INTRODUCTION
Helicobacter pylori is a major cause of gastroduodenal diseases like gastritis, peptic ulcer, gastric carcinoma (GC) and mucosal associated lymphoid tissue lymphoma [Reference Parsonnet1, Reference Nakamura2]. The prevalence of H. pylori is very heterogeneous in Asia with some populations having extremely low prevalence, e.g. Malays [Reference Lee3]. In developing countries, like Pakistan and Bangladesh, infection with H. pylori is more frequent in the general population and is acquired at an early age. It has been shown previously that H. pylori is acquired by most individuals in early childhood [Reference Jafri4]. In Pakistan, the prevalence of H. pylori seropositivity in children aged 11–15 years was 53·5% [Reference Jafri4]. As H. pylori is transmitted via the gastro-oral and faeco-oral route, overcrowding, poor sanitation, lower socioeconomic status and poor water supply are the major factors that result in its acquisition at a higher frequency and lower age in less developed Asian countries [Reference Mazumder and Ghoshal5, Reference Sarker6]. By contrast, in industrialized and developed countries like the United States, the prevalence of H. pylori has decreased [Reference Everhart7]. In Asian countries like Japan, Singapore and China, the frequency of H. pylori infection has been reported to be somewhat lower [Reference Miwa8]. By contrast, people living in less developed countries of Asia with high frequency of H. pylori infection [Reference Jafri4–Reference Sarker6] acquired at an earlier age have the lowest risk of developing GC [Reference Miwa8]. It is interesting to note that in Japan despite a lower frequency of H. pylori infection, this country has the highest frequency of GC.
Genetic diversity among H. pylori strains and host characteristics play a role in the varying clinical outcomes in persons colonized with H. pylori. Candidate markers for distinguishing disease-associated H. pylori strains from less virulent strains include the presence of the cag pathogenicity island, vacA alleles s1/m1, induced on contact with epithelium (iceA types 1/2), intact outer immunoprotein A (oipA) alleles, outer membrane protein (OMP) Q (hopQ), etc. [Reference Wotherspoon9–Reference Kersulyte13]. However, presence of these OMPs and virulence markers does not always correlate well with gastroduodenal diseases [Reference Abadi and Lee14].
The inflammatory immune response during acute H. pylori infection of the gastric epithelium triggers a mutation burst and an increased frequency of mutation and recombination events occur in H. pylori OMP genes [Reference Linz15, Reference Linz16]. It is known that changes in immunogenic OMPs facilitates rapid adaptation of H. pylori to an individual host, evasion of the host's immune system resulting in chronic infection [Reference Linz16]. Horizontal transmission of H. pylori is common in populations living in low socioeconomic conditions found in developing countries and is known to be associated with infection by multiple H. pylori strains [Reference Schwarz17, Reference Ghose18]. In view of the high frequency of DNA transformation and lateral gene transfer reported in H. pylori strains we wanted to see whether there was any differences in the H. pylori strains isolated from overseas Pakistani residents (expatriates) compared to local residents. Existence of differences may suggest that expatriates’ H. pylori genome acquires characteristics of the country of temporary residence. It is also possible that these newly acquired H. pylori genomic characteristics may be transfered to the H. pylori gene pool of their country of origin once individuals return. There is a high prevalence of virulent H. pylori strains in countries like China, South Korea and Japan that are known to be associated with severe gastroduodenal diseases.We studied the prevalence of H. pylori, its virulence marker, e.g. cagA, cagA promoter, vacA alleles s1a/1b, m1, m2 and s2, iceA types 1 and 2 and OMP Q (hopQ) in expatriates and compared them with those in local residents.
