Colorectal cancer is the third most common cancer type, with 1·4 million cases diagnosed worldwide in 2012( Reference Ferlay, Soerjomataram and Dikshit1 ). While the incidence, in general, has been increasing, the mortality has been decreasing in most regions( Reference Ferlay, Soerjomataram and Ervik2 , Reference Siegel, Miller and Fedewa3 ), and thus, there are large number of colorectal cancer survivors. In the USA alone, there are more than 1·2 million colorectal cancer survivors, and the number is expected to increase to more than 1·5 million by 2024( Reference DeSantis, Lin and Mariotto4 ). While there is strong evidence of diet and physical inactivity playing a role in colorectal cancer( 5 ), the influence of dietary and lifestyle risk factors on survival among colorectal cancer patients remains much less studied. There are indications of a Western dietary pattern being associated with higher overall mortality( Reference Schwedhelm, Boeing and Hoffmann6 ). Specifically, in the American cohorts Nurses’ Health Study and Health Professionals Follow-up Study, intake of whole grains has been associated with better survival among people diagnosed with colorectal cancer( Reference Song, Wu and Meyerhardt7 ).
Incidence rates of colorectal cancer are higher among men than among women, and it has been hypothesised that this is partly due to a protective effect of oestrogen. Two types of oestrogen receptors (ER) have been identified, ERα and ERβ, with the latter predominantly expressed in both malignant and normal colonic epithelium, but with decreasing expression with cancer progression( Reference Caiazza, Ryan and Doherty8 , Reference Niv9 ). Furthermore, ERβ is shown to inhibit tumour cell proliferation, whereas ERα may increase cell proliferation( Reference Kennelly, Kavanagh and Hogan10 ). Phyto-oestrogens have also been suggested as protective against colorectal cancer due to the oestrogen-like structure, and phyto-oestrogens have higher affinity for ERβ than ERα ( Reference Kennelly, Kavanagh and Hogan10 ). In Europe and North America, plant lignans are the main type of phyto-oestrogens consumed deriving from, for example, whole grains and fibre-rich vegetables; however, seeds such as sesame seed and flaxseed have the highest content, but are less consumed( Reference Zamora-Ros, Knaze and Lujan-Barroso11 – Reference de Kleijn, van der Schouw and Wilson13 ). In Asia, isoflavonoids from, for example, soya products remain the most studied, and a high intake has been related to lower colorectal cancer risk in Asian populations( Reference Jiang, Botma and Rudolph14 ). A previous study based on the same cohort as the present study found enterolactone, the main microbial-derived metabolite of plant lignans, to be associated with lower colorectal cancer incidence among women, but with a higher risk among men( Reference Johnsen, Olsen and Thomsen15 ). A recent meta-analysis, which investigated the association between enterolactone concentration, plant lignan intake and colorectal cancer risk( Reference Jiang, Botma and Rudolph14 ) found no significant associations. However, the results were based on only four cohort studies. Thus, few studies have been conducted, and colorectal cancer survivorship remains almost unstudied.
When plant lignans are ingested, they are converted into primarily enterolactone by the gut microbiota. Therefore, the circulating level of enterolactone depends on the intake of lignans and on the capacity of the gut microbiota to convert plant lignans to mainly enterolactone. Microbial disturbing factors such as antibiotics may affect the enterolactone production( Reference Bolvig, Kyrø and Norskov16 ), and other factors like body fatness and smoking have also been related to lower concentrations( Reference Kilkkinen, Stumpf and Pietinen17 , Reference Johnsen, Hausner and Olsen18 ). Phyto-oestrogens share structural similarities with endogenous oestrogens and can exert agonist or antagonist effects on the ER( Reference Mueller, Simon and Chae19 ). Another much more studied type of exogenous oestrogen is menopausal hormone therapy. Use of exogenous oestrogens has been related to lower risk( Reference Lin, Cheung and Lai20 ), especially for ERβ-positive cancer( Reference Rudolph, Toth and Hoffmeister21 ). A recent cohort study found the use of menopausal hormone therapy postdiagnosis to be associated with lower risk of colorectal cancer mortality( Reference Ji, Sundquist and Sundquist22 ). However, overall the role of menopausal hormone therapy in colorectal cancer aetiology and progression is complex and remains largely undetermined.
From both epidemiological and mechanistic studies, it seems plausible that enterolactone could play a role in colorectal cancer progression. The aim of the present study was to investigate the association between prediagnosis plasma concentrations of enterolactone among persons diagnosed with colorectal cancer in relation to all-cause and cause-specific mortality. We hypothesised that high enterolactone concentrations were associated with lower all-cause and colorectal cancer-specific mortality.
