Carbon monoxide (CO) is a colourless, odourless gas produced as a by-product of incomplete combustion of carbon-containing compounds such as coal, wood, peat, charcoal and petroleum products (Reference Hopkins and WoonHopkins 2006).
Carbon monoxide poisoning is usually presented with non-specific symptoms similar to flu-like illnesses (Reference Hopkins and WoonHopkins 2006). It may also present with diverse neurological signs and the level of exposure seems to be the key determinant. The lethal level of carboxyhaemoglobin (COHb) is variable and difficult to predict. There is evidence of oxidation injury following lipid peroxidation in the central nervous system (Reference ThomThom 1990a). With oxidative stress, nerve conduction becomes impaired and transmission of nerve impulses is delayed as myelin and basic protein are altered (Reference Thom, Bhopale and FisherThom 2004). Neuronal cell death occurs as a result of different mechanisms such as hypoxia (Reference Caine and WatsonCaine 2000), release of excitatory amino acids (e.g. glutamate) and apoptosis (Reference Piantadosi, Zhang and LevinPiantadosi 1997).
The cause of CO poisoning is heterogeneous, with both accidental and intentional poisoning considered to be common. Victims of CO poisoning are usually young and middle-aged adults (Reference Hopkins and WoonHopkins 2006). Reference Cobb and EtzelCobb & Etzel (1991) reported that intentional CO poisoning is more common in spring and accidental poisoning is more common in winter. Common causes of accidental poisoning include faulty items: furnaces, forklifts, gas-powered saws, water heaters, boats, room heaters, stoves, methylene chloride in paint removers, and fire (Reference WeaverWeaver 1999). Carbon monoxide poisoning also occurs when motor vehicles are trapped in the snow with their exhaust pipe buried (Reference Geehr, Salluzzo and BoscoGeehr 1989). In the USA, CO poisoning results in approximately 40 000 accident and emergency department visits (Reference HampsonHampson 1999) and 800 deaths every year (Reference Piantadosi, Zhang and LevinPiantadosi 1997). The true prevalence may be even higher as some cases have only been discovered during post-mortem examination. Middle-aged adults and those with comorbid cardiac and pulmonary disorders are vulnerable to CO-related mortality (Reference Cobb and EtzelCobb 1991).
History of CO poisoning
In the UK
Historical records have shown that the Romans used CO to execute criminals (Reference Prockop and ChichkovaProckop 2007). In the early 1900s, exposure to fires in closed spaces were common during winter in Great Britain; more recently, faulty gas appliances have often led to accidental CO poisoning (Reference BlumenthalBlumenthal 2001).
Early medical literature describing neuropsychiatric symptoms related to CO poisoning date back to a case report written by Starkey published in 1914 in the Journal of Mental Science.
‘A 56-year-old man was poisoned by CO gas from a defective stove. He was normal during the first few days. Then he developed progressive stupor, apathy, poor attention and complete disorientation. On neurological examination, he had an unsteady gait, tremulous tongue, exaggerated knee-jerk reflexes and incontinence. After 2 months, his cognitive and neurological abnormalities subsided.’
During the Second World War, acute and chronic CO poisoning were common, as wood was used as the main source of fuel on the war front (Reference Tvedt and KjuusTvedt 1997). On the home front, Reference Steele and HegartySteele & Hegarty (1950) commented that suicide attempts by coal-gas poisoning were common in England in the 1940s. They reported that a 35-year-old man attempted suicide with coal gas, of which CO is the principal constituent. Subsequently, the patient developed complex parieto-occipital syndrome, including Gerstmann’s syndrome. In contrast to the patient reported in 1914, this patient did not recover from his illness (Reference Steele and HegartySteele 1950). In the 1960s and 1970s, the switch from coal gas to CO-free natural gas caused a dramatic reduction in accidental CO poisoning (Reference BlumenthalBlumenthal 2001).
In the USA
In the USA, there have been reports of CO poisoning in enclosed vehicle cabins. Reference Griffin, Ward and TerrellGriffin et al (2008) reported that a 52-year-old lorry driver felt ill with ‘flu-like’ symptoms for a few days before being found dead in his secure lorry cabin. He had unknowingly been exposed to a constant level of CO through an exhaust leak in the enclosed cabin. An autopsy report showed that he had developed ischaemic heart failure prior to his death. This was explained by the fact that CO has a high affinity for cardiac myogloblin and such binding causes myocardial depression, hypotension and arrhythmia (Reference BlumenthalBlumenthal 2001).
Other sources of CO
Carbon monoxide poisoning also occurs without direct inhalation of CO. Methylene chloride (dichloromethane) is an industrial solvent and is found in paint remover. Inhaled or ingested methylene chloride is metabolised to CO by the liver (Reference Ernst and ZibrakErnst 1998; Reference Chang, Yang and DengChang 1999).