MATERIALS AND METHODS
Patients
Three hundred and nine patients were enrolled from an endoscopy suite providing upper gastrointestinal endoscopy for upper gastrointestinal symptoms and who were positive by rapid urease test for H. pylori infection at endoscopy. There were 236 (76%) males and 73 (24%) females with a mean age of 45 ± 13 years (range 18–79 years). These patients attended the gastroenterology outpatient and endoscopy suite from January 2013 to December 2014. Of these, 207 (67%) were local residents with a mean age of 46 ± 14 years (male:female ratio 148:59) while 102 (33%) were expatriates with a mean age of 44 ± 9 years (male:female ratio 88:14). There was no significant difference in the mean age of the two groups. There were significantly (P = 0·004) fewer female patients in the expatriate group compared to local residents due to males being the family earner and travelling abroad in the Pakistani culture. These expatriate patients had lived abroad for more than 10 years for various reasons and had moved in their mid-twenties to a foreign country. These expatriates included 48 (15%) from China; 43 (14%) from South Korea and 11 (4%) from Japan. These expatriates had lived abroad for more than 10 years and had paid infrequent visits to their country of origin for social reasons or in seeking medical healthcare. The study was approved by the Ethics Review Committee of Aga Khan University. All patients gave informed consent for endoscopy and participation in the study. None of the patients had received antibiotics, acid reducing drugs such as H2 receptor antagonists, proton pump inhibitors, non-steroidal anti-inflammatory drugs or bismuth compounds in the last 8 weeks. The clinical symptoms at the time of presentation and endoscopic findings were noted. Gastric biopsy specimens were taken from an area of inflammation in the antrum and corpus. Two biopsy specimens were taken for each of: rapid urease test (Pronto Dry, Medical Instruments Corporation, Switzerland), histology and polymerase chain reaction (PCR). Two gastric biopsy specimens were used for a rapid urease test (Pronto Dry). Specimens for histology were dispatched in formalin and for PCR in 0·9% normal saline. PCR for cagA 5′-terminal, cagA promoter region, vacA alleles for the signal (s), i.e. s1a, s1b, s2 and middle (m), i.e. m1, m2, iceA (types 1 and 2) and hopQ alleles (types 1 and 2) were analysed.
Bacterial culture
The specimens were transported immediately in sterile normal saline to isolate H. pylori. Each specimen was homogenized in a sterile Eppendorf tube with electric homogenizer and inoculated onto Columbia blood agar (Oxoid, UK) medium supplemented with 10% defibrinated sheep blood and Dent's supplement (containing vancomycin, trimethoprim, cefsulodin and amphotericin B) and incubated at 37 °C under microaerobic conditions using anaerobic jars and strips (Campygen strips, Oxoid) for isolation and growth for 5–7 days. Plates were then examined for bacterial growth and typical colonies were selected for identification. The identity of H. pylori was confirmed by Gram stain, and production of urease and catalase. H. pylori isolates were defined as Gram-negative spiral-shaped bacilli that were catalase positive and rapidly (<30 min) urease positive.
Histology
Gastric biopsy specimens for histopathology were stained with haematoxylin and eosin (H&E) stain for the detection of H. pylori and degree of gastritis. The degree of gastritis as determined on H&E stain was scored in accordance with the Sydney system [Reference Price19].
Extraction of genomic DNA
The bacterial cells on chocolate agar plate were washed twice with phosphate-buffered saline (PBS, pH 8·0) then centrifuged at 1008 g for 20 min. H. pylori DNA was extracted by the phenol/chloroform method similar to a method described previously [Reference Yakoob20].
PCR
cagA and vacA genotyping
Amplification of cagA, cagA promoter, and vacA alleles by PCR was performed in a volume of 25 µl containing 10 mmol/l Tris–HCl (pH 8·3), 50 mmol KCl, 1·5–2·5 mmol/l MgCl2, 200 μmol/l deoxynucleoside triphosphates, 2 U Taq DNA polymerase (Promega, USA) and 25 pmol of both forward and reverse primers (MWG automatic synthesizer, Germany) (Table 1) as used previously [Reference Covacci and Rappuoli21]. PCR was performed in a PerkinElmer 9700 thermal cycler (PerkinElmer, USA). The amplification cycles for cagA, cagA promoter, and vacA alleles are given in Table 1. Positive and negative reagent control reactions were performed with each batch of amplifications. DNA from H. pylori strains ATCC 43504 (vacA s1am1, cagA positive), ATCC 51932 (vacA s2m2, cagA negative) and ATCC 43526 (vacA s1bm1, cagA positive) was used to define the accuracy of the cagA and vacA alleles [Reference Covacci and Rappuoli21]. After PCR, the amplified PCR products were electrophoresed in 2% agarose gels containing Tris/acetate/EDTA acid, stained with ethidium bromide, and visualized under a short wavelength ultraviolet light source.
Table 1. Oligonucleotide primers used in typing of Helicobacter pylori

Source: Covacci & Rappuoli [Reference Covacci and Rappuoli21]; Van Doorn et al. [Reference Van Doorn22]; Cao & Cover [23].
iceA genotyping
For analysis of the iceA genotype, primers previously described [Reference Van Doorn22] were used. Primers iceA1 F and iceA1R yielded a fragment of 247 bp for the iceA1 allele, and primers iceA2 F and iceA2R yielded a fragment of 229 or 334 bp, respectively, according to the existence of repeated sequences of 105 nt.
hopQ genotyping
The hopQ genotype (types 1 and 2) were determined by PCR methods [Reference Cao and Cover23]. Primers and conditions used for PCR amplification of hopQ sequences of types 1 and 2 are shown in Table 1. Primers used for PCR amplification of hopQ alleles are given in (Table 1) [Reference Cao and Cover23].