Methods
Study population
The ongoing Danish cohort study ‘Diet, Cancer and Health’ consists of 57 053 men and women (with response rates of 34 and 37 %, respectively). The participants were recruited in 1993–1997, and they were 50–64 years of age at invitation( Reference Tjonneland, Olsen and Boll23 ). At recruitment, participants completed a validated 192-item FFQ( Reference Tjonneland, Overvad and Haraldsdottir24 ), a lifestyle questionnaire, and had anthropometric measurements taken by trained personnel. From each participant, a total of 30 ml blood was drawn, spun and divided into plasma, serum and ‘buffy coat’. The samples were processed and frozen within 2 h at −20°C and thereafter stored in liquid N2 (maximum −150°C)( Reference Tjonneland, Olsen and Boll23 ).
Incidence of colorectal cancer
In all, 57 053 participants were followed from recruitment (1993–1997) for colorectal cancer incidence until end of 2009. Through linkage to the Danish Cancer Registry( Reference Gjerstorff25 ), which holds information on all cancer cases in Denmark, 1003 first incident primary colorectal cancer cases with plasma samples available in the biobank were identified. The colorectal cancer cases were identified as codes C18–C20 in the 10th revision of the International Statistical Classification of Disease, Injury and Causes of Death-10. Proximal colon cancers include cancers of the caecum, appendix, ascending colon, hepatic flexure, transverse colon and splenic flexure (C18 : 0–C18 : 5); distal colon cancer includes the descending (C18 : 6) and sigmoid colon (C18 : 7). Overlapping (C18 : 8) and unspecified lesions (C18 : 9) were grouped as ‘overlapping/unspecified’, and cancers of the rectosigmoid junction (C19 : 9; region at the transition between colon and rectum) of the colon were grouped among all colon cancers and grouped as ‘rectosigmoid junction’ (C18 : 0–C18 : 9). Cancers of the rectum (C20) were grouped as rectal cancer.
Laboratory analyses: plasma concentrations of enterolactone
Plasma concentration of enterolactone was successfully measured in 1002 of the 1003 plasma samples from participants later diagnosed with colorectal cancer. A high-throughput liquid chromatography–tandem mass spectrometry (LC–MS/MS) was used to measure enterolactone as glucuronide-conjugated, sulphate-conjugated and in free form( Reference Norskov, Kyrø and Olsen26 ). Previous studies have used a fluoro-immunoassay method that applies enzymatic hydrolyses for deconjugation of enterolactone and further time-consuming diethyl ether extraction( Reference Adlercreutz, Wang and Lapcik27 ). The method used in the present study consists of simple solid-phase extraction using ninety-six-well plates combined with short LC–MS/MS run, which offers the handling of 192 samples per d. The used LC–MS/MS method furthermore has superior selectivity and sensitivity and can quantify enterolactone in its intact forms as conjugate of glucuronic and sulphonic acids and as free enterolactone without any modification of sample( Reference Norskov, Kyrø and Olsen26 ).
Endpoints and clinical characteristics
Information on vital status, date of death and cause of death was obtained from The Danish Civil Registration System and The Danish Register of Causes of Death( Reference Helweg-Larsen28 , Reference Pedersen29 ). Information on date of disappearance or emigration was from The Danish Civil Registration System( Reference Pedersen29 ). End of follow-up was 31 December 2015.
From the database of the Danish Colorectal Cancer Group (DCCG), the following information was available: physical status of the patient at time of diagnosis according to the American Society of Anesthesiologists (ASA) physical status classification system (ASA score)( 30 ), Charlson comorbidity index( Reference Charlson, Szatrowski and Peterson31 ), clinical characteristics (cancer stage) and information on cancer treatment received (surgery with curative aim)( Reference Ingeholm, Gogenur and Iversen32 ). The DCCG holds population-based clinical data since May 2001. Study participants of the present study that were not subsequently confirmed to have primary colorectal adenocarcinoma in DCCG (e.g. benign tumour, tumour located in anus or small intestine) were excluded (n 36) leaving 966 participants. About 71 % (681 participants of the 966 in total) were diagnosed later than May 2001 and thus had clinical information from DCCG available.
Participants with missing information on important co-variates (n 13) were excluded. Of the remaining 953 participants (416 women and 537 men), 535 died (210 women, 325 men) during follow-up (until 31 December 2015), 385 from colorectal cancer (170 women, 215 men) and 150 for cause other than colorectal cancer (40 women and 110 men) (see online Supplementary Fig. S1 for flow chart).
Antibiotics use
From the Danish National Prescription Registry( Reference Kildemoes, Sorensen and Hallas33 ), information on redeemed prescriptions from Danish community pharmacies since 1995 is available. For the present study, antibiotics use up to 12 months before blood sample (blood sampling at recruitment, prediagnosis) was obtained. The use of antibiotic was categorised into three groups based on the most recent filling of antibiotic prescriptions (as done in a previous study( Reference Bolvig, Kyrø and Norskov16 )): 0–3 months before recruitment, 3–12 months before recruitment or no use. Since the database was not available until 1995, 12-month prior use of antibiotics could only be obtained from those that were recruited after 1 January 1996. Thus, analyses accounting for antibiotics use were only possible for those recruited from 1 January 1996 (n 523, 55 %).
