medicine for treating relapses of P. vivax and P. ovale malaria, due to its specific activity against malarial hypnozoites (WHO, 2016). Despite a reduced sensitivity of these parasites in some countries, requiring increased doses, primaquine has remained highly effective for preventive therapy since its introduction in 1952. For elimination of P. vivax and P. ovale liver-stage infections (radical cure), primaquine must be given at a higher dose, 0·25–0·5 mg base kg−1 body weight daily for 14 days, in addition to the antimalarial medicine that cures the blood-stage infection. In glucose-6-phosphate dehydrogenase (G6PD)-normal patients, this dose of primaquine is remarkably safe, well tolerated and highly efficacious in preventing relapse. The medicine induces dose-dependent acute haemolytic anaemia in individuals with G6PD deficiency (G6PDD), a genetically X-linked disorder (WHO, 2016). According to the national G6PDD investigation conducted targeting some population in high, medium and low transmission areas by the WHO support in 2013, the rate ranges from 0·45 to 1·36% in DPR Korea (Technical Report on G6PDD rate in DPR Korea was submitted to the WHO country office in 2013) and it leads primaquine to be safely used as a main drug for radical treatment of vivax malaria and mass chemoprevention. We conducted the study aiming at disturbing malaria transmission by finding the characteristics of incubation and relapse of indigenous vivax malaria prevailing in the country and establishing relevant chemoprevention method.
Methods
Epidemiological characteristics of vivax malaria
Questionnaires from 312 cases who first experienced malaria among population living in non-transmission areas but travelled highly malarious areas in malaria transmission season were used for the analysis of incubation form and period. Incubation period was determined by investigating minimum and maximum days of duration between the day bitten by malaria mosquitoes and the attack day of malaria symptoms, and by calculating arithmetic mean. We investigated the malaria cases who first experienced malaria within a month after travelling high malaria transmission areas among population living in non-malarious areas and recorded them as short-form cases. Malaria cases who manifested malaria symptoms 5 months after travelling malarious areas were recorded as long-form cases. Then we summed up two forms of malaria cases by month and year to calculate percentage.
For investigation of relapse, we targeted cases with vivax malaria after travelling malarious areas and recorded monthly relapse cases after administering chloroquine regimen to them. According to the WHO classification criteria (Markell, Reference Markell1971), short relapse recurs 2 months after primary infection of vivax malaria and remote relapse 6 months later. We followed the criteria for investigation of short and remote relapses by each long form and short form of vivax malaria in the country context.
Effectiveness of chemoprevention with primaquine
To find a reasonable regimen for chemoprevention with primaquine, we selected five high malarious areas with similar incidences in the previous year to conduct pilot study. We administered primaquine (IPCA Laboratories Ltd., Kandivli Mumbai, Maharashtra, India. 7·5 mg tablet−1, provided by the WHO) based on age and body weight for different periods of time (once a day for 5-, 10-, 14-day regimens and twice a day for 7-day regimen) to persons of 6 years or older in four high malarious areas (study groups) before malaria transmission season. Persons of 16 years or older were given two 7·5 mg tablets day−1. For children, the doses were reduced to 1·5 tablets day−1 (13–15 years, 40–49 kg), one tablet day−1 (10–12 years, 30–39 kg) or 0·5 tablet day−1 (6–9 years, 22–29 kg). Persons in one area were not given primaquine (control group).
To find the efficacy of chemoprevention with primaquine, 64 814 of population in high malarious areas were targeted, and household doctors in each community were responsible for administration under direct observation. (A household doctor is responsible for each 150 households to care their health and this is a predominant system based on free medical care system of our country.) Pregnant or lactational women, under 5 children and contraindicative individuals were omitted in the study.
In total, 42 509 of population (16 915 for 7-day regimen and 26 594 for 14-day regimen) were targeted for investigation on adverse effects by primaquine. Questionnaires on adverse effects were agreed with the WHO technical advisor of design, and household doctors were instructed themselves to interview with people taking primaquine and fill in the questionnaires.
To find the impact of chemoprevention with primaquine on asymptomatic carriers, we assigned the population in the areas with implemented chemoprevention to study group and control group was selected from non-implemented areas. We conducted microscopical test of the parasites in peripheral blood to detect asymptomatic carriers. The tests were conducted 15 days after chemoprevention (2974 persons for 14-day regimen, 2227 for 7-day regimen and 3322 for control) and from 15 to 30 September, the period when malaria transmission is ended.
