Elevated concentrations of dietary cholesterol as well as high cholesterol concentrations in the serum are both associated with an increased risk of atherosclerosis and CHD. A reduction as small as 1 % in serum cholesterol concentrations has been shown to decrease the risk of CHD in human subjects by 2–3 %(Reference Manson, Tosteson and Ridker1). In addition to endogenously synthesised cholesterol, the absorption of dietary cholesterol and the reabsorption of biliary cholesterol in the small intestine also contribute to the regulation of plasma cholesterol concentrations(Reference Wilson and Rudel2). Moreover, reducing the intestinal absorption of dietary and biliary cholesterol can decrease plasma cholesterol concentrations(Reference Gylling and Miettinen3).
It has been demonstrated that the molecular mechanism responsible for intestinal cholesterol absorption requires the Niemann-Pick C1-Like 1 (NPC1L1) protein(Reference Altmann, Davis and Zhu4). Mice lacking NPC1L1 display a substantial reduction in cholesterol absorption(Reference Altmann, Davis and Zhu4), and are completely resistant to both diet-induced hypercholesterolaemia and apoE deficiency-induced atherosclerosis(Reference Davis, Hoos and Tetzloff5, Reference Davis, Zhu and Hoos6). In rats, the expression level of NPC1L1 along the length of the small intestine has been correlated with the efficiency of cholesterol absorption, with the highest level being found in the proximal intestine (duodenum and jejunum) and lower expression being found in the distal intestine (ileum)(Reference Altmann, Davis and Zhu4).
Lactic acid bacteria such as Lactobacillus acidophilus are important, beneficial micro-organisms in the intestines of healthy human subjects(Reference Macfarlane and Cummings7), and they have been associated with several probiotic effects in both human subjects and animals(Reference Fuller8). In fact, the hypocholesterolaemic effects of probiotics have already been demonstrated in various animal and human trials(Reference Simons, Amansec and Conway9–Reference Xiao, Kondo and Takahashi14). Among the beneficial effects that have been described, the reduction of blood cholesterol concentrations is of particular interest(Reference Ebringer, Ferencík and Krajcovic15). It has been proposed that the mechanisms underlying the hypocholesterolaemic activity of probiotics can be attributed to the inhibition of the absorption of exogenous cholesterol in the small intestine by two mechanisms: through the binding and incorporation of cholesterol by bacterial cells, and through the suppression of bile acid reabsorption mediated by bacterial bile salt hydrolysis(Reference Liong and Shah16–Reference Buck and Gilliland19).
Lactobacillus acidophilus is one of the most well-known species of beneficial bacteria that predominantly reside in the small intestine(Reference Robins-Brown and Levine20). Our previous in vitro study indicated that L. acidophilus 4356 reduces cholesterol absorption by down-regulating the expression of NPC1L1(Reference Huang and Zheng21). The present study investigated whether L. acidophilus American Type Culture Collection (ATCC) 4356 affects cholesterol concentrations and NPC1L1 expression in vivo, which may provide a possible molecular mechanism for the modulation of cholesterol concentrations.
Materials and methods
Source and maintenance of bacterial cultures
Lactobacillus acidophilus strain ATCC 4356 was obtained from ATCC (Rockville, MD, USA). Stock culture was stored in 40 % (v/v) glycerol at − 80°C. The organism was subcultured three times before use in sterile de Man, Rogosa, Sharpe broth using 1 % inoculum, and was allowed to grow for 16 h at 37°C. The inoculum was stored at 4°C between transfers.
Preparation of freeze-dried Lactobacillus acidophilus American Type Culture Collection 4356
Lactobacillus acidophilus ATCC 4356 was grown in de Man, Rogosa, Sharpe broth at 37°C for 16 h. The cells were harvested by centrifugation at 2000 g for 20 min, washed twice with sterile distilled water and frozen at − 80°C overnight. The cells were dried under vacuum for 24 h in a freeze dryer (DC400; Yamato, Tokyo, Japan).