Sample size
Two different sample sizes were calculated in keeping with the aim of the study. The first sample size was calculated to estimate the prevalence of H. pylori in overseas Pakistani residents (expatriates), taking the prevalence of 58% (as reported in the Pakistani population) that gives the maximum sample size, with 95% level of confidence and 6% bound on the error of estimation [Reference Jafri4]. After accounting for a non-response rate of about 10%, the minimum sample size required is about 285 participants using the formula [Reference Eng24]:
The second sample size of 160 was derived using the formula
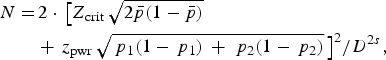 $$\eqalign{N = &\,2 \cdot \,\big[{Z_{{\rm crit}}}\,\sqrt {2\bar p(1 - \bar p)} \cr & + \,{z_{{\rm pwr}}}\,\sqrt {{\,p_1}(1 - {\,p_1})\, + \,{\,p_2}(1 - {\,p_2})} \,{\big]^2}/{D^{2s}},}$$
$$\eqalign{N = &\,2 \cdot \,\big[{Z_{{\rm crit}}}\,\sqrt {2\bar p(1 - \bar p)} \cr & + \,{z_{{\rm pwr}}}\,\sqrt {{\,p_1}(1 - {\,p_1})\, + \,{\,p_2}(1 - {\,p_2})} \,{\big]^2}/{D^{2s}},}$$
assuming that 73 patients in each group will help achieve a 5% significance level using a two-sided equivalence test of proportions [Reference Eng24]. This number of patients would provide the study with the ability to detect a 20% difference in the H. pylori virulence marker and HopQ protein in local residents and expatriates, with a power of 80%. Taking into account a dropout rate of 10%, the final sample size of 300 participants would suffice for both objectives.
Statistical analysis
The descriptive analysis was performed for demographic and clinical features. Results were presented as mean ± standard deviation for quantitative variables and number (percentage) for qualitative variables. Differences in proportions were assessed using Pearson's χ 2, Fisher's exact or the likelihood ratio tests, as appropriate. To assess the univariate association between the outcomes and potential factors, odds ratios (ORs) and their 95% confidence intervals (CIs) were computed by logistic regression analysis. All significant factors in the univariate analysis were considered for inclusion in the multivariable logistic model. A P value of <0·05 was considered statistically significant. All P values were two sided. Statistical interpretation of data was performed using the computerized software program SPSS v. 19.0 (IBM Corp., USA).
RESULTS
Abdominal pain was significantly more common in local residents (183, 88%) compared to expatriates (72, 71%) (P < 0·001), while GC was diagnosed in 30 (29%) expatriates compared to 24 (12%) local residents (P < 0·001) (Table 2).
Table 2. Comparison of residents symptoms, diagnosis and histological changes in local citizens and expatriates

Values given are n (%).
H. pylori OMP (hopQ) types
In local residents, hopQ type 1 was associated with abdominal pain in 84 (86%, P = 0·001), gastric erythema on endoscopic examination (GST) in 63 (64%, P < 0·001), chronic active gastritis in 65 (66%, P = 0·002), and cagA was positive in 56 (57%, P = 0·005) individuals. hopQ type 2 was also associated with abdominal pain in 158 (90%, P = 0·02), GST in 122 (69%, P = 0·014) and vacA s1a in 119 (68%, P = 0·016) local residents.
In expatriates, hopQ was associated with abdominal pain in 62 (70%, P = 0·001) and weight loss in 22 (25%, P = 0·001) individuals, GST in 32 (36%, P = 0·019) and GC in 30 (34%, P = 0·009) individuals, respectively. hopQ type 1 was associated with histological mild gastritis in 43 (49%, P < 0·001), cagA in 82 (93%, P < 0·001) and cagA promoter in 81 (92%, P < 0·001) individuals, respectively. hopQ type 2 was associated with abdominal pain in 46 (77%, P < 0·001), and GST in 28 (47%, P < 0·001) individuals. On histology, hopQ type 2 was associated with histological mild gastritis in 35 (58%, P < 0·001) local residents, and in expatriates, it was associated with vacA s1a in 34 (57%, P < 0·001) individuals.