Long impact of chemoprevention with primaquine on malaria incidence was evaluated by calculating malaria incidence per 1000 persons in areas where mass chemoprevention with primaquine was provided. We implemented chemoprevention once a year, before malaria season (early April) with 14-day regimen and analysed the reduction in incidence rate per 1000 persons every year.
Results
Epidemiological characteristics of vivax malaria
We analysed the proportion of incubation form of vivax malaria among 312 travellers who travelled to malaria transmission areas. Throughout the year, short incubation form cases accounted for 31% and long form cases 69%. For monthly proportion of malaria cases, according to the incubation form, 100% of cases reported before June and after September resulted from long form and it tended to reduce since June. While short form cases were reported since 20 June and increased gradually till September (Fig. 1).
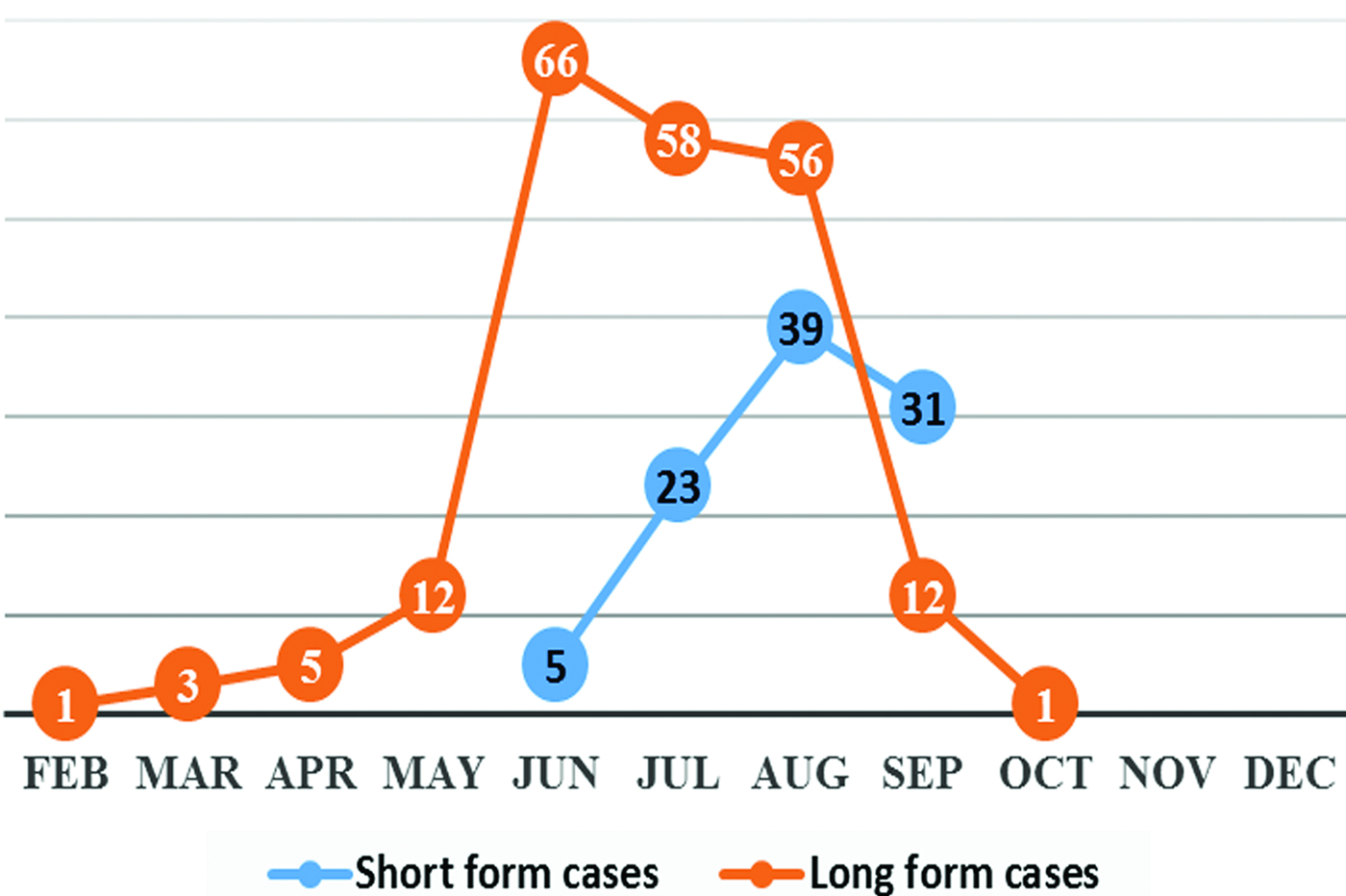
Fig. 1. Monthly trend of vivax malaria by incubation forms in DPR Korea.