Animal feeding and grouping
Twenty male Sprague–Dawley rats (4 weeks of age) were obtained from the National Animal Breeding and Research Centre, Beijing, China. The rats were fed a commercial chow (Kangqiao, Inc., Beijing, China), which included 32 % protein, 5 % fat, 2 % fibre, 1·8 % Ca, 1·2 % P and 59 % N-free extract for 1 week. After this adaptation period, rats were divided into two groups consisting of ten rats each. Group A was fed a high-cholesterol diet only, which included 1 % cholesterol (Aoboxing Biotech, Beijing, China), 10 % lard, 5 % sucrose, 0·3 % sodium cholate (Aoboxing Biotech) and 78·5 % chow. Group B was fed a high-cholesterol diet that was identical to that fed to group A supplemented with freeze-dried L. acidophilus ATCC 4356 at a dose of 109 colony-forming units per d. Rats were individually housed in metal cages at a controlled temperature (23 ± 3°C) and humidity (55 ± 10 %) under a 12 h light–dark cycle. The rats were fed for 4 weeks, during which time, body weight and food intake were recorded daily. After the feeding period, the rats were fasted overnight and used for subsequent testing. Animal experiments were conducted in compliance with the Guide for Care and Use of Laboratory Animals from the National Institutes of Health(22).
Assay for serum lipids
Blood samples were collected from the tail veins of the rats under diethyl ether anaesthesia on days 0, 7, 14, 21 and 28. Approximately, 1 ml of blood was taken from each rat, transferred to sterile tubes and kept on ice for 30 min. The tubes were then centrifuged at 2000 g for 20 min at 4°C. Collected serum samples were analysed to determine serum total cholesterol (TC), HDL-cholesterol, LDL-cholesterol (LDL-C) and TAG concentrations using a commercial kit (Biosino Biotechnology and Science, Beijing, China).
Assay for liver total cholesterol and TAG
After 4 weeks on the appropriate diet, all the rats were euthanised. The livers were removed, rinsed with normal saline solution, blot dried with a filter paper and weighed. The liver TC and TAG levels were determined according to the method of Chiu et al. (Reference Chiu, Lu and Tseng11).
Preparation of samples for RNA and protein measurements
After the 4-week feeding period, rats were euthanised with diethyl ether. The small intestines were removed, flushed with ice-cold PBS and cut into three sections of equal length. The sections were slit lengthwise, and the mucosa was gently scraped, frozen in liquid N2 and stored at − 80°C. Total RNA was extracted from the tissue samples using the RNA STAT-60 (Tel-Test, Friendswood, TX, USA). Total protein was recovered from the organic phase that remained after RNA isolation by precipitating with isopropanol, washing with 0·3 m-guanidine hydrochloride in 95 % ethanol and resuspending the protein pellet in 1 % SDS and 50 mm-Tris–HCl, pH 8·8(Reference Banerjee, Smallwood and Chambers23). The RNA concentration was determined by absorbance at 260 nm, and the protein concentration was determined using the Bio-Rad Protein Assay Kit (Hercules, CA, USA).
Measurement of Niemann-Pick C1-Like 1 mRNA in the small intestines by quantitative real-time PCR
Complementary DNA was synthesised using the complementary DNA Synthesis Kit obtained from Invitrogen (Carlsbad, CA, USA) according to the manufacturer's protocol. Semi-quantitative, SYBR Green-based real-time PCR (Applied Biosystems, Foster City, CA, USA) was used to detect transcripts. Forward and reverse primers were mixed in equal amounts, and were used at a final concentration of 0·2 μm. The following primer sequences were used for PCR: rat NPC1L1, forward: 5′-AACAGCGAGAGGCTCACATT-3′ and reverse: 5′-AGTGGCGTTCATGCCTGCCT-3′; and rat β-actin, forward: 5′-ATTGTGATGGACTCCGGAGA-3′ and reverse: 5′-CAGCTCATAGCTCTTCTCCA-3′. Each experiment was carried out with duplicate samples. The mRNA level in each sample was determined in triplicate. Real-time PCR was performed using the ABI 7500 System for data acquisition, and was analysed using the ABI 7500 System Sequence Detection software. Data are presented as means and standard deviations.
Western blot
Total protein obtained from the lysates of tissue samples was size fractionated on a 6 % SDS-PAGE (40 μg/lane), transferred electrophoretically to a nitrocellulose membrane and then analysed by Western blot. Rabbit anti-rat NPC1L1 and anti-rat β-actin polyclonal antibodies were purchased from Novus Biologicals (Littleton, CO, USA). Immunodetection was carried out with appropriate secondary peroxidase-conjugated antibodies (Pierce Biotechnology, Rockford, IL, USA) and chemiluminescence detection (enhanced chemiluminescence; Amersham, Poole, UK).
