High producing dairy goats must be fed diets with a large amount of starchy concentrates to support their energy requirements (National Research Council, 2007), if they are to attain maximum levels of lactation performances. Such feeding practice has been shown to interfere with rumen function and to trigger a shift from the trans-11 to the trans-10 pathway of ruminal biohydrogenation of polyunsaturated fatty acids (FA), leading to the production of trans-10, cis-12 18:2 (Zheng et al., Reference Zheng, Wu, Shen, Han, Jin, Chen, Zhao, Cao and Yao2020). This conjugated linoleic acid isomer is known to down-regulate genes involved in mammary lipogenesis, thus inhibiting milk fat synthesis in dairy goats (Lock et al., Reference Lock, Rovai, Gipson, De Veth and Bauman2008; Zheng et al., Reference Zheng, Wu, Shen, Han, Jin, Chen, Zhao, Cao and Yao2020).
Relative to the bovine, the caprine appears to be less sensitive to diet-induced milk fat depression (Chilliard et al., Reference Chilliard, Toral, Shingfield, Rouel, Leroux and Bernard2014). As a result, feeding varying lipid sources rich in unsaturated FA has been shown to increase milk fat secretion in lactating dairy goats, with little impact on milk yield (Chilliard et al., Reference Chilliard, Ferlay, Rouel and Lamberet2003). In this regard, dietary addition of extruded flaxseed as a source of α-linolenic acid (cis-9, cis-12, cis-15 18:3) increased milk fat concentration (Renna et al., Reference Renna, Lussiana, D'Agostino, Mimosi and Fortina2013; Bennato et al., Reference Bennato, Ianni, Innova, Grotto, D'Onofrio and Martino2020) or yield (Chilliard et al., Reference Chilliard, Rouel and Guillouet2013; Bernard et al., Reference Bernard, Toral, Rouel and Chilliard2016) with varying effects on milk production under different experimental conditions.
High-palmitic acid (16:0) lipid supplements have been extensively investigated to determine their feeding value for dairy cows (Mosley et al., Reference Mosley, Mosley, Hatch, Szasz, Corato, Zacharias, Howes and McGuire2007; Piantoni et al., Reference Piantoni, Lock and Allen2013; Rico et al., Reference Rico, De Souza, Allen and Lock2017) and were shown in these experiments to increase milk fat concentration and yield when compared with low-fat control diets. However, studies evaluating the impact of dietary lipids rich in 16:0 on milk yield and fat secretion in lactating dairy goats are scarce. In a rare comparison published several decades ago, Astrup et al. (Reference Astrup, Steine and Robstad1985) observed that milk yield was not affected, whereas milk fat concentration was increased by 1.14 percentage units (+32%) in goats receiving 100 g/d of 16:0. This experiment was conducted with goats in the last third of their lactation, yet Chilliard et al. (Reference Chilliard, Ferlay, Rouel and Lamberet2003) stated that the impact of lipid supplementation on milk fat secretion could be of lower magnitude in late than in early lactation. Therefore, the objective of the current trial was to further document the potential of fat supplementation to improve lactation performances in high-producing dairy goats. We hypothesised that dietary lipid addition would increase milk fat concentration and yield, the response being of greater magnitude with saturated than with unsaturated FA sources.
Materials and methods
Goats, experimental design and dietary treatments
The experimental procedures involving dairy goats followed the guidelines of the Canadian Council on Animal Care (2009) and were approved by the local animal care committee. The experiment was conducted at the Centre de Recherche en Sciences Animales de Deschambault, QC, Canada in a pen facility equipped with Calan gate feeders (American Calan, Northwood, NH, USA). Goats were housed on wood shavings, to prevent consumption of litter material, and had free access to water at all times.
Thirty Alpine goats (6 primiparous, 24 multiparous) were enrolled at kidding. They were first fed a pretreatment total mixed ration (TMR), with a forage-to-concentrate ratio of 55:45 (27.7% NDF and 17.9% starch) on a dry matter (DM) basis (Table 1). This TMR was based on alfalfa silage and ground barley, and was formulated to meet or exceed energy and nutrient requirements using the Small Ruminant Nutrition System (version 1.9.6; Tedeschi et al., Reference Tedeschi, Cannas and Fox2010). Daily TMR was offered in two equal meals at 10:00 and 18:00 h. As a precaution to prevent ruminal disturbance at the initiation of lactation, goats were offered 100 g/d of grass hay before the morning feeding during the pretreatment period. Alfalfa silage was sampled once a week and dried at 55°C for 72 h to determine its DM content, and to adjust the proportion of feed ingredients in TMR on an as-fed basis. Goats were milked twice daily at 7:00 and 17:00 h. In order to facilitate goat handling, the cracked corn portion of the diet was offered at the parlour in two equal meals, at the time of milking.