The rising trend of CO poisoning as a suicide method in Asia
In 1998, a 38-year-old woman took her own life by burning barbecue charcoal in her bedroom in Hong Kong. The sealed room created a CO chamber and the media portrayed this method as a painless way to die, without following the World Health Organization’s guidelines on reporting suicide (Reference Liu, Beautrais and CaineLiu 2007). This news spread to other Asian countries and created a ‘copycat phenomenon’. In Hong Kong, it became the second most common method of suicide 4 years after the first reported case (Reference Liu, Beautrais and CaineLiu 2007). In Taiwan, the incidence of charcoal-burning suicide rose from 0.22 per 100 000 in 1999 to 6.48 per 100 000 in 2006 (Reference Pan, Liao and LeePan 2009). In Singapore, we have also witnessed an increase in the incidence of CO intoxication among individuals who attempt suicide in clinical practice.
Assessment of CO poisoning
Diagnosis
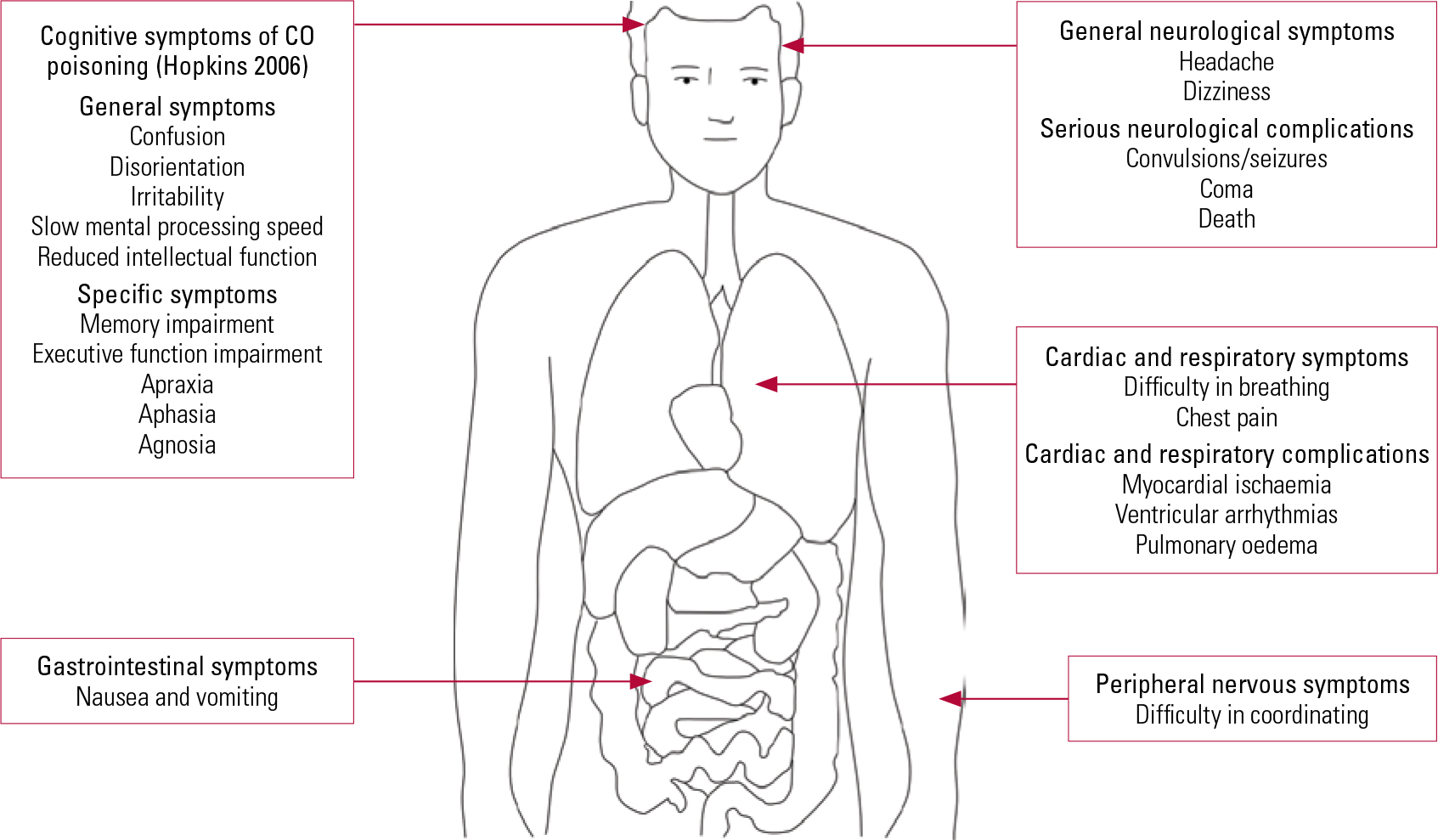
The diagnosis of CO poisoning is essentially established by clinical assessment and further confirmed by laboratory investigations. The clinician should enquire about the symptoms associated with CO poisoning in a systematic manner as illustrated in Fig. 1, but there is great variation in clinical presentation. On the one hand, patients may present with subtle physical signs which conceal a significant degree of CO exposure due to delayed neuropsychiatric syndrome. On the other hand, some people may already be unconscious when they arrive at hospital. In such cases, individuals have usually sealed off windows to create a closed chamber while the charcoal is burning and have ingested benzodiazepines.