Statistical analyses
Characteristics from time of recruitment (prediagnosis) and from time of diagnosis are presented as medians with corresponding percentiles (5th and 95th) or as percentages.
Cox proportional hazards models were used to estimate the association between prediagnosis plasma concentrations of enterolactone in relation to all-cause and cause-specific mortality (colorectal cancer-specific mortality and other causes of death). Time since diagnosis (defined as time elapsed from date of diagnosis until exit) was used as underlying time scale. The participants were followed from date of diagnosis of colorectal cancer until date of death (n 535), disappearance (n 0), emigration (n 2) or end of follow-up (31 December 2015), whichever came first. For colorectal cancer-specific mortality and other causes of death, deaths from the other cause(s) were censored. Linearity of the associations for enterolactone (exposure) and all continuous co-variates were evaluated using linear splines with three knots placed at the quartile cut-off points among deceased participants( Reference Greenland34 ). Enterolactone concentration was log-transformed in order to obtain linearity; none of the other evaluated variables showed departures from linearity. Based on a priori assumption, separate analyses were performed by sex.
The exposure, enterolactone was investigated both as a continuous variable and as a categorical variable. The continuous variable was the log-transformed concentration so one unit difference corresponds to a doubling in concentration. The categorical variable corresponded to sex-specific quartiles among all participants with the lowest quartile as reference. The results are presented as hazard ratios (HR) with 95 % CI. Two models were conducted; a minimally adjusted model (model 1a) (adjusted for age at diagnosis) and a model adjusted for potential confounders (model 1b). In model 1b, age at diagnosis, smoking status and length of schooling were included a priori. The following variables were further evaluated using a change-in-estimator criterion with a cut-off of 10 % of the continuous estimate of enterolactone, pack-years, fasting status, BMI, waist circumference, alcohol intake, participation in sports, hours a week spent on sport activities, intake of processed meat, intake of red meat, intake of dairy products, total Ca intake and frequency of bowel movements and for women, menopausal status and use of menopausal hormones. For BMI and waist circumference, both alone changed the association more than 10 %. However, adding BMI to the analysis adjusted for waist circumference did not change the association more than 10 %, and thus, the analyses were only adjusted for waist circumference. The following were thus included in model 1b for both men and women; age at diagnosis, smoking status (current, former, never), schooling (as measure of socio-economic status, ≤7, 8–10, ≥11 years), quantification of cigarette smoking, that is, pack-years (lifetime average number of cigarettes smoked multiplied by the number of years smoked divided by 20. One pack of cigarettes has twenty cigarettes), waist circumference (cm), alcohol intake (abstainer yes/no, continuous intake), intake of processed meat (g/d, all consumed processed meat) and frequency of bowel movements (≤4 times/week, 5–6 times/week, 1 time/d, ≥2 times/d).
Analyses restricted to those with diagnosis after May 2001 (and thus clinical data available from DCCG) were made; one analysis was adjusted for general health at time of diagnosis, that is, Charlson comorbidity index (0, 1, ≥2) and ASA score (II, II, III, IV); furthermore, one analysis was made with adjustment for treatment, that is, radical surgery performed (yes, no). Information on chemotherapy was unfortunately unavailable for most of the participants in the present study.
In order to test whether the use of antibiotics up to 12 months before blood sampling (exposure measurement) lead to differential associations, analyses including only participants recruited after 1 January 1996 (n 523, 55 %) were performed. Possible differential associations by antibiotics use was investigated by associating plasma enterolactone with events allowing for different associations for each of the two categories of antibiotics use (binary, used antibiotics 0–12 months before blood sampling, yes/no). Comparison of the regression coefficients was done using a Wald test for the hypothesis of equal regression coefficients. We furthermore investigated the association between plasma levels of log-transformed enterolactone as dependent variable and estimated log-transformed lignan intake (estimated using dietary intake from the FFQ and the Phenol Explorer( Reference Kyrø, Zamora-Ros and Scalbert35 )) depending on the use of antibiotics within 12 months before blood sampling. Results are reported as the percentage change in enterolactone concentration (with corresponding 95 % CI and P values) per doubling in lignan intake according to prior use of antibiotics.
Possible differential associations with plasma enterolactone by tumour subsite and Union for International Cancer Control (UICC) stage were tested allowing different associations with the log-transformed enterolactone concentration for each category of tumour subsite/UICC stage. Comparison of the regression coefficients was done using a Wald test for the hypothesis of equal regression coefficients. For the analyses for UICC stage, it was only possible to conduct the analysis on a subset (68 %). Lastly, possible differential associations by time between blood sampling and diagnosis was investigated (0–5, >5–10, >10 years). The above analyses were performed for all-cause mortality only due to limited statistical power for cause-specific mortality.