Table 1. Composition of pretreatment and experimental diets

a CONT, Unsupplemented control diet; PALM, Diet supplemented with palmitic acid; FLAX, Diet supplemented with extruded flaxseed.
b Goats were offered 100 g/d of grass hay in addition to the basal diet.
c Fed at the milking parlour in two equal meals daily.
d Palmit 80®; Natu'oil Services Inc., Port Coquitlam, BC, Canada.
e Val 160TM; Extruded mixture of 30% wheat bran and 70% whole flaxseed; Valorex, Combourtillé, France.
f Contained 200 g/kg Ca, 20 g/kg P, 95 g/kg Na, 50 g/kg Mg, 1 g/kg K, 14 g/kg S, 45 mg/kg I, 840 mg/kg Fe, 600 mg/kg Cu, 2000 mg/kg Mn, 3000 mg/kg Zn, 25 mg/kg Se, 20 mg/kg Co, 200 mg/kg F, 300 000 IU vitamin A, 100 000 IU vitamin D, and 1500 IU vitamin E.
g Calculated using Small Ruminant Nutrition System (version 1.9.6; Tedeschi et al., Reference Tedeschi, Cannas and Fox2010).
After 23 ± 5 d on the pretreatment TMR, goats were allocated to 1 of 10 blocks according to parity and milk fat concentration. Within each block, goats were randomly assigned to one of three dietary treatments (Table 1; DM basis) as follows: (i) a basal diet with a F:C ratio of 45:55 used as control (CONT); (ii) the basal diet supplemented with 2% of a 16:0-enriched fat product (Palmit®, Natu'oil Services Inc., Port Coquitlam, BC, Canada; PALM); and (iii) the basal diet supplemented with 7% of an extruded mixture of 30% wheat bran and 70% whole flaxseed (Val-160™, Valorex, Combourtillé, France; FLAX) as a source of α-linolenic acid (cis-9, cis-12, cis-15 18:3). The experimental period was 41 d in length.
Experimental measurements, samplings and analyses
Data collected on the last 4 d of the pre-treatment period (referred to as day 0) were used as covariates. In addition, goats were subjected to repeated measures during the experimental period on days 7–10, 17–20, and 38–41 (referred to as days 10, 20, and 41, respectively). At each of these periods, goats were weighed on 3 consecutive days after the morning milking. Dry matter intake was determined on 4 consecutive days, and samples of TMR and refusals were collected. At the end of each period, TMR were pooled by treatment and refusals were pooled by goat. Samples of TMR were analysed for residual moisture, neutral detergent fibre, acid detergent fibre, crude protein, organic matter, starch and ash concentrations as described in the online Supplementary File, Materials and methods. Samples of TMR and refusals were analysed for FA profiles using gas chromatography as described by Villeneuve et al. (Reference Villeneuve, Lebeuf, Gervais, Tremblay, Vuillemard, Fortin and Chouinard2013). Milk yield was recorded for 6 consecutive milkings using Waikato MKV milk meters (Waikato Milking Systems LP, Hamilton, New Zealand). Milk samples were harvested at each milking and pooled by goat on a daily basis, proportionately to milk yield. A first set of subsamples was stored at 4°C with 2-bromo-2-nitropropane-1,3-diol as preservative. These subsamples were sent to a commercial laboratory (Lactanet, Ste-Anne-de-Bellevue, QC, Canada) where they were analysed for fat, protein, lactose and urea-N concentration by infrared absorption spectroscopy using a Foss MilkoScan FT 6000 (Foss, Hillerød, Denmark), as well as for somatic cell count determination using a Fossomatic FC (Foss). A second set of subsamples without preservative was stored at −20°C until FA analysis following the procedure described by Boivin et al. (Reference Boivin, Gervais and Chouinard2013), and reported in the online Supplementary File, Material and methods.