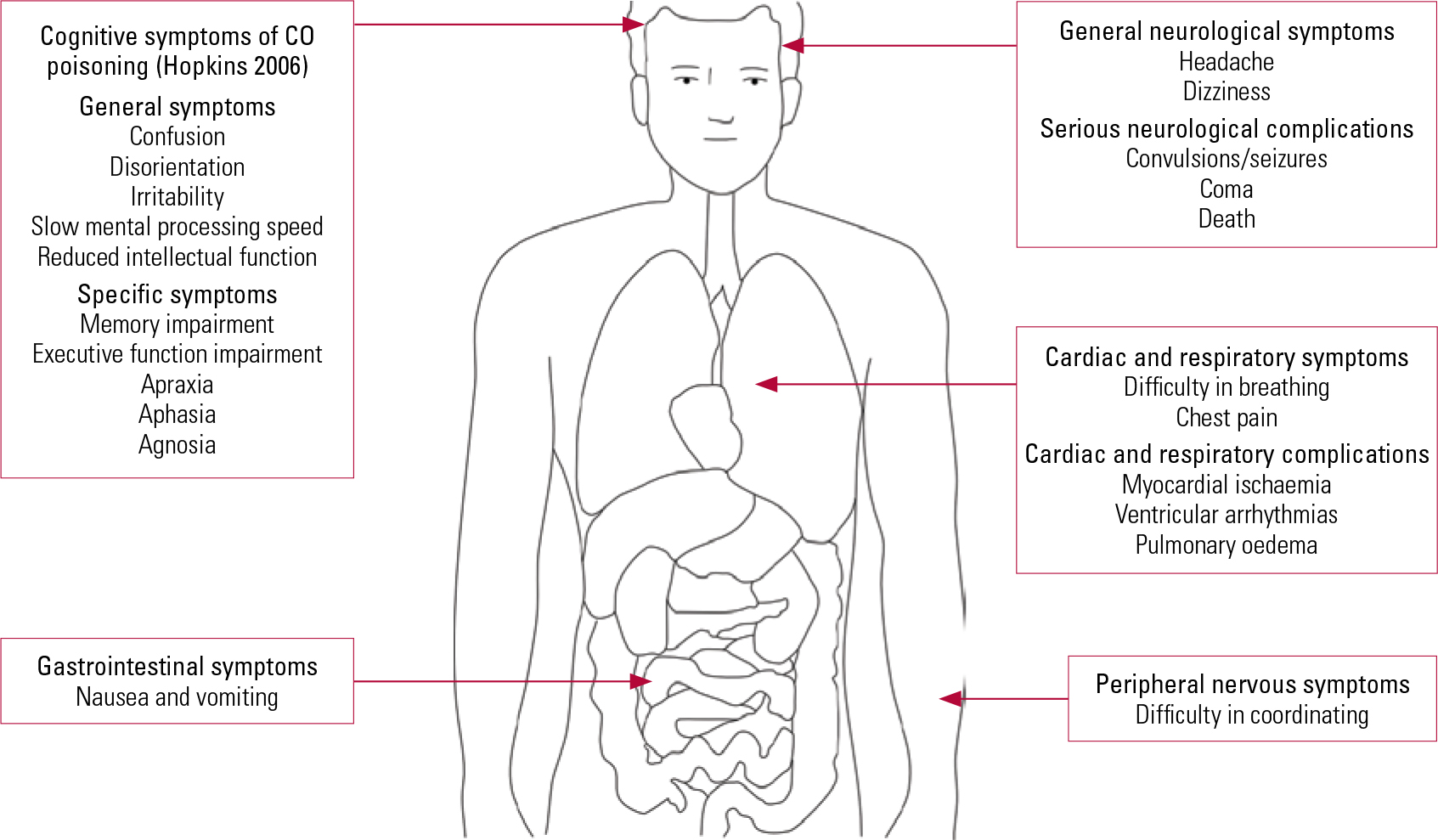
FIG 1 Signs and symptoms of carbon monoxide (CO) intoxication.
COHb levels
The level of COHb is useful to confirm exposure to CO. However, one limitation is that COHb levels drop rapidly after the person starts to inhale atmospheric air (which contains about 21% oxygen). Other laboratory tests that may help to determine COHb levels include arterial blood gases (looking for metabolic acidosis) and blood lactate levels. Informants should be interviewed, as people who have attempted suicide are sometimes reluctant to disclose information. They may have difficulty in recalling the details of the antecedents, the actual suicide attempt and the consequences. An informant close to the patient may also be able to shed valuable light on the usage of faulty devices in cases of accidental poisoning.