Before conducting the study, power calculations were made to make sure that the number of participants and events were sufficient to measure the expected effect size. The power calculations are based on the assumption of a log-rank two-sample test comparing those above to those below the median enterolactone concentration and are based on an anticipated number of colorectal cancer cases of 1038 with an expected 5-year survival of 55 % among those with levels below the median. With a statistical power of 90 % and a two-sided significance level of 0·05, a risk reduction of 25 % can be detected (or with 80 % power, a risk reduction of 22 %).
SAS® statistical software release 9.4 was used for statistical analyses. The PHREG procedure was used for the Cox proportional hazard models and the ‘assess ph’ option was used to test for proportionality, and no violation was found. The ‘test’ statement in PHREG was used to perform the Wald test. The GLM procedure was used to investigate associations between enterolactone and estimated lignan intake depending on antibiotics use. The power analysis was made using the POWER procedure. The UNIVARIATE and FREQ procedures were used for descriptive analyses. Statistical significance level was P<0·05.
Ethics
The present study has obtained approvals from The National Committee on Health Research Ethics (Den Videnskabsetiske Komité for Region Hovedstaden) and the Danish Data Protection Agency. Approval to link with the database of DCCG was obtained through the Danish Clinical Registries (‘Regionernes Kliniske Kvalitetsudviklingsprogram’; RKKP). Linkage with the Danish National Prescription Registry was approved by the Danish authorities (Sundhedsdatastyrelsen), and made through Statistics Denmark.
Results
Of the 416 women and 537 men diagnosed with colorectal cancer, 210 women (50 %) and 325 men (61 %) died during follow-up (170 women and 215 men had colorectal cancer as cause of death, and the remaining forty women and 110 men died due to other causes). Those with an enterolactone concentration in the highest quartile had a lower prediagnosis BMI, higher intake of whole grains and had longer educations. Additionally, more reported to have constipation (frequency of bowel movements ≤4 times/week) (Table 1). Those who died during follow-up had more advanced disease and more comorbidities. For prediagnosis lifestyle, those that died during follow-up were more likely to be smokers. The prediagnosis median enterolactone concentration was 19 (5th–95th percentile 2–83) nmol/l for women and 18 (5th–95th percentile 3–90) nmol/l for men. Among women, those that died during follow-up tended to have lower enterolactone concentrations, whereas no apparent difference was observed for men (online Supplementary Table S1).
Table 1 Characteristics of women and men diagnosed with colorectal cancer for all and according to prediagnosis quartile (Q) of plasma enterolactone concentration* (Numbers and percentages; medians and 5th–95th percentiles (P5–P95))
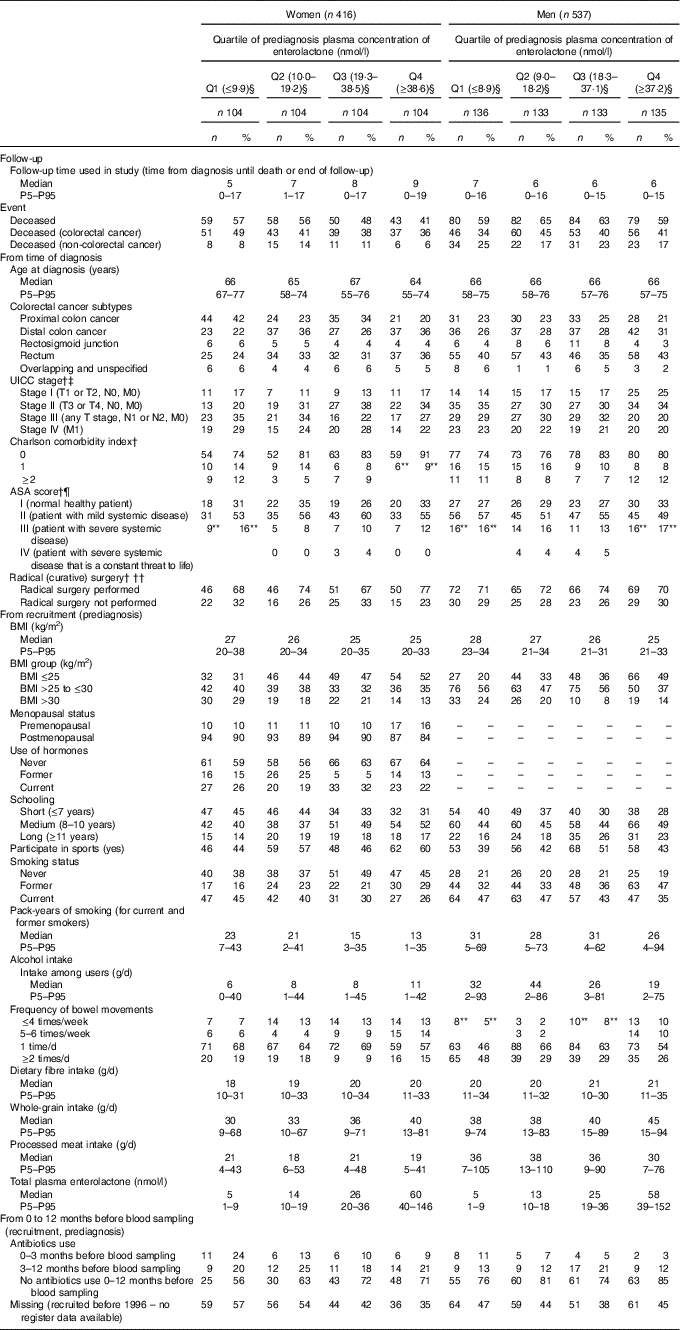
UICC, Union for International Cancer Control; ASA, American Society of Anesthesiologists.