Assay for small intestinal and faecal microflora
The mucosal samples were scraped from the small intestine for isolation and enumeration of bacteria. Faecal samples for microbial analysis were collected weekly in separate sterile tubes, and were analysed within 1 h of harvest. Each sample was homogenised using sterile peptone and water diluents. Subsequently, tenfold serial dilutions of each sample were plated in triplicate. Eosin methylene blue agar was used for Escherichia coli, whereas de Man, Rogosa, Sharpe agar was used for lactic acid bacteria, and Bifidobacterium-selective medium agar was used for bifidobacteria. Eosin methylene blue agar and Bifidobacterium-selective medium agar were obtained from Luqiao Tech (Beijing, China).
Statistical analysis
Data are expressed as the means and standard deviations. The statistical significance of the difference between two means at one particular time point was evaluated using Student's t test. Two-way ANOVA with repeated measures was used to compare the effects over time between the two groups. In these tests, values of P < 0·05 were considered significant.
Results
Weight and food intake
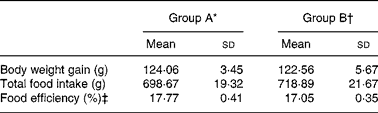
All rats appeared healthy throughout the feeding period. No significant differences in body weight gain, total food intake or food efficiency (P>0·05) were seen between the two groups of rats (Table 1).
Table 1 Body weight gain, total food intake and food efficiency of rats fed a high-cholesterol diet for 4 weeks
(Mean values and standard deviations)
* Group A, high-cholesterol diet only.
† Group B, high-cholesterol diet+Lactobacillus acidophilus American Type Culture Collection 4356.
‡ Food efficiency (%) = (body weight gain/food intake) × 100.
Blood lipid analysis
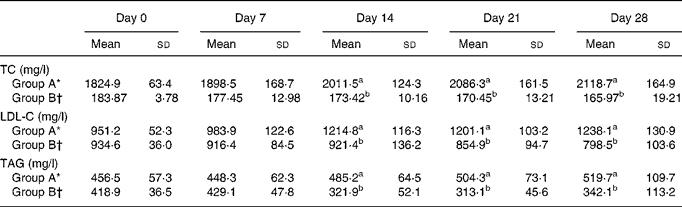
Table 2 shows the effects of dietary cholesterol and L. acidophilus ATCC 4356 on serum cholesterol and TAG levels in rats. The TC, LDL-C and TAG concentrations observed in group B were significantly decreased than those observed in group A. The HDL-cholesterol concentrations, however, did not show a significant difference between the groups.
Table 2 Serum total cholesterol (TC), LDL-cholesterol (LDL-C) and TAG levels in rats fed a high-cholesterol diet
(Mean values and standard deviations)
a,b Mean values with unlike superscript letters were significantly different as assessed by ANOVA (P < 0·05).
* Group A, high-cholesterol diet only.
† Group B, high-cholesterol diet+Lactobacillus acidophilus American Type Culture Collection 4356.
Liver lipid analysis
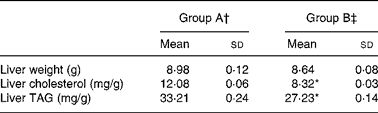
Table 3 shows the data on weight and lipid content of the liver. Average liver weight was not significantly different between the two groups. However, liver cholesterol and TAG concentrations were significantly lower in group B than in group A (P < 0·05).
Table 3 Hepatic cholesterol and TAG levels in rats fed a high-cholesterol diet
(Mean values and standard deviations)
* Mean values were significantly different when compared with the control group by Student's t test (P < 0·05).
† Group A, high-cholesterol diet only.
‡ Group B, high-cholesterol diet+Lactobacillus acidophilus American Type Culture Collection 4356.
Lactobacillus acidophilus American Type Culture Collection 4356 inhibits Niemann-Pick C1-Like 1 expression in the small intestine
To address the mechanism underlying L. acidophilus 4356-mediated inhibition of cholesterol absorption, mRNA levels of NPC1L1 in the small intestines were assayed (Fig. 1(a)). The level of NPC1L1 mRNA varied in different segments of the rat intestine, with the peak expression being observed in the proximal jejunum. In the duodenal and jejunal segments, NPC1L1 mRNA levels detected in group B were significantly lower than those detected in the control group. These decreases in NPC1L1 levels in group B were also confirmed at the protein level (Fig. 1(b)). In contrast, there was no significant difference in NPC1L1 mRNA levels in the ileal segment.