Neuropsychiatric syndrome
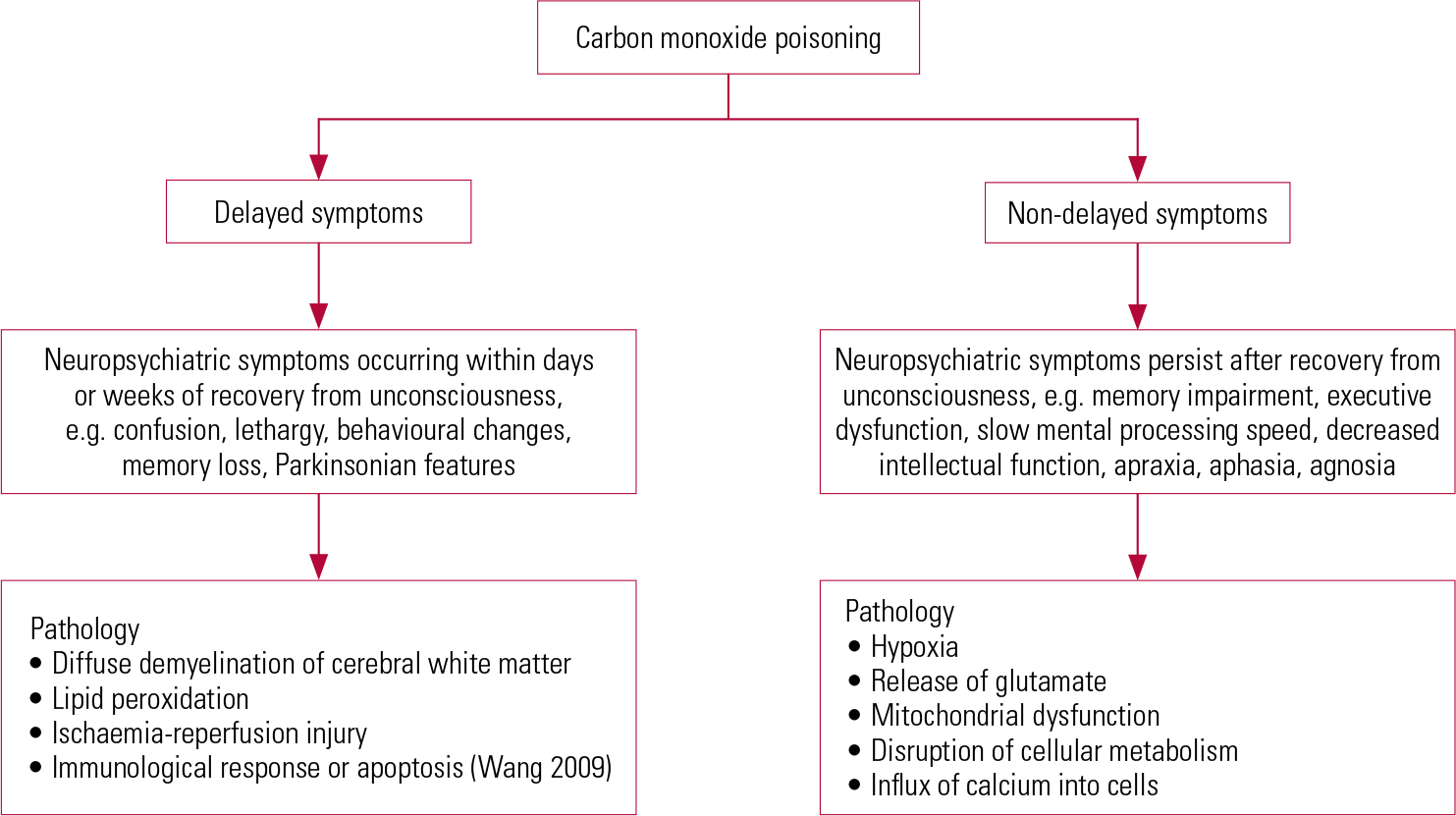
The man poisoned by the defective stove in 1914 (Reference StarkeyStarkey 1914) demonstrated delayed neuro-psychiatric syndrome. The onset of the syndrome ranges from 2 to 240 days (Reference ChoiChoi 1983) after acute CO exposure. Patients may show subtle abnormalities such as personality changes, mild cognitive deficit to severe dementia and even psychosis (Reference Mimura, Harada and SumiyoshiMimura 1999; Reference ChoiChoi 2000). Figure 2 illustrates the delayed and non-delayed neuro-psychiatric symptoms after CO poisoning, with the underlying aetiologies. Clinicians should explore somatic symptoms such as throbbing in the temples, severe headache, generalised weakness, fatigue, sleepiness and dizziness. Physical examination may reveal supporting neurological features such as pseudo-Parkinsonism and evidence of incontinence.