A single hopQ type was present in 105 (58%) local residents compared to 56 (55%) expatriates (P = 0·563), while multiple types of hopQ in were present in 76 (42%) local residents compared to 46 (45%) expatriates (P = 0·563). Both types were absent in one (0·5%) local resident (P = 0·563).
cagA and cagA promoter
H. pylori virulence marker cagA was positive in 97 (47%) local residents compared to 86 (84%) expatriates (P < 0·001), while cagA-P was positive in 72 (35%) local residents compared to 87 (85%) expatriates (P < 0·001) (Table 3).
Table 3. Comparison of Helicobacter pylori virulence markers in the study groups

Values given are n (%).
* P < 0·05 significant.
vacA alleles
There was no difference noted in the distribution of vacA allele s1a, s1b and m1 in local residents and expatriates (Table 3).
iceA types
iceA was positive in 157 (76%) and iceA type 2 in 81 (39%) local residents, respectively (Table 4). iceA type 1 was associated with gastritis (non-ulcer dyspepsia; NUD) in 95 (61%) and GC in 24 (15%) (P < 0·001) individuals. It was also associated with intestinal metaplasia in 32 (80%, P < 0·001) individuals. iceA type 1 was associated with cagA, cagA promoter and vacA s1a in 86 (55%, P < 0·001), 68 (43%, P < 0·001), and 95 (60%, P = 0·047), individuals, respectively.
Table 4. Multivariable model for factor predicting expatriate Helicobacter pylori virulence

OR, Odds ratio; CI, confidence interval.
Values given are n (%).
* P < 0·05 significant.
In expatriates distribution of iceA type 1 was 45 (44%) and iceA type 2 was 86 (84%), respectively. iceA type 1 was associated with abdominal pain, lymphoid aggregates, intestinal metaplasia and mild gastric mucosal inflammation while iceA type 2 was associated with GST and GC. On histology iceA type 2 was also associated with chronic active gastritis, lymphoid aggregates and mild to moderate degree of inflammation. iceA type 1 was associated with cagA [44 (98%), P = 0·001] and cagA promoter [45 (100%), P < 0·001], respectively.
Multiple iceA type was positive in 29 (28%) expatriates compared to 33 (18%) local residents (P = 0·044). iceA types were absent in 11 (6%) local residents compared to none in expatriates (P = 0·009). The H. pylori genotype cagA iceA2 in local residents was 40 (19%) compared to 67 (66%) in expatriates (P < 0·001).
Multivariate analysis showed that expatriates’ H. pylori strains were associated with moderately severe mucosal inflammation (OR 4·75, 95% CI 1·49–15·08, P = 0·008), lymphoid aggregates (OR 7·25, 95% CI 2·10–25·0, P = 0·002), hopQ type 1 (OR 3·19, 95% CI 1·06–9·63, P < 0·03), cagA promoter (OR 22·54, 95% CI 7·53–67·40, P < 0·001), vacA m2 (OR 0·075, 95% CI 0·02–0·23, P < 0·001), vacA s2 (OR 8·77, 95% CI 2·87–26·74, P < 0·001) and iceA type 1 (OR 0·03, 95% CI 0·01–0·10, P < 0·001) (Table 4).
DISCUSSION
This study shows that patients in both groups presented with abdominal pain associated with NUD. Weight loss was common in expatriates and was associated with GC. Gastric mucosal changes of H. pylori infection were evident in expatriates as chronic active inflammation while mild chronic inflammation was more common in local residents. The virulence marker cagA and cagA promoter region positive H. pylori infection were frequent in expatriates and was associated with severe H. pylori related pathology (Table 3). No difference was noted in the distribution of vacA alleles s1a, s1b and m1 in the two groups (Table 3). H. pylori infection in expatriates was predominantly with iceA type 2 and it was significantly associated with vacA s1a [58 (67%), P = 0·036]. However, in this group, iceA type 1 was also seen to be associated with cagA [44 (98%), P = 0·001] and cagA promoter region [45 (100%), P < 0·001] (Table 3). In local residents, hopQ type 2 was predominant compared to hopQ type 1 in expatriates (Table 2). In both groups there were H. pylori strains that demonstrated multiple types of hopQ. Their number was greater in expatriates compared to local residents suggesting co-infection was marked in expatriate patients.
The implications of this study are that NUD with H. pylori infection was also common in expatriates residing abroad for a lengthy period. Gastric ulcer was marginally significant in expatriates compared to local residents, whereas duodenal ulcer was not. GC in expatriates was associated with chronic active inflammatory changes and intestinal metaplasia.