Fig. 1 Consumption of Lactobacillus acidophilus American Type Culture Collection (ATCC) 4356 down-regulates Niemann-Pick C1-Like 1 (NPC1L1) expression in the small intestines of rats. (a) Real-time PCR of NPC1L1 mRNA in the small intestines of rats fed L. acidophilus ATCC 4356 (group B) was compared to that in the small intestines of the controls (group A). The data are presented as the means and standard deviations. * Mean values were significantly different compared with the control group by Student's t test (P < 0·05). (b) Protein expression of NPC1L1 in the small intestines of rats fed L. acidophilus ATCC 4356 (group B) was compared to that in the small intestines of the control group using Western blot. Actin expression serves as a loading control.
Microbial populations in the small intestine
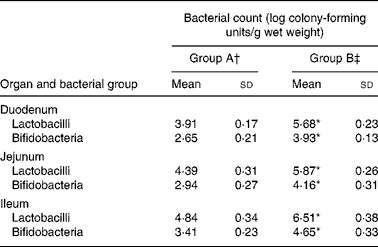
Table 4 shows the counts of total lactobacilli and bifidobacteria in the duodenum, jejunum and ileum in rats fed either the control or the L. acidophilus ATCC 4356-supplemented diet. In both the groups, there was a trend for lactobacilli and bifidobacteria counts to be highest in the ileum than in the more proximal regions of the intestine. In the duodenal, jejunal and ileal segments, both lactobacilli and bifidobacteria counts were significantly greater in rats fed L. acidophilus ATCC 4356 than in rats in group A.
Table 4 Bacterial counts in different regions of the small intestine of rats fed a high-cholesterol diet
(Mean values and standard deviations)
* Mean values were significantly different when compared with the control group by Student's t test (P < 0·05).
† Group A, high-cholesterol diet only.
‡ Group B, high-cholesterol diet+Lactobacillus acidophilus American Type Culture Collection 4356.
Microbial populations in the faeces
Table 5 shows the effect of L. acidophilus ATCC 4356 on the faecal microflora of rats fed a high-cholesterol diet. Significant increases in the total lactobacilli and bifidobacteria populations were observed in the faecal samples of rats fed L. acidophilus ATCC 4356 than in those of the control group. However, faecal levels of lactobacilli and bifidobacteria in rats fed a cholesterol-enriched diet without L. acidophilus 4356 supplementation remained unchanged throughout the experimental period. The total E. coli count did not significantly change between the two groups during the entire experimental period.
Table 5 Population of lactobacilli, bifidobacteria, and Escherichia coli from faeces of rats fed a high-cholesterol diet
(Mean values and standard deviations)
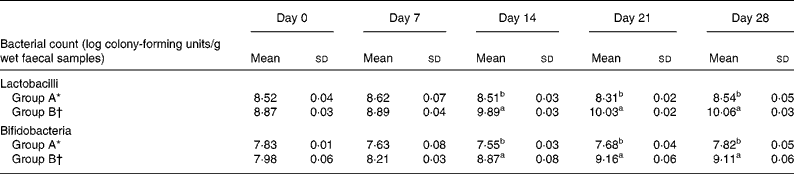
a,b Mean values with unlike superscript letters were statistically different as assessed by ANOVA (P < 0·05).
* Group A, high-cholesterol diet only.
† Group B, high-cholesterol diet+Lactobacillus acidophilus American Type Culture Collection 4356.
Discussion
High concentrations of TC and LDL-C are strongly associated with an increased risk of CHD. A reduction in the TC and LDL-C concentrations in hypercholesterolaemic men has been shown to reduce the incidence of CVD(Reference Probstfield and Rifkind24). The modification of diet, such as the inclusion of fermented dairy products or lactic acid bacteria-containing dairy products, is one way through which serum cholesterol can be reduced(Reference Park, Kim and Shin12). In agreement with previous reports(Reference Danielson, Peo and Shahani25–Reference Usman and Hosono27), the present study demonstrated that ingestion of L. acidophilus ATCC 4356 results in a reduction of the serum TC, LDL-C and TAG concentrations of rats fed a high-cholesterol diet. Since significant reductions in cholesterol and TAG concentrations were also observed in the liver of rats in the L. acidophilus ATCC 4356-fed group, it can be suggested that the cholesterol concentration was actually reduced, and not merely re-distributed between the blood and liver. No significant differences in HDL-cholesterol concentrations were observed in our study in agreement with previous results in rats and human subjects reported by Abd El-Gawad et al. (Reference Abd El-Gawad, El-Sayed and Hafez28), Fukushima & Nakano(Reference Fukushima and Nakano29) and Wang et al. (Reference Wang, Xu and Xi13).