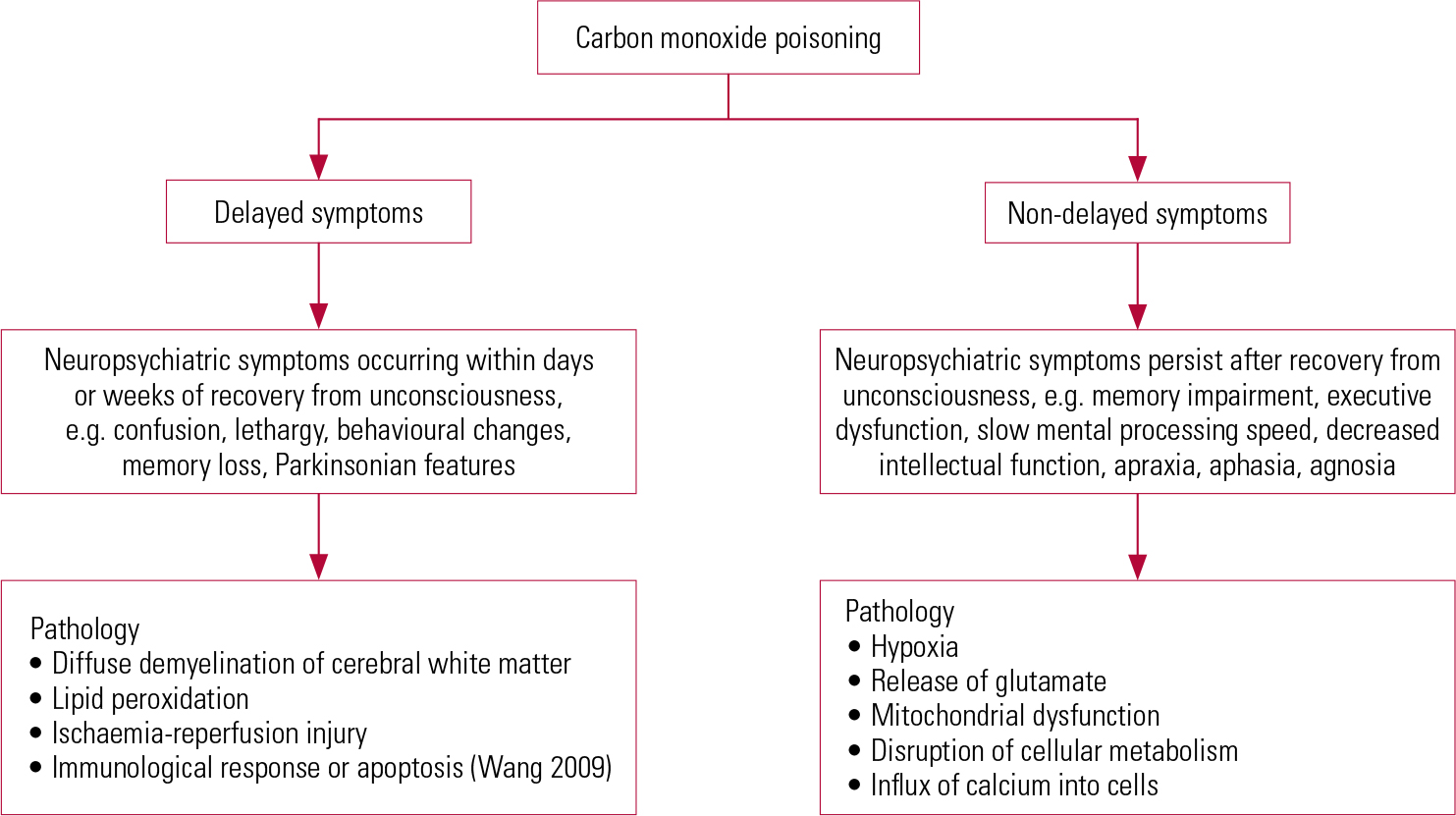
FIG 2 Delayed and non-delayed neuropsychiatric symptoms and the underlying pathology (Reference Mannaioni, Vannacci and MasiniMannaioni 2006).
Neuropathological changes following CO poisoning
There are a number of reasons why the brain and heart are vulnerable to developing complications after CO poisoning (Reference Prockop and ChichkovaProckop 2007). Both the brain and heart have a high metabolic rate, and as mentioned earlier, CO’s high affinity for cardiac myogloblin causes myocardial depression, hypotension and arrhythmia (Reference BlumenthalBlumenthal 2001).
Carbon monoxide toxicity may increase the brain’s constitutional vulnerability to psychiatric symptoms. Figures 3 and 4 show coronal and sagittal views of the neuroanatomical areas affected by CO poisoning, and the neuropathological changes found in different neuroanatomical areas reported in the literature.
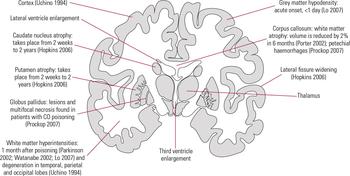
FIG 3 Coronal view of neuroanatomical areas affected by carbon monoxide (CO) poisoning and the associated neuropathological changes with time of onset.

FIG 4 Sagittal view of neuroanatomical areas affected by carbon monoxide poisoning and the associated neuro-pathological changes.
In CO poisoning, neuroimaging changes correspond to physical signs, and one well-reported neuroanatomical area in which these occur is the bilateral globus pallidus (Reference Sawada, Takahashi and OhashiSawada 1980; Reference Miura, Mitomo and KawaiMiura 1985; Reference Jones, Lagasse and ZimmermanJones 1994; Reference Silver, Cross and FoxSilver 1996; Reference Kao and NanagasKao 2004). In animal models, the development of low-density lesions in bilateral basal ganglia correlate with the severity of CO exposure through reduction in local blood flow (Reference Song, Okeda and FunataSong 1983), metabolic acidosis (Reference Kao and NanagasKao 2004) and hypotension (Reference Ginsberg, Myers and McDonaghGinsberg 1974; Reference Koehler, Jones and TraystmanKoehler 1982). The cerebellum shows acute neuropathological changes but the corpus callosum may develop neuropathological changes 2 years after the initial episode of CO poisoning (Reference Lo, Chen and LeeLo 2007). On the other hand, both acute and chronic neurological changes occur in the caudate nucleus and putamen (Reference Hopkins and WoonHopkins 2006).