Ruminal fluid was collected during one day at all collection periods, except the one conducted on day 10. Samples were harvested at 0, 2, 4, 6 and 8 h after the morning meal. Collections were performed using a flexible plastic tube connected to a metal sieve with 1-mm openings to filter the rumen fluid. This sampling apparatus was inserted into the rumen through the mouth. The liquid was aspirated with a 60-ml syringe, and immediately used for pH measurement (Accumet pH Meter 925, Fisher Scientific, Hampton, NH, USA). Two 10-ml samples were then prepared into 20-ml glass vials containing 200 μl of H2SO4 (50%, v/v), and stored at −20°C for subsequent analysis of volatile FA (VFA) profile by gas chromatography as described by Alfonso-Avila et al. (Reference Alfonso-Avila, Charbonneau, Chouinard, Tremblay and Gervais2017).
Statistical analysis
Data were analysed using the REPEATED statement in the MIXED procedure of SAS (version 9.4; SAS Institute Inc., Cary, NC) according to the following model:
where μ is the overall mean, T i denotes the fixed effect of the ith treatment, B j is the random effect of the jth block (j = 1 to 10), G k:j is the random effect of the kth goat (k = 1 to 3) within the jth block, D l refers to the fixed effect of the lth sampling time (l = 1 to 3), TDil is the interaction term of the ith treatment and the lth sampling time, C is the covariate adjustment for each goat (C = 1–30), and ɛijkl denotes the residual error. Goat within block was included in the model as the subject of the repeated statement.
Because no differences were observed between the last two collection periods, data were combined, and the reduced following model was fitted:
Least-squares means pairwise comparisons were performed with Tukey's adjustment. Results are reported as least-squares means. Significant differences were declared at P ≤ 0.05. Values differing non-significantly at 0.05 < P ≤ 0.10 are mentioned as numerical differences in Results.
Results
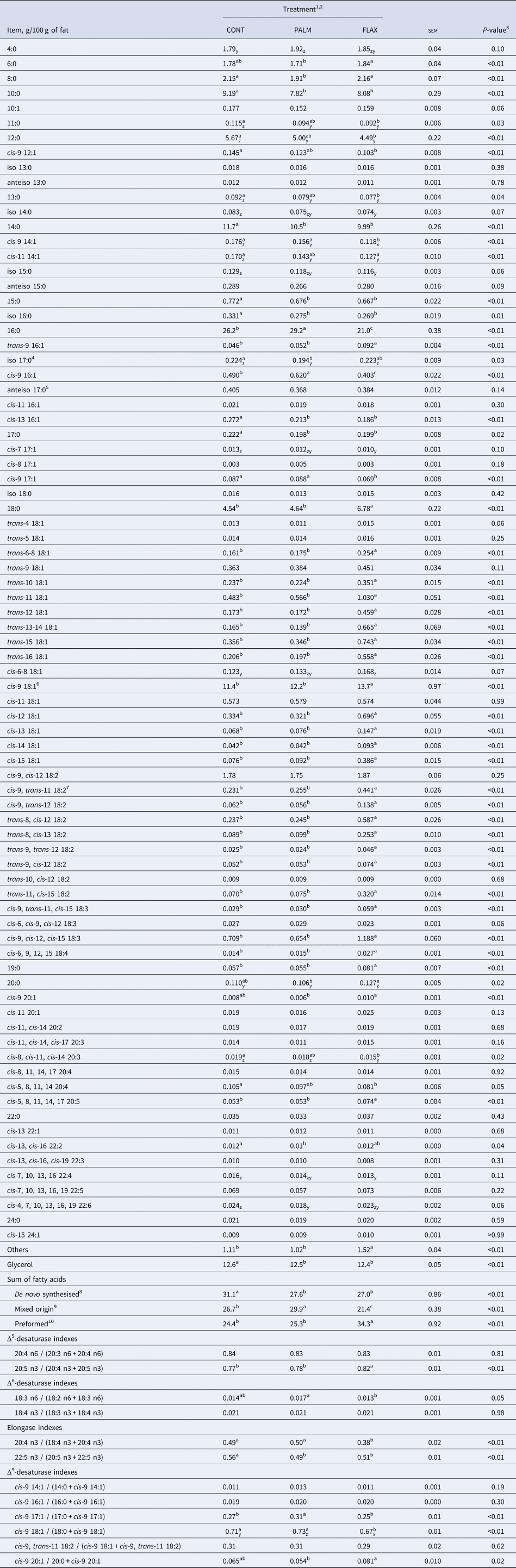
Supplementing the TMR with both experimental lipids, at the expense of ground barley, slightly decreased starch concentration compared with CONT (-5,2% on average; Table 1). Adjustment to the supply of corn gluten meal allowed us to obtain experimental diets with similar crude protein concentrations. Both lipid supplements increased the FA concentration of TMR by 76%. Supplementing the TMR with PALM increased the concentration of 16:0, which reached 49% of total FA, whereas dietary addition of FLAX increased the concentration cis-9, cis-12, cis-15 18:3 up to 37.6% of total dietary FA (Table 1).