In contrast to the present results, some researchers(Reference Thompson, Jenkins and Amer30, Reference St-Onge, Farnworth and Savard31) have not observed hypocholesterolaemic effects from lactic acid bacteria consumed by mice or human subjects. Many researchers(Reference Akalin, Gönç and Düzel32, Reference Taranto, Medici and Perdigon33) have suggested that these conflicting results may be due to the different properties of the cultures used (e.g. acid and bile tolerance). Other important factors that may affect the results include the amount of bacteria ingested, the cholesterol content of the diet under study, the animal used and the length of the feeding period.
Since cholesterol absorption primarily occurs in the duodenum and proximal jejunum, with little absorption by the ileal segment of the intestine(Reference Grundy34, Reference Borgström35), we investigated NPC1L1 mRNA expression along the duodenum–ileum axis. The level of NPC1L1 mRNA varied in the different segments of rat intestine, with the peak expression being detected in the proximal jejunum, a result that is in agreement with a study done by Altmann et al. (Reference Altmann, Davis and Zhu4). Lactobacillus and Bifidobacterium counts in the small intestines of rats fed L. acidophilus ATCC 4356 were significantly greater than those in the small intestines of rats solely fed the high-cholesterol diet. Moreover, NPC1L1 mRNA levels in the duodenal and jejunal segments of rats in the L. acidophilus ATCC 4356-fed group were significantly lower than those in the duodenal and jejunal segments of the control group. In contrast to previous studies, the present results indicate that L. acidophilus ATCC 4356 is able to reduce cholesterol absorption by inhibiting NPC1L1 mRNA transcription in the small intestine.
In our study, the number of faecal lactobacilli and bifidobacteria in the L. acidophilus ATCC 4356-fed group is greater than that observed in the high-cholesterol group. This indicates that L. acidophilus ATCC 4356 can successfully tolerate the gastric acid and bile salts of the small intestines, and still retain biological activity. As Donnet-Hughes et al. (Reference Donnet-Hughes, Rochat and Serrant36) have suggested, bacterial survival in the faeces following oral administration reflects successful bowel colonisation and proliferation that are required for the initiation of biological effects.
Many studies have shown that lactic acid bacteria inhibit the proliferation of pathogenic bacteria, improve the intestinal microflora composition, reduce the risk of diseases and promote the health of the host(Reference Fuller8, Reference Seow, Cai and Rahmat37, Reference van Winsen, Keuzenkamp and Urlings38). However, in rats of both group A and group B, the faecal E. coli level was constant throughout the feeding period. These results indicate that the growth of E. coli is not inhibited by L. acidophilus ATCC 4356 in vivo.
In conclusion, in addition to contributing to a healthy microbial balance in the bowels, L. acidophilus ATCC 4356 also exerted a significant hypocholesterolaemic effect on rats fed a high-cholesterol diet through inhibition of NPC1L1 expression in the small intestines. These findings suggest a novel mechanism that may underlie probiotic-mediated cholesterol reduction. The mechanism proposed here differs from conclusions drawn by previous studies, which have implicated the incorporation of cholesterol into cellular membranes or the deconjugation of bile salts during bacterial growth as the underlying mechanism. However, it will be necessary to conduct more extensive animal studies, using varying doses of bacteria over longer times, to fully assess the long-term cholesterol-lowering potential of L. acidophilus ATCC 4356.
Acknowledgements
The authors gratefully acknowledge financial support from the Scientific Foundation of Jilin Province (grant no. 2008026). The authors performed this experiment together, and contributed to the completion of the manuscript. Y. H. designed the study, performed the data analysis and contributed to the drafting of the paper. Y. Z., J. W. and Y. C. performed the data analysis. The authors declare no conflict of interest.