We collected information on the length of stay in the field and bite by Anopheles mosquitoes from malaria cases who travelled to malarious areas in transmission season and manifested malaria symptoms after coming back to non-malarious areas, and estimated incubation period of vivax malaria, in which short incubation period was 10 days in minimum and 29 days in maximum, on average 17 days, and long incubation was minimum 5·5 months and maximum 16 months, on average 8–13 months.
Following treatment with chloroquine, relapse cases were reported throughout the year – increasing drastically since May and decreasing after July (peak month) – and 90% out of relapse cases were reported between May and September when the activity of Anopheles mosquitoes is strengthened in the country (Fig. 2). Short-form vivax malaria occurred in only remote relapse, while long form occurred in both short relapse (59%) and remote relapse (41%) (Table 1). Among whole relapse cases, remote relapse accounted for 70% and short relapse 30%. Considering relapse duration, for exclusive short relapse in long-form vivax malaria, was 32–163 days (on average 63 days) after chloroquine treatment of primary infection, i.e. 1–5·5 months. For remote relapse, the duration was 190–482 days (on average 366 days), i.e. 6·5–16 months in long incubation form and 187–463 days (on average 329 days) corresponding 6–15·5 months in short incubation form. Based on the analysis on duration of relapse, for remote relapse, first relapse appeared in January of next year after primary infection, increased since April, peaked at July and disappeared after October. Patients who experienced primary infection in the first half of the year were relapsed after 10–16 months, and when primarily infected in the second half of the year, patients were relapsed after 6–13 months. Short relapse appeared since May and peak at July. Patients who first manifested malaria symptoms between January and March were relapsed after 3–5 months and patients between April and September relapsed after 1·5–2 months.

Fig. 2. Monthly trend of relapse among vivax malaria cases occurred in a year.
Table 1. Relapse types and ratio by vivax malaria incubation forms in DPR Korea

Effectiveness of chemoprevention with primaquine
In total, 53 296 persons except pregnant or lactation women and contraindicative persons among total 64 814 persons in target areas were involved in the study. Six hundred and forty-one persons (1·2%) were withdrawn from the study processing due to severe adverse effects including blood or brown urine and thus 52 655 persons (98·8%) finished chemoprevention with primaquine.
We observed preventive effects by preliminary various methods to find out reasonable chemoprevention method in small size of population, in which standard 14-day course group (given primaquine 0·25 mg base kg−1 body weight day−1 for 14 days) and 7-day group (0·5 mg kg−1 day−1 for 7 days) showed higher preventive effects than other two groups (P < 0·05) and significant difference between 14- and 7-day groups was not shown (P > 0·05) (Table 2).
Table 2. Vivax malaria preventive efficacy by chemoprevention with primaquine in various groups

Five-day group; group taken primaquine of 0·25 mg kg−1 day−1 for 5 days.
Ten-day group; group taken primaquine of 0·25 mg kg−1 day−1 for 10 days.
Fourteen-day group; group taken primaquine of 0·25 mg kg−1 day−1 for 14 days.
Seven-day group; group taken primaquine of 0·5 mg kg−1 day−1 for 7 days.
During chemoprevention with primaquine, we observed transient adverse effects including headache (1·44%), dizziness (0·54%), anorexia (0·52%), dyspepsia (1·10%), nausea (0·37%), abdominal pain (0·79%), diarrhoea (0·94%), pain in right upper quadrant (0·17%) and myalgia (0·33%), and the incidence of adverse effect was 1·4–1·7 times more in the 7-day group than in the 14-day group for almost all indicators. When we gave primaquine according to the dosing based on age, the incidence of adverse effects was significantly higher in the 7-day group than in the 14-day group among males, females and adults. On the other hand, we gave primaquine on the basis of body weights of ≥6 years children, and among them adverse effects appeared significantly lower than in adults, with no difference between two regimens (Table 3).