Similar body weight, DM intake, actual and fat-corrected milk yield as well as feed efficiency were observed between treatments on days 20 and 41 of the feeding trial (Table 2). During the same period, feeding PALM and FLAX increased milk fat concentration, and PALM produced a non-significant numerical increase in milk fat yield as compared with CONT (Table 2). Milk fat concentration gradually declined over the experimental period, when the level of concentrate was increased in the TMR, and as goats reached their lactation peak, when compared with the pretreatment period (Fig. 1a). The decrease was of a lower magnitude when TMR was supplemented with PALM or FLAX as compared with CONT on days 20 and 41 of the feeding trial. Milk protein concentration was greater with FLAX, intermediate with CONT, and lesser with PALM (Table 2). These differences gradually developed over the course of the experimental period (Fig. 1b). Milk protein yield as well as lactose concentration and yield were similar among treatments. Milk urea-N was numerically greater with CONT than with FLAX, whereas an intermediate concentration was observed with PALM. Milk somatic cell score was numerically greater with FLAX than with PALM, whereas an intermediate score was observed with CONT. Ruminal pH (![]() $\bar{{\rm x}}$ = 6.33), total concentration of VFA (
$\bar{{\rm x}}$ = 6.33), total concentration of VFA (![]() $\bar{{\rm x}}$ = 82.8 mmol/l), relative proportions of acetate (
$\bar{{\rm x}}$ = 82.8 mmol/l), relative proportions of acetate (![]() $\bar{{\rm x}}$ = 62.6 mol/100 mol), propionate (
$\bar{{\rm x}}$ = 62.6 mol/100 mol), propionate (![]() $\bar{{\rm x}}$ = 20.7 mol/100 mol), and butyrate (
$\bar{{\rm x}}$ = 20.7 mol/100 mol), and butyrate (![]() $\bar{{\rm x}}$ = 12.4 mol/100 mol), as well as the acetate-to-propionate ratio (
$\bar{{\rm x}}$ = 12.4 mol/100 mol), as well as the acetate-to-propionate ratio (![]() $\bar{{\rm x}}$ = 3.14) were not different between treatments (online Supplementary File, Table S1).
$\bar{{\rm x}}$ = 3.14) were not different between treatments (online Supplementary File, Table S1).

Fig. 1. Concentrations of (a) milk fat and (b) milk protein in dairy goats fed different lipid supplements over a 41-d experimental period. ■: Unsupplemented control diet (Cont); ◆: Diet supplemented with palmitic acid; and ⬤: Diet supplemented with extruded flaxseed; referred to as CONT, PLAM, and FLAX, respectively, in text and tables. Table values and statistical comparisons are presented in the online Supplementary File, Table S2.
Table 2. Average body weight, dry matter intake, milk yield, and milk composition in dairy goats fed different lipid supplements from days 20 and 41 of the experimental period

1 CONT, Unsupplemented control diet; PALM, Diet supplemented with palmitic acid; FLAX, Diet supplemented with extruded flaxseed.
2 Within a row, means without a common superscript letter differ (a,b; P ≤ 0.05) or tend to differ (z,y; 0.05 < P ≤ 0.10.
3 Interaction of time × treatment: *P ≤ 0.05 (see Fig. 1).
4 4% FCM = actual milk yield, kg/d × [0.411 + (0.147 × milk fat, %)]; Mavrogenis and Papachristoforou (Reference Mavrogenis and Papachristoforou1988).