* Characteristics are from recruitment (before diagnosis) and at time of diagnosis. The Diet, Cancer and Health cohort.
† n, women=138 (33 %) and n, men=138 (33 %) have missing information due to diagnosis before May 2001.
‡ n, women=14 (3 %) and n, men=15 (3 %) missing (unknown reason).
§ Enterolactone concentrations are expressed in nmol/l.
¶ n, women=26 (6 %) and n, men=30 (6 %) missing (unknown reason).
** Two categories merged into one due to n<3 in one of the categories.
†† n, women=7 (2 %) and n, men=15 (3 %) missing (unknown reason).
For women, enterolactone concentrations were borderline associated with all-cause mortality (HRmodel 1b, per doubling in concentration: 0·92, 95 % CI 0·84, 1·00, P=0·0509) (Table 2). For colorectal cancer-specific mortality, a 12 % lower risk per doubling in enterolactone concentration was observed (HRmodel 1b, per doubling in concentration: 0·88, 95 % CI 0·80, 0·97, P=0·0123), and no association was found for non-colorectal cancer mortality. The above continuous associations is also illustrated by the women with enterolactone concentrations in the highest quartile on average having a 37 % lower colorectal cancer-specific mortality (HRmodel 1b: 0·63, 95 % CI 0·41, 0·99) compared with women with enterolactone concentrations in the lowest quartile. For men, no statistically significant association was found between enterolactone and all-cause mortality or other non-colorectal cancer-specific mortality. For colorectal cancer-specific mortality, for men, a doubling in enterolactone was associated with a 10 % higher risk of colorectal cancer-specific mortality (HRmodel 1b, per doubling in concentration: 1·10, 95 % CI 1·01, 1·21, P=0·0379). No tendencies of dose–response relationships illustrated by quartile estimates were observed for men for any of the outcomes.
Table 2 Association between prediagnosis plasma concentrations of enterolactone and risk of all-cause, colorectal cancer-specific mortality, and non-colorectal cancer-specific mortality – women and men diagnosed with colorectal cancer from the Diet, Cancer and Health cohort (n 953)* (Hazard ratios (HR) and 95 % confidence intervals)
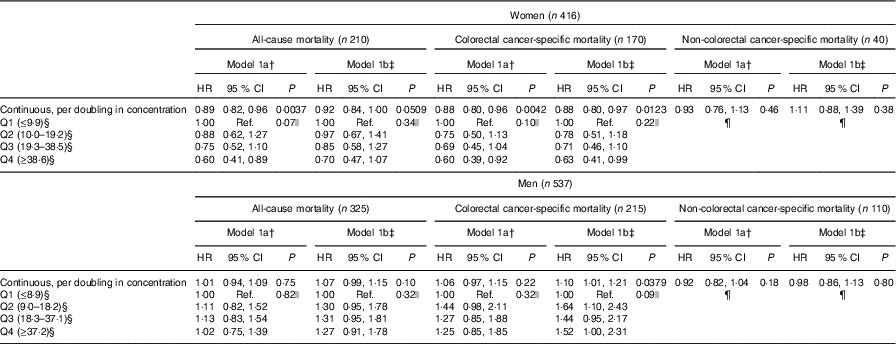
Q, quartiles; Ref., reference.
* HR and 95 % CI were obtained by the Cox proportional hazards model.
† Model 1a: adjusted for age.
‡ Model 1b: additionally adjusted for smoking status (current, former, never), schooling (as measure of socio-economic status, ≤7, 8–10, ≥11 years), quantification of cigarette smoking, that is, pack-years, waist circumference (cm), alcohol intake (abstainer yes/no, continuous intake), intake of processed meat (g/d) and frequency of bowel movements (≤4 times/week, 5–6 times/week, 1 time/d, ≥2 times/d).
§ Enterolactone concentrations are expressed in nmol/l.
|| Overall P value for quartiles (main effect of the categorical variable).
¶ Numbers of events too small for analysis.