Table 3. Incidence of adverse effects by primaquine chemoprevention in DPR Korea

Regimen 1 group were given 2·5 mg kg−1 primaquine for 14 days and regimen 2 group given for 7 days. Children group included 6–16 years old children with body weight 22–50 kg. P value indicates the significant difference in appearance of adverse effects between regimens 1 and 2.
We investigated asymptomatic carriers among the population in the study and control areas after the chemoprevention and down to the end of malaria transmission season and observed no carrier in the study areas, compared with 0·27–0·29% of slide-positive rate in the control areas.
We evaluated the impact of vivax malaria incidence in the areas with implemented mass chemoprevention. If malaria incidence before conducting mass chemoprevention with primaquine was 100, after implementation it reduced dramatically, with 46·6 for the first year, second year 15·0, third year 5·4 and fourth year 0·3.
Discussion
Plasmodium vivax is prevalent between latitude 63 degrees North and latitude 40 degrees South (even in these zones, no malaria in highlands and deserts) because it can develop in the body of Anopheline in the zones where monthly average temperature is above 16 °C (Belding, Reference Belding1965). There are tens of strains including Greece strain, Chesson strain, Madagascar strain, Mediterranean strain, Pacific ocean strain, Romania strain, Korean strain in P. vivax (Song-Jung, Reference Song-Jung1956). Some authors suggested that P. vivax can be classified into two forms according to their incubation period, in which one is southern form with short incubation of 10–21 days (on average 13·4 days) and another is northern form with long incubation of 8–14 months, and former is P. vivax and latter is P. vivax hibernans (Yukio , Reference Yukio1996). To find correctly the incubation period and distribution status by different forms (short form and long form) of P. vivax strain is a very important problem for controlling malaria on the planned basis as different P. vivax strains have different incubation periods.
To investigate the proportion by incubation period of vivax malaria prevailing in DPR Korea, we targeted populations living in non-malarious areas who travelled to transmission areas in malaria season. This was based on the inference that they never suffered from malaria before. We investigated malaria cases among the travellers by different forms of incubation, in which long incubation form showed a trend of much more ratio in northern areas (80%) than in southern areas and short form was 31% and long form 69% by nationwide mean.
We identified the proportion of long- and short-form cases reported monthly during the malaria transmission season under the condition that the proportion by incubation forms of vivax malaria in the country was defined, in which all cases reported before 20 June and after September were due to long incubation form. Monthly proportion of malaria cases by long and short forms from 20 June to the end of September was 92·9:7·1 in June, 71·6:28·4 in July, 59:41 in August and 28:72 in September (Fig. 1). As described above, vivax malaria in the country is prevalent by P. vivax strain containing both predominant long and short incubations.
Vivax malaria is characteristic to relapse due to dormant hypnozoites in the liver.
Based on the analysis on monthly relapse of vivax malaria, relapse cases were reported throughout the year, which drastically increased since May, peak at July and rapidly reduced after then. Ninety per cent of relapse cases were reported between May and September (Fig. 2). As shown in Table 1, short incubation form of vivax malaria occurs in only remote relapse and long form occurs in both short and remote relapses.
From the features mentioned above, vivax malaria in DPR Korea shows high seasonality in reporting malaria cases.
On the basis of clarification of incubation form and relapse characteristics, we could determine the practical significance and implementing point of time for chemoprevention with primaquine corresponding to vivax malaria property prevailing in the country. However, the study was based on epidemiological investigation including travelling history to find incubation and relapse characteristics, and thus further findings should be obtained by molecular parasitological methods such as use of PCR.
Malarial infectious source can be controlled by chemoprevention with treatment of malaria cases from biological characteristics of P. vivax.
As vivax malaria in the country has characteristics of predominant long incubation form, conducting mass chemoprevention with primaquine that acts strongly on hypnozoites of vivax malaria is a reasonable way to reduce the number of cases and to disturb malaria transmission. Primaquine is currently the only medicine for treating relapses of P. vivax and P. ovale malaria, due to its specific activity against malarial hypnozoites, but the medicine induces dose-dependent acute haemolytic anaemia in individuals with G6PDD, a genetically X-linked disorder (WHO, 2016). So investigation on G6PDD should be proceeded in order to implement mass chemoprevention with this medicine safely.