5 Somatic cell score = log2(somatic cell count/100 000) + 4; (Shook, Reference Shook1982).
Feeding FLAX increased the concentration of preformed FA in milk fat as compared with CON and PALM (Table 3). The rise was gradual over the first 20 d of the experimental period (Fig. 2a), remained stable thereafter and was compensated by a concomitant decrease in concentrations of FA of mixed origins (i.e., 16:0 + cis-9 16:1; Fig. 2b) and of de novo synthesised FA (Fig. 2c). On the other hand, dietary addition of PALM increased milk fat concentration 16:0 + cis-9 16:1, lowered the concentrations of de novo synthesised FA, but did not affect the proportions of preformed FA as compared with CONT.

Fig. 2. Milk fat concentrations of (a) preformed fatty acids (sum of all fatty acids with a carbon chain length of 18 or more), (b) fatty acids of mixed origin (16:0 + cis-9 16:1), and (c) de novo synthesised fatty acids (sum of straight even-chain fatty acids from C6 to C14) in dairy goats fed different lipid supplements over a 41-d experimental period. ■: Unsupplemented control diet, ◆: Diet supplemented with palmitic acid, and ⬤: Diet supplemented with extruded flaxseed; referred to as CONT, PLAM, and FLAX, respectively, in text and tables. Table values and statistical comparisons are presented in the online Supplementary File, Table S3.
Table 3. Average milk fat composition in dairy goats fed different lipid supplements from days 20 and 41 of the experimental period
1 CONT, Unsupplemented control diet; PALM, Diet supplemented with palmitic acid; FLAX, Diet supplemented with extruded flaxseed.
2 Within a row, means without a common superscript letter differ (a,b,c; P ≤ 0.05) or tend to differ (z,y,x; 0.05 < P ≤ 0.10).
4 Coelution with minor concentration of trans-10 16:1.
5 Coelution with minor concentration of cis-10 16:1.
6 Coelution with minor concentration of cis-10 18:1.
7 Coelution with minor concentration of trans-7, cis-9 18:2.
8 Sum of straight even-chain fatty acids from C6 to C14.
9 16:0 + cis-9 16:1.
10 Sum of all fatty acids with a carbon chain length of 18 or more.
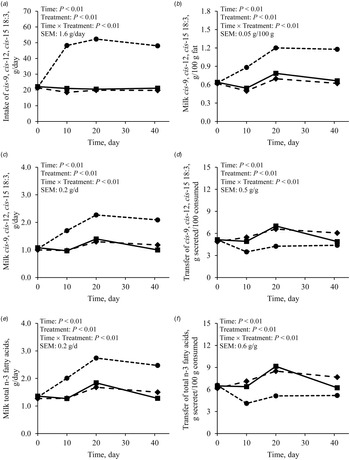
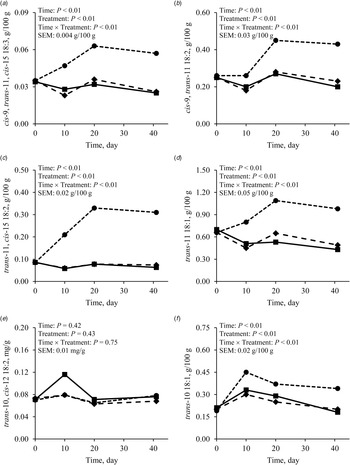
As the intake of cis-9, cis-12, cis-15 18:3 was greater with FLAX than with CONT or PALM (Table 4; Fig. 3a), concentration and yield of this FA increased in milk fat (Tables 3 and 4, Fig. 3b and 3c). Treatments did not differ in the yield of FA of the n-3 family other than cis-9, cis-12, cis-15 18:3 (Table 4). Dietary supply of FLAX also increased milk fat concentration of cis-9, trans-11, cis-15 18:3, trans-11, cis-15 18:2, cis-9, trans-11 18:2, trans-11 18:1, and trans-10 18:1, but did not affect the proportion of trans-10, cis-12 18:2 (Table 3, Fig. 4). The apparent transfer efficiency, from diet to milk, of cis-9, cis-12, cis-15 18:3 (Fig. 3d) and total n-3 FA (Fig. 3f) was lower with FLAX as compared with CONT and PALM (Table 4). Intake, cis-9, cis-12 18:2 was also increased with FLAX, but its secretion remained unchanged as compared with CONT and PALM. The transfer efficiency of this n-6 FA was least with FLAX, intermediate with CONT and greatest with PALM.