Investigation of possible differential associations between events and plasma enterolactone depending on use of antibiotics up to 12 months before blood sampling (enterolactone measurement) was possible on a subset (n 523, 55 %) (Table 3). For women, the association with all-cause mortality and colorectal cancer-specific mortality seemed more pronounced among those that did not use antibiotics 0–12 months before blood sampling, although the test for interaction was statistically insignificant. For men, no sign of interaction was found, although signs of a direct association was most pronounced among those that used antibiotics. The association between plasma enterolactone concentrations and estimated intake of lignans was investigated according to antibiotics use (data not shown). For women that did not use antibiotics, a doubling in estimated lignan intake was associated with a 56 % higher enterolactone concentration (56 %, 95 % CI 15, 110, P=0·0041), and for those who used antibiotics 0–12 months before blood sampling, no association was found. For men, no association was found among those that did not used antibiotics, whereas a surprising inverse association was found among those that did use antibiotics 0–12 months before blood sampling (−49 %, 95 % CI −72, −6, P=0·0317).
Table 3 Association between prediagnosis plasma concentrations of enterolactone as continuous (per doubling in concentration) and risk of all-cause, colorectal cancer-specific mortality, and non-colorectal cancer-specific mortality – separately for those that used antibiotics 0–12 months before blood sampling and those that did not, respectively – women and men diagnosed with colorectal cancer from the Diet, Cancer and Health cohort*† (Numbers, hazard ratios (HR) and 95 % confidence intervals)
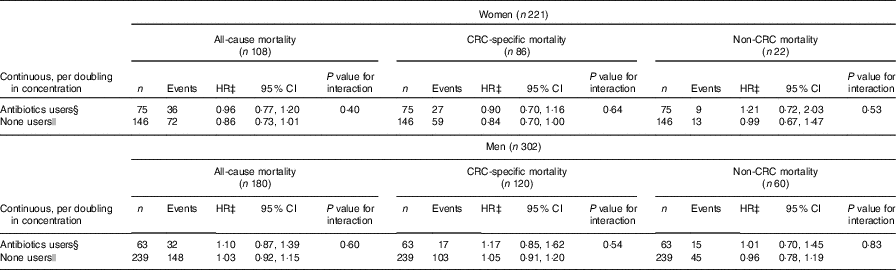
CRC, colorectal cancer.
*On a subset, those recruited from 1 January 1996 (n 523).
† HR and 95 % CI were obtained by the Cox proportional hazards model.
‡ All analyses adjusted for age, smoking status (current, former, never), schooling (as measure of socio-economic status, ≤7, 8–10, ≥11 years), quantification of cigarette smoking, that is, pack-years, waist circumference (cm), alcohol intake (abstainer yes/no, continuous intake), intake of processed meat (g/d) and frequency of bowel movements (≤4 times/week, 5–6 times/week, 1 time/d, ≥2 times/d).
§ Used antibiotics ≤12 months before blood sampling, that is, enterolactone measurement.
|| Did not take antibiotics ≤12 months before blood sampling, that is, enterolactone measurement.
Potential differences by tumour site and UICC stage was investigated (Table 4). For tumour subsites, the P value for interaction was statistically insignificant for men and women. For women, however, the inverse association between enterolactone and all-cause mortality appeared more pronounced proximal colon cancer subtypes, and the association appeared less pronounced the closer the tumour was to the rectum. For UICC stage, an interaction was observed for both women and men. For women, the association between enterolactone and mortality endpoints seemed most pronounced among women with UICC stages II and III, and for men, those with stages I and IV had a point estimate much higher than 1·00.
Table 4 Analyses by tumour subsite and UICC stage of the association between prediagnosis enterolactone concentrations as continuous (per doubling) and all-cause mortality – women and men diagnosed with colorectal cancer from the Diet, Cancer and Health cohort* (Numbers, hazard ratios (HR) and 95 % confidence intervals)
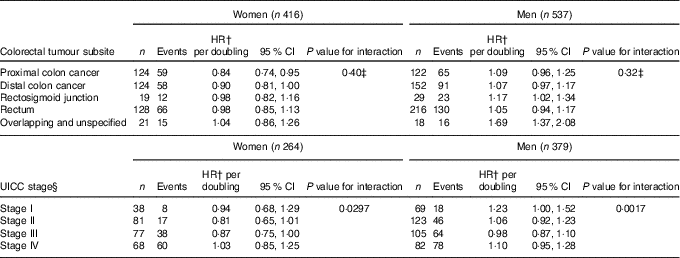
UICC, Union for International Cancer Control.
* HR and 95 % CI were obtained by the Cox proportional hazards model, and HR are expressed as linear per doubling in enterolactone concentration.
† All analyses adjusted for age, smoking status (current, former, never), schooling (as measure of socio-economic status, ≤7, 8–10, ≥11 years), quantification of cigarette smoking, that is, pack-years, waist circumference (cm), alcohol intake (abstainer yes/no, continuous intake), intake of processed meat (g/d) and frequency of bowel movements (≤4 times/week, 5–6 times/week, 1 time/d, ≥2 times/d).
‡ Testing differential associations by tumour subsite, overlapping and unspecified not included in test.