The onset of neuropathological changes varies from one neuroanatomical area to another and this may underpin the delayed and non-delayed neuropsychiatric symptoms after CO poisoning. Other mechanisms leading to delayed neuropsychiatric sequelae include lipid peroxidation by toxic oxygen species generated by xanthine oxidase (Reference Goldbaum, Ramirez and AbsalonGoldbaum 1975; Reference ThomThom 1990b,Reference Thom, Xu and Ischiropoulos1997,Reference Thom, Bhopale and Fisher2004; Reference Zhang and PiantadosiZhang 1992), ischaemia-reperfusion injury and exposure to hyperoxia which may exacerbate the initial oxidative damage (Reference TomaszewskiTomaszewski 1999; Reference WeaverWeaver 1999).
In the acute setting, computed tomography is helpful to rule out other causes of neurological decompensation (Reference Clardy, Manaker and PerryClardy 2010). For patients with delayed neuropsychiatric sequelae, a magnetic resonance imaging scan may show abnormalities in the globus pallidus and deep white matter (Reference Zagami, Lethlean and MellickZagami 1993; Reference ChoiChoi 2000; Reference Teksam, Casey and MichelTeksam 2002; Reference Kim, Chang and SongKim 2003; Reference Chu, Jung and KimChu 2004).
Carboxyhaemoglobin
Carbonmonoxide has a 200 times greater affinity for haemoglobin than oxygen. A small concentration of CO can cause significant hypoxia by reducing oxygen release, especially in neuroanatomical areas which require large oxygen consumption such as the basal ganglia (Reference BlumenthalBlumental 2001). Hypoxia may lead to mitochondrial dysfunction and lipid peroxidation, resulting in reversible demyelination. Since the demyelinating action of CO poisoning affects white matter (Reference Prockop and ChichkovaProckop 2007) and causes other neuropathological changes in grey matter, cognitive dysfunction associated with CO poisoning may lead to both cortical and subcortical dementia. This gives rise to diverse neuropsychiatric presentations. Figure 5 illustrates the relationship between neuropsychiatric sequelae and COHb levels.
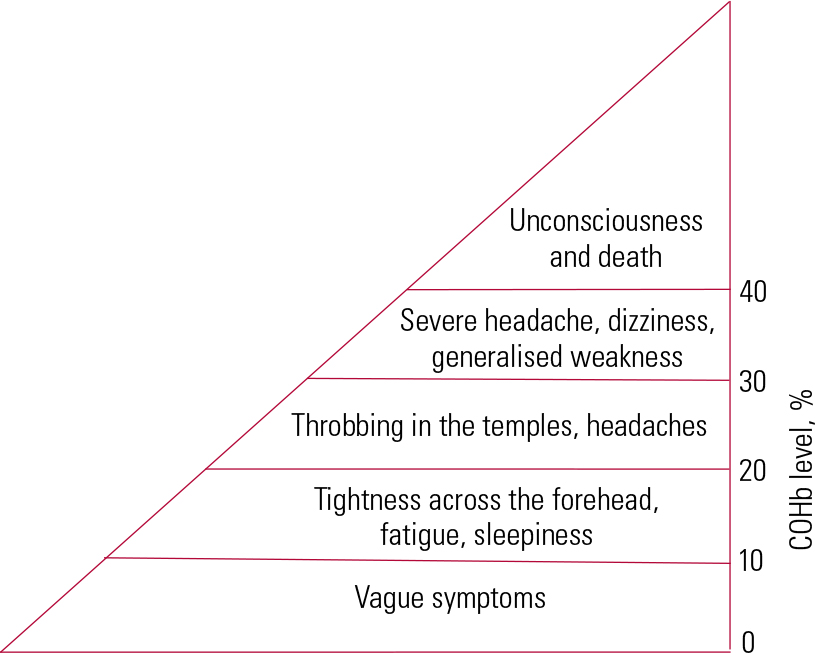
FIG 5 The relationship between neuropsychiatric sequelae after carbon monoxide poisoning and carboxyhaemoglobin (COHb) levels.
At baseline, non-smokers’ COHb levels range from 0 to 5%, whereas the baseline COHb levels of smokers range from 10 to 15% (Reference Ernst and ZibrakErnst 1998). Baseline COHb levels are also elevated in people with chronic obstructive pulmonary disease. More specific symptoms may emerge if the COHb level is greater than 20%, and life-threatening consequences may occur if it is higher than 40%. Carboxyhaemoglobin levels are poorly correlated with the degree of poisoning and are not predictive of delayed neuropsychiatric sequelae. Levels of COHb are significantly higher in individuals who die from suicide compared with individuals with accidental CO poisoning (Reference Hopkins and WoonHopkins 2006), and the blood alcohol level has a positive correlation with COHb level. Lethal COHb levels are significantly lower in people with concomitant cardiac or pulmonary diseases.
Other investigations
A chest radiograph (looking for non-cardiogenic pulmonary oedema), drug levels such as paracetamol and salicylate, blood lactate levels, arterial blood gases (looking for metabolic acidosis) and an electrocardiogram (looking for arrhythmia, changes associated with myocardial ischaemia) should be performed in the accident and emergency department. The presence of metabolic acidosis (Reference Kao and NanagasKao 2004) and raised lactate levels (Reference Sokal and KralkowskaSokal 1985) correlate well with severity of poisoning.