Fig. 3. Effect of different lipid supplements on cis-9, cis-12, cis-15 18:3 intake (a), concentration (b) and secretion (c) in milk fat, and apparent transfer efficiency from diet to milk (d), along with total n-3 fatty acid secretion in milk fat (e), and transfer efficiency from diet to milk (f) in lactating dairy goats over a 41-d experimental period. ■: Unsupplemented control diet, ◆: Diet supplemented with palmitic acid, and ⬤: Diet supplemented with extruded flaxseed; referred to as CONT, PLAM, and FLAX, respectively, in text and tables. Table values and statistical comparisons are presented in the online Supplementary File, Table S2.

Fig. 4. Milk fat concentrations of (a) cis-9, trans-11, cis-15 18:3, (b) trans-11, cis-15 18:2, (c) cis-9, trans-11 18:2, (d) trans-11 18:1, (e) trans-10, cis-12 18:2, and (f) trans-10 18:1 in dairy goats fed different lipid supplements over a 41-d experimental period. ■: Unsupplemented control diet, ◆: Diet supplemented with palmitic acid, and ⬤: Diet supplemented with extruded flaxseed; referred to as CONT, PLAM, and FLAX, respectively, in text and tables. Table values and statistical comparisons are presented in the online Supplementary File, Table S3.
Table 4. Average intake, milk secretion and apparent transfer efficiency, from diet to milk, of polyunsaturated fatty acids in dairy goats fed different lipid supplements from days 20 and 41 of the experimental period

1 CONT, Unsupplemented control diet; PALM, Diet supplemented with palmitic acid; FLAX, Diet supplemented with extruded flaxseed.
2 Within a row, means without a common superscript letter differ (P ≤ 0.05).
3 Interaction of time × treatment: **P ≤ 0.01; *P ≤ 0.05 (see Fig. 3 and online Supplementary Table S2).
4 Sum of cis-6, cis-9, cis-12 18:3, cis-11, cis-14 20:2, cis-8, cis-11, cis-14 20:3, cis-5, cis-8, cis-11, cis-14 20:4, cis-13, cis-16 22:2, and cis-7, cis-10, cis-13, cis-16 22:4.
5 Sum of cis-6, cis-9, cis-12, cis-15 18:4, cis-11, cis-14, cis-17 20:3, cis-8, cis-11, cis-14, cis-17 20:4, cis-5, cis-8, cis-11, cis-14, cis-17 20:5, cis-13, cis-16, cis-19 22:3, cis-7, cis-10, cis-13, cis-16, cis-19 22:5, and cis-4, cis-7, cis-10, cis-13, cis-16, cis-19 22:6.
As compared with CONT, feeding PALM and FLAX significantly reduced (15:0 and 17:0) and caused a non-significant numerical reduction (11:0 and 13:0) in milk fat concentrations of odd-chain FA (Table 3). Moreover, dietary supply of FLAX decreased the concentration of iso 16:0 and caused a numerical decrease in iso 14:0 and iso 15:0, whereas it did not affect the proportions of other identified branched-chain FA (iso 13:0, anteiso 13:0, anteiso 17:0, anteiso 15:0, iso 17:0, and iso 18:0) relative to CONT.
Discussion
The lack of effect of lipid supplements on milk yield in the current trial is in accordance with previous experiments studying the impact of extruded flaxseed (Renna et al., Reference Renna, Lussiana, D'Agostino, Mimosi and Fortina2013; Bernard et al., Reference Bernard, Toral, Rouel and Chilliard2016; Klir et al., Reference Klir, Castro-Montoya, Novoselec, Molkentin, Domacinovic, Mioc, Dickhoefer and Antunovic2017) or 16:0 supplement (Astrup et al., Reference Astrup, Steine and Robstad1985) in dairy goats. Moreover, the substitution of lipid for starch did not seem to have affected ruminal fermentation pattern, as pH and relative proportions of VFA were not different between treatments. Data on the effects of fat supplementation on ruminal fermentation in dairy goats are scarce and inconsistent. While no information is available regarding 16:0 supplements, Kholif et al. (2016) have shown that feeding flaxseed oil reduced ruminal pH and relative proportion of acetate, but increased the concentration of total volatile FA and the relative proportion of propionate. Such impacts on rumen environment were not observed in the current trial.