§ UICC stage analyses only conducted on subset due to missing data: women n 264 of 416 (63 %), men n 379 of 537 (71 %).
Adjusting for associations between plasma enterolactone and all-cause mortality for general health status and cancer treatment did not change estimates (online Supplementary Table S2). When investigating possible differential associations by time between blood sampling (enterolactone measurement) and diagnosis, no sign of interaction was found among women. Among men, a borderline significant interaction was observed (P=0·0513), where a direct association between enterolactone and all-cause mortality was found among those with the shortest time between blood sampling and diagnosis (0–5 years) (online Supplementary Table S3).
Discussion
High prediagnosis enterolactone concentrations were associated with lower colorectal cancer-specific mortality among women in the present study. For men, on the contrary, enterolactone was associated with higher colorectal cancer-specific mortality, although no sign of a dose–response relationship was found.
The present study had several potential weaknesses but also strengths that should be considered before interpreting the findings. The major strengths of the present study include the prospective design and detailed diet and lifestyle information (prediagnosis) and clinical data. The outcomes, all-cause and cause-specific mortality, were based on register information. For all-cause mortality, The Danish Civil Registration System is nearly complete. For cause-specific mortality, where information is derived from the Danish Register of Causes of Death( Reference Helweg-Larsen28 ), there is risk of sticky-diagnosis bias (when deaths from an uncertain cause likely are attributed to the previous cancer diagnosis), and furthermore, lower validity is expected due the low autopsy rate in Denmark (<10 %)( Reference Helweg-Larsen28 ).
The exposure, enterolactone, was measured in plasma. The objective nature of biomarkers as opposed to questionnaires is a main advantage. However, biomarkers are also subject to measurement errors, for example, due to the analytical method, effects of storage and diurnal variation( Reference Jenab, Slimani and Bictash36 ). The plasma samples were prediagnosis and taken several years before diagnosis, and thus may not reflect the time under study (from diagnose and onwards). In a previous study in the same cohort, we found moderate accordance between prediagnosis and diagnostic measurement of enterolactone (Spearman’s correlation coefficient of 0·44)( Reference Kyrø, Hansen and Frederiksen37 ). Our hypothesis was that the long-term enterolactone exposure is the relevant measure. In this regard, it may be advantageous that the exposure is measured before disease occurrence, because the disease progression may affect, for example, the microbiota and thus the enterolactone concentration. For men, the unexpected direct association between enterolactone and all-cause mortality was found only among those with 0–5 years between blood sampling and diagnosis, suggesting the disease may have affected the plasma enterolactone concentration. Another factor known to affect the enterolactone concentration is antibiotics use. The importance of the microbiota was also shown from our analyses for women, where lignan intake was associated with enterolactone concentrations only among those that did not use antibiotics before blood sampling (enterolactone measurement), and moreover, that the association between enterolactone and all-cause mortality seemed most pronounced among those that did not use antibiotics. For men, the findings of the associations between lignans intake and enterolactone were not as expected, there was no association among those that did not use antibiotics. Furthermore, among those who did use antibiotics, lignan intakes were associated with lower enterolactone. We have no obvious biological explanation for this.
An advantage of present study is that the Danish Diet, Cancer and Health cohort may be a suitable population for research in plant lignans and enterolactone due to a large between-subject variation in plant lignan intake and circulating concentration of enterolactone compared to, for example, cohorts in the USA( Reference Xie, Tworoger and Franke38 ). High quality information on outcomes and clinical information on tumour characteristics and cancer treatment was available in the present study, with the latter being rare in a cohort setting. The clinical information on treatment was limited; however, cancer stage and other disease characteristics may serve as proxies for cancer treatment. Unfortunately, we had no data on ERα and ERβ status, which would have enabled us to gain insights into the possible differential associations with enterolactone depending on the receptor status. We thoroughly considered potential confounders, and adjusted the analyses. However, by design, observational studies are prone to confounding, and thus residual confounding cannot be ruled out. Lastly, one additional weakness of studies on cancer survival is that if the exposure under study also is associated with risk of the disease, then we may get a selection into the case group. This would introduce a type of selection bias similar to the so-called obesity paradox( Reference Preston and Stokes39 ). However, it may not be a major concern in the present study, because no strong association between enterolactone concentrations and colorectal cancer risk has been found in prospective studies( Reference Jiang, Botma and Rudolph14 ).