Baseline and regular neuropsychological assessment
Neuropsychological assessment should be carried out once the patient’s physical condition is stable. Baseline neuropsychological assessment after CO poisoning is certainly useful as it will allow clinicians to measure improvement or deterioration at regular intervals. This will also guide appropriate rehabilitation strategies.
The Carbon Monoxide Neuropsychological Screening Battery (CONSB) was designed specifically for patients with CO poisoning (Reference Messier and MyersMessier 1991). This battery consists of six domains assessing general orientation, digit span, trail making, digit symbol, aphasia and block design. Alternatively, neuropsychologists should assess major cognitive functions including retrieval and global memory (Rey Auditory Verbal Learning Test), visuospatial activities (Rey Complex Figure Test), attention and concentration (Wechsler Adult Intelligence Scale), difficulties with abstract thinking (proverb interpretation) and psychomotor speed (Wechsler Adult Intelligence Scale) (Reference Mak, Ho and LauMak 2009).
Predictors for neuropsychiatric sequelae
One study has looked at the prevalence of cognitive impairment in CO poisoning. Reference Weaver, Valentine and HopkinsWeaver et al (2007) studied 163 patients with CO poisoning but not receiving treatment: 42% developed neuro-psychiatric sequelae. Risk factors for sequelae were loss of consciousness after CO poisoning, being aged 36 years and above, and COHb levels ≥25%. Patients with abnormal neuroimaging findings are more likely to have poorer outcomes but there is no specific neuroimaging marker which indicates prognosis (Reference Sawada, Takahashi and OhashiSawada 1980; Reference Jones, Lagasse and ZimmermanJones 1994; Reference Silver, Cross and FoxSilver 1996; Reference Kao and NanagasKao 2004).
Reference Lee and LeungLee & Leung (2008) conducted a similar study with 69 patients after charcoal-burning suicide attempts. The presence of past psychiatric treatment, history of suicide attempt and physical complications were associated with psychiatric comorbidity, whereas loss of consciousness and abnormal blood acidity were associated with medical comorbidity.
Management
Diagnosis is the most important step – being highly aware that the patient’s ill health may be due to CO exposure. This, in itself, can be of tremendous therapeutic benefit, since it will prompt the paramedics and accident and emergency doctors to start appropriate treatment as early as possible.
The first step of management is stabilisation of the patient’s vital status (airway, breathing and circulation). Immediate resuscitation should be performed if the patient is in a critical condition. The second step is assessment of the airway, breathing and circulation. Intubation is indicated in patients in a coma or in those who are severely drowsy. We recommend the immediate use of a 100% oxygen mask once a patient is suspected of CO poisoning. We also recommend strict bed rest to reduce oxygen demand and consumption. Patients with respiratory distress and decreased level of consciousness should be intubated and ventilated.
Oxygen therapy
The most common complication of CO poisoning is hypoxia. Oxygen therapy is effective as most symptoms resolve with high flow of oxygen. The half-life of CO is significantly reduced from 300 min in atmospheric air to 30 min in 100% hyperbaric oxygen (Reference Clardy, Manaker and PerryClardy 2010).
There are two types of therapy using 100% oxygen. In hyperbaric oxygen therapy, the oxygen is at an atmospheric pressure two to three times that of atmospheric pressure at sea level, whereas in normobaric oxygen therapy, the pressure is equivalent to atmospheric pressure at sea level. The main reported benefit of hyperbaric oxygen therapy is its ability to raise the partial pressure of oxygen in arterial blood while decreasing the half-life of CO, thus facilitating its dissociation from haemoglobin. This allows more oxygen to attach to the free-binding sites of haemoglobin, reducing lipid peroxidation and neuropsychiatric sequelae associated with CO poisoning (Reference Prockop and ChichkovaProckop 2007). In addition, hyperbaric oxygen offers more benefits to the brain compared with normobaric oxygen by improving energy metabolism and decreasing neutrophil adherence (Reference StollerStoller 2007). The indications for hyperbaric oxygen treatment include a COHb level above 25% (regardless of symptomatology), evidence of ongoing end-organ ischaemia (regardless of COHb level), profound metabolic acidosis (pH <7.1), loss of consciousness and, in pregnant women, a COHb level >15% or evidence of fetal distress (Reference Hampson, Dunford and KramerHampson 1995; Reference Ernst and ZibrakErnst 1998; Reference WeaverWeaver 1999; Reference Kao and NanagasKao 2004). A typical regime involves administering hyperbaric oxygen treatment at 2.5–3.0 atm for about 45 min in a monoplace chamber.
Despite its potential benefits, hyperbaric oxygen therapy is associated with adverse effects such as reversible myopia, cataracts, tracheobronchial symptoms, self-limited seizures and barotraumas to the middle ear, the cranial sinuses or the lungs. Another limitation is that not all hospitals are equipped with such a chamber. For patients with mild CO poisoning (COHb level <20%), a different regime involving 100% normobaric oxygen for 6 h would be appropriate.