The distribution of H. pylori virulence markers in expatriates was different from local residents in that H. pylori were 84–85% cagA and cagA promoter positive compared to the distribution of 45–51% previously described in local residents [Reference Yakoob25, Reference Khan26]. The expatriates’ H. pylori strain increase in cagA and cagA promoter region positivity is in keeping with H. pylori strains described in East Asian countries. In a previous local study that looked at the intactness of the cag pathogenecity activity island (cag-PAI) in 115 clinical strains of H. pylori only 31 (28%) were positive for the five cag-PAI loci [Reference Yakoob27]. It has been reported that the iceA allelic type distribution was independent of cagA and vacA status. Previously, a significant association between the presence of the iceA type 1 allele and peptic ulcer disease has been described [Reference Ilver28]. The iceA type 1 allele is reported to be predominant in Japan and Korea, and the iceA type 2 allele in the United States and Colombia [Reference Van Doorn29]. In expatriates, infection with H. pylori strains demonstrating multiple iceA types was frequent at 28% compared to 18% in local residents.
The wide CI of factors in the multivariable analysis suggests that the study may be underpowered (Table 4). However, the findings from the multivariable analysis support our conclusion that expatriates have more virulence markers and also more gastroduodenal diseases. Further, significance of vacA m2 and iceA type 1 with ORs of 0·075 and 0·03, respectively, in local resident suggests that they are associated with nonulcer gastritis and less florid H. pylori associated disease in keeping with our previous results [Reference Yakoob25, Reference Yakoob30].
There was clearly a difference in the distribution of virulence marker and hopQ types in local residents and expatriates. This is in keeping with the exogenous DNA taken up by H. pylori and its integration into the chromosome by homologous recombination or replication as a plasmid [Reference Suerbaum and Josenhans31]. For survival and persistent growth in the presence of a constant immune response permanent adaptation of the bacteria is required [Reference Suerbaum and Josenhans31]. Such adaptive processes include mechanisms of reversible or irreversible genome changes. It is known that clonal transmission is followed by a rapid adaptation to the new host, so that H. pylori isolates from different subjects are almost always unique [Reference Suerbaum and Josenhans31]. Each H. pylori isolate contains a distinct set of strain-specific genes often located in plasticity regions. They generally contain complete sets of genes required to produce type IV secretion machineries, as well as genes encoding different DNA-processing proteins [Reference Fischer32–Reference Kersulyte34] that are mobile genetic elements capable of horizontal gene transfer between bacterial cells. In an earlier study which was conducted in the Malaya population [Reference Alfizah35], the virulence genotype of H. pylori strain was different in the immigrant Chinese and Indian population but the native Malays showed a mixture of both [Reference Alfizah35].
The limitation of this study includes an absence of information regarding smoking and salt intake that are independent risk factors for GC. Dietary intake of red meat, high fat, and heavy alcohol use positively influences carcinogenesis while fresh fruit, vegetables and vitamin C reduce the risk. Certain dietary constituents of local cuisine reduce H. pylori viability, colonization and infection may also reduce the GC risk [Reference Yakoob36–Reference Lee and Derakhshan38]. In this study, the chance of possible selection bias could not be ignored. All the patients attending the endoscopy suite of the tertiary-care private hospital in the study period were included. All expatriates and residents were sufficiently well off financially to access healthcare services at this hospital. There is a possibility of information bias in regards to the length of time expatriates had spent in the foreign country. The stated length of time all expatriates were away was >10 years and all left their country of origin as adults. An earlier study from China foundthat children aged 5–6 years and adults had comparable rates of H. pylori infection at ~70% [Reference You39]. A review of H. pylori prevalence in USA found that individuals who immigrated as adults (aged >20 years) had a rate of infection, consistent with their country of origin [Reference Jones40]. Moreover, spontaneous elimination of H. pylori infection was found rarely in adults [Reference Lin and Koskella41]. Socioeconomic factors play a distinct role in transmission due to differences in waste disposal, hygiene, and practices such as sharing of eating utensils, e.g. chopsticks and premastication by parents [Reference Dowsett and Kowolik42]. There is a possibility that non-viable oral H. pylori participate in horizontal gene transfer that is increased in unsanitary, crowded environments, making multiple infection and recombination among strains more common in populations living in crowded conditions [Reference Jones40, Reference Kodaman43]. Hence, these expatriates were likely to have maintained their H. pylori infection and experienced change in their infecting H. pylori strains’ dynamic genome.
In conclusion, the local residents and expatriates demonstrated differences in the distribution of H. pylori virulence marker cagA, cagA promoter, iceA and hopQ types. In expatriates severe gastroduodenal disease could be explained by the distribution of virulence markers, although the causes of GC and gastroduodenal diseases are multifactorial in nature.
DECLARATION OF INTEREST
None.