We found enterolactone to be associated with lower all-cause mortality and colorectal cancer-specific mortality among women only. In a previous study based on the present cohort, we have found prior antibiotics use to mostly affect enterolactone levels in women( Reference Bolvig, Kyrø and Norskov16 ) and higher enterolactone levels to be associated with lower risk of colorectal cancer among women, but with a higher risk among men( Reference Johnsen, Olsen and Thomsen15 ). Overall in other cohort studies, no association between enterolactone and risk of colorectal cancer has been found( Reference Jiang, Botma and Rudolph14 , Reference Ko, Yeo and Yoon40 ). To the best of our knowledge, enterolactone and survival after colorectal cancer has not previously been studied. Just one previous study investigated dietary intakes of plant lignans in relation to survival after colorectal cancer and found no association and also no indications of effect modification by sex( Reference Zamora-Ros, Guino and Henar Alonso41 ). In the present study, a higher colorectal cancer-specific mortality with higher enterolactone levels among men was observed, which is in contradiction to what we hypothesised. A higher risk was observed in both the second and the fourth quartiles, as compared with the first quartile. The characteristics for those with an enterolactone concentration in the lowest quartile, differed, as expected, by reported lower whole-grain intake (plant lignan source), but also, in general, were of worse physical condition (higher Charlson index) and had more unhealthy lifestyle. Adjustment for potential confounders, for example, frequency of bowel movements especially affected the estimates for men. Thus, one could speculate that the analyses for men especially may suffer from residual confounding.
In general, the evidence, although limited, points towards enterolactone being of relevance especially in relation to women’s health including hormone-related cancers( Reference Seibold, Vrieling and Johnson42 , Reference Zhang, Feng and Qluwakemi43 ). In the present study, an association between high enterolactone concentrations and lower colorectal cancer-specific mortality was found among women only. Phyto-oestrogens have been shown to have higher affinity for ERβ than ERα, and ERβ may inhibit cell growth( Reference Kennelly, Kavanagh and Hogan10 ), and thus activation of ERβ may be related to improved survival among colorectal cancer patients. A previous study investigated oestrogen ERα and ERβ in normal colorectal mucosa and in colorectal tumours in women and men( Reference Nussler, Reinbacher and Shanny44 ). They found that for colorectal tumours, the average expression of ERβ was higher for women than for men. This may explain why enterolactone was observed to have an inverse association with colorectal cancer-specific mortality among women only. For colorectal cancer subsites, ERβ is more expressed tumours located in the proximal (right-sided) than the distal colon (left-sided). Proximal colon cancer is also the colon cancer subsite found more frequent in women than in men( Reference Koo and Leong45 ) (also seen in the present study cohort). In the analysis of the associations for women between enterolactone and risk of mortality endpoints by colorectal cancer subsite, the inverse associations seemed to be most pronounced among those with proximal tumours (right-sided). ERβ expression has been shown to decline in parallel with cancer progression( Reference Konstantinopoulos, Kominea and Vandoros46 ). This may explain the interaction by UICC stage found for women with signs of an inverse association between enterolactone and all-cause mortality was found only among stages I, II and II (and not the advanced stage IV). Interaction by UICC stage was also found for men, but with an direct association found among extreme stages only (UICC stages I and IV). The biological explanation for the finding in men seems less obvious. Furthermore, it seems counter-intuitive that enterolactone seems to be related to survival, but not to incidence, since ERβ is especially expressed in healthy colonic tissue. However, as mentioned, the biological action of ERβ may be especially in relation to cell growth( Reference Kennelly, Kavanagh and Hogan10 ) and thereby both disease progression and initiation.
One of the largest sources of plant lignans in Denmark is whole grains, and a previous study found whole grains to be associated with lower mortality among patients with colorectal cancers( Reference Song, Wu and Meyerhardt7 ). In fact, the association found could possibly be ascribed to whole grains as such, to the bulking properties diluting potential toxic compounds( Reference Cummings, Bingham and Heaton47 ), and the myriad of other molecules deriving from whole grains( Reference Fardet48 ) rather than to enterolactone. As expected, adjustment for whole-grain intake attenuated the association with enterolactone, but it remained statistically significant in relation to colorectal cancer-specific mortality among women. Due to the observational study design, it is not straightforward to investigate whether enterolactone is solely a biomarker of a high whole-grain intake or whether enterolactone is the biological compound responsible.
Main dietary sources of lignans include whole grains, which, in general, have been related to improved survival among persons diagnosed with colorectal cancer as well as among the general population( Reference Song, Wu and Meyerhardt7 , Reference Aune, Keum and Giovannucci49 ). However, more research in this area is needed before a conclusion can be drawn and recommendations can be made for enterolactone and lignans. Specifically, the observed sex difference, and the finding of a higher risk of colorectal cancer-specific mortality among men found in the present study warrants further investigation.
The present study supports that the phyto-oestrogen, enterolactone, may play a role in colorectal cancer survivorship among women.
Acknowledgements
The authors would like to thank Nick Martinussen and Katja Boll for collection of data and assistance on data management and Jytte Fogh Larsen for administrative assistance.
This work was supported by Innovation Fund Denmark (Project ELIN: the effects of enterolignans in chronic disease: 0603-00580B); and Danish Cancer Society.
C. K. prepared the initial draft of the manuscript and conducted the statistical analysis, with statistical assistance from K. F., N. P. N. was responsible for the laboratory analyses. All authors helped conceptualise the study and provided feedback on various drafts of the manuscript and read and approved the final manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114518002143