The characteristics of Korean people as a homogeneous nation and the result of nationwide G6PDD investigation (0·45–1·36%: national investigation supported by the WHO in 2013) support a possibility that primaquine can be safely used for mass chemoprevention in the country.
In total, 17·8% of target population did not participate in chemoprevention with primaquine due to various reasons (pregnant or lactational women, and under 5 children). According to malaria epidemiology in the country, malaria proportion among children aged 1–5 years accounts for about 1·5%. This low proportion and toxicity of primaquine let us decide to exclude under 5 children from the chemoprevention. In 1·2% of population, administration of primaquine was not complete due to severe adverse effects including blood urine or brown urine during the chemoprevention process. This figure is similar to G6PDD rate in the country. These results will provide basic information on expecting benefit from chemoprevention and estimating drug needs with national malaria control programme.
Reference Pukrittayakamee, Chantra, Simpson, Vanijanonta, Clemens and LooareesuwanPukrittayakamee et al. compared different durations of treatment for vivax malaria in Thailand and showed that a 30 mg adult dose given twice daily for 7 days was not inferior to the standard 14-day course of 30 mg day−1 once daily and was not associated with a greater incidence of adverse effects (Pukrittayakamee et al., Reference Pukrittayakamee, Imwong, Chotivanich, Singhasivanon, Day and White2010). Our results were inconsistent with Pukrittayakamee et al’s results, in which we observed the incidence of adverse effects by two effective methods (14-day and 7-day regimens) and 1·4–1·7 times more incidence in the 7-day group than in the standard 14-day group. A greater incidence of adverse effects was shown in the female group than the male group (Table 3).
Seven-day regimens are used commonly in the Americas (WHO, 2015). Durand et al. evaluated the efficacy of three primaquine regimens – 0·5 mg kg−1 body weight day−1 for 5 days (150 mg total), 0·5 mg kg−1 body weight day−1 for 7 days (210 mg total) or 0·25 mg kg−1 day−1 for 14 days (210 mg total) – to prevent P. vivax malaria relapses in Loreto, Peru. The relapse rates were similar with the 7- and 14-day regimens (16/156 = 10·3% and 22/162 = 13·6%, P = 0·361) and higher in the 5-day group (48/169 = 28·4%, P < 0·001 and P = 0·001, respectively) (Durand et al., Reference Durand, Cabezas, Lescano, Galvez, Gutierrez and Arrospide2014). Our results are consistent and reconfirm that antirelapse efficacy is directly related to the total dose of primaquine.
We could not find out even an asymptomatic carrier in areas given mass chemoprevention with primaquine. We believe that it is because primaquine would act on blood-stage parasites. We did microscopical test to find asymptomatic carriers 15 days after completion of chemoprevention with only primaquine, and thus we assume that this result is attributable to primaquine. This would support the data that primaquine also has weak but significant activity against blood-stage parasites (Pukrittayakamee et al., Reference Pukrittayakamee, Imwong, Chotivanich, Singhasivanon, Day and White2010).
We observed yearly malaria incidence administering chemoprevention with primaquine in the same season once a year to find a long impact of chemoprevention on malaria incidence. Observation during 4 years showed dramatic reduction of malaria incidence by 0·3% in the fourth year. In conclusion, mass chemoprevention with primaquine before malaria transmission season targeting long-form vivax malaria and relapse in high malarious areas brought about remarkable reduction in malaria incidence of the year.
Study limitation
The study did not establish regimen of chemoprevention with primaquine for people with G6PDD, pregnant or lactational women, under 5 children and contraindications. We did not introduce sophisticated test such as molecular biological method in detecting asymptomatic carriers to increase the detection.
Acknowledgements
The authors are also thankful to the responsible person in National Malaria Control Program who organized epidemiological investigation and provided study conditions possible, and the WHO country office who provided technical support.
Financial support
This work was supported by the Ministry of Public Health, Democratic People's Republic of Korea.
Conflict of interest
None.
Ethical standards
All subjects were adequately informed of the study, and written informed consent for participation was obtained from all subjects.