Role of donepezil in CO poisoning
Donepezil, an acetylcholinesterase inhibitor which is commonly used in the treatment of Alzheimer’s disease, has been shown to improve memory in CO poisoning in a case report (Reference Wang, Zeng and ChiWang 2009). This report evaluated a 60-year-old man with a COHb level of 11.2%. He developed neuropsychological impairment after 15 days. Donepezil (10 mg/day) treatment showed improvement in the Mini-Mental State Examination after 2 months. Nevertheless, the overall benefits of donepezil remain unknown. A large, multicentre investigation of the therapeutic effects of donepezil or other acetylcholinesterase inhibitors in CO poisoning is warranted.
Role of cognitive rehabilitation in CO poisoning
There is no well-established rehabilitation programme for patients with cognitive impairment associated with CO poisoning. A suitable programme will have to be created from existing strategies for other neuropsychiatric disorders such as head injury:
-
• individualised treatment with particular focus on the patient’s background, addressing the emotional responses after CO poisoning and developing coping skills for long-term adjustment (Reference Mateer, Sira and O'ConnellMateer 2005);
-
• components such as psychoeducation on the neuropsychiatric sequelae of CO poisoning, identification of behavioural deficits (Reference Mangaoang and LuceyMangaoang 2007), reinforcing previously learned behaviour, establishing compensatory mechanisms for cognitive deficits (Reference Bergquist and MalecBergquist 1997), and applying the strategies learnt to suit the patient’s need through repeated practice;
-
• comparing baseline and post-rehabilitation neuropsychological assessments, helping to monitor treatment progress and giving feedback to the patient and caregivers.
Conclusions
Carbon monoxide poisoning has been reported throughout medical history and its presentation has changed from accidental poisoning due to faulty stoves in the UK almost a century ago to the recent intentional poisoning by individuals who attempt suicide in the Far East. Analyses of case series and advances in imaging techniques have helped clinicians understand the neuropathology of CO poisoning. There is great variation in the onset and clinical presentation as CO poisoning affects both cortical and subcortical brain regions.
All patients who are suspected of having CO poisoning should be given 100% oxygen via a face mask in the accident and emergency department. It should now be clear that the management of CO poisoning does not stop at acute treatment but continues with personalised long-term management in a multidisciplinary setting. All patients should undergo baseline and follow-up neuropsychological assessment. Cognitive rehabilitation targeted at maintaining residual cognitive functions and developing compensatory strategies will be beneficial. Further studies will be required to investigate the effects of acetylcholinesterase inhibitors on cognitive impairment after CO poisoning.
MCQs
Select the single best option for each question stem
-
1 A 20-year-old man murdered his girlfriend and attempted suicide by connecting the exhaust pipe into his car. He was found by a passerby and sent to accident and emergency (A&E) by ambulance. You suspect that he may be suffering from CO poisoning. The following signs and symptoms are commonly found in people after CO poisoning except for:
-
a confusion
-
b memory impairment
-
c respiratory distress
-
d abdominal distension
-
e vomiting.
-
-
2 After you have assessed the patient, you need to advise the A&E doctors, who are not familiar with the management of CO poisoning. The following advice is appropriate except for:
-
a immediate administration of 100% of oxygen via face mask is recommended
-
b blood investigations should include COHb level, blood lactate levels and arterial blood gases
-
c electrocardiogram is necessary as the cardiovascular system is usually affected in CO poisoning.
-
d a chest X-ray is recommended
-
e immediate consultation for a renal physician to arrange urgent dialysis to remove the CO from the haemoglobin.
-
-
3 The laboratory has returned the COHb level results. The A&E doctors want to consult you on the likely neuropsychiatric sequelae in relation to the COHb level. Which of the following statements is incorrect?
-
a patients usually present with vague symptoms if the COHb level is less than 10%
-
b patients usually present with tightness across the forehead, fatigue and sleepiness if the COHb level is between 10 and 20%
-
c the COHb level is expected to be lower in people who have died by suicide than those with accidental poisoning
-
d patients usually present with severe headaches, dizziness and generalised weakness when the COHb level is between 30 and 40%
-
e patients may become unconscious when the COHb level is above 40%.
-
-
4 The patient has been stabilised on the medical ward. The consultant physician has asked you to review the patient and give your professional opinion. Which of the following actions is inappropriate?
-
a obtaining the underlying reason and consequences of the suicide attempt from both the patient and informants
-
b exploring his state of mind when the homicide took place and assessing him for current feelings of guilt
-
c transferring him to police custody once he is free from confusion or cognitive impairment
-
d administering hyperbaric oxygen treatment if the facility is available
-
e regular monitoring of COHb levels.
-
-
5 The patient develops neuropsychiatric complications. His sister would like to discuss with you the long-term management of his case. Which of the following statements would be wrong?
-
a baseline and follow-up neuropsychological assessments are necessary in this case
-
b an assessment of his fitness to plead will be necessary before he appears in court
-
c cognitive rehabilitation to develop compensatory strategies should be helpful
-
d on admission, his COHb level was 35%, putting him at risk of developing neuropsychiatric sequelae
-
e acetylcholinesterase inhibitors have a strong evidence base in treating cognitive impairment after CO poisoning.
-
MCQ answers

| 1 | d | 2 | e | 3 | c | 4 | c | 5 | e |






eLetters
No eLetters have been published for this article.