Both lipid supplements increased milk fat concentration to a similar extent when compared with CONT, whereas PALM produced a numerical but non-significant increase in milk fat yield. Feeding PALM, therefore, appears to be slightly more efficient than FLAX in promoting milk fat secretion of dairy goats in the current study. However, milk protein concentration was lower with PALM than with FLAX. This difference could be explained by a dilution effect, as milk yield was numerically greater with PALM, and as PALM and FLAX did not differ in protein yield.
Feeding PALM, as a source of 16:0, increased the level of cis-9 16:1 in milk fat. This augmentation is the result of an active desaturation process taking place in goat mammary gland (Bickerstaffe and Annison, Reference Bickerstaffe and Annison1970). The increase in milk fat concentration of 16:0 + cis-9 16:1 was mostly at expense of de novo synthesised FA, as the proportion of preformed FA, taken up from the bloodstream by mammary gland, was not significantly affected by PALM. Moreover, feeding PALM did not affect the relative proportion of cis or trans isomers of 18:1 and 18:2, suggesting the dietary 16:0 was inert in the rumen, and did not seem to interfere with the activity of microorganisms responsible for biohydrogenation processes.
A lower apparent transfer, from diet to milk, of cis-9, cis-12, cis-15 18:3 observed with FLAX, when compared with CONT or PALM, suggests that this variable follow a Michaelis–Menten kinetics, that is to say, as the provision of this essential FA increases, the amount recovered in milk also increases but at progressively lowered efficiency. A similar phenomenon has been observed in dairy cows by Benchaar et al. (Reference Benchaar, Romero-Pérez, Chouinard, Hassanat, Eugene, Petit and Côrtes2012) when feeding increasing levels of flaxseed oil from 0 to 4% of DM, leading to a linear decrease in the apparent recovery of cis-9, cis-12, cis-15 18:3 in milk from 10.1 to 2.4%.
The low overall transfer efficiency of unsaturated FA from diet to milk is largely explained by the biohydrogenation taking place within the rumen. The main pathway in the hydrogenation of cis-9, cis-12, cis-15 18:3 involves an initial isomerisation where the cis-12 double bond is converted to a trans-11 double bond, producing cis-9, trans-11, cis-15 18:3 (Harfoot, Reference Harfoot and Christie1981). The second and third steps consist of the hydrogenation of cis-9 and cis-15 double bonds to sequentially produce trans-11, cis-15 18:2, then trans-11 18:1. After its absorption, trans-11 18:1 can also be desaturated to cis-9, trans-11 18:2 by the action of the enzyme Δ9-desaturase (Griinari and Bauman, Reference Griinari, Bauman, Yurawecz, Mossoba, Kramer, Pariza and Nelson1999). Concentrations of these FA were increased in milk fat by the dietary supply of FLAX in the current experiment. The production of FA intermediates of the trans-10 pathway has also been reported during the biohydrogenation of cis-9, cis-12, cis-15 18:3 (Lee and Jenkins, Reference Lee and Jenkins2011). However, no significant amounts of trans-10, cis-12 18:2 appear to be directly produced during this process, which could explain the lack of difference between treatments on milk fat concentration of this FA in the current experiment.
Despite ruminal biohydrogenation, the concentration of cis-9, cis-12, cis-15 18:3 in milk fat was increased by 67.6% when feeding FLAX as compared with CONT. In this regard, studies have shown positive impacts on blood lipid profile in human subjects consuming food products, including milk, from FLAX-fed animals (Weill et al., Reference Weill, Schmitt, Chesneau, Daniel, Safraou and Legrand2002; Malpuech-Brugère et al., Reference Malpuech-Brugère, Mouriot, Boue-Vaysse, Combe, Peyraud, LeRuyet, Chesneau, Morio and Chardigny2010). Dietary cis-9, cis-12, cis-15 18:3 escaping ruminal biohydrogenation can be elongated and desaturated to produce longer chain highly unsaturated FA of the n-3 family, which are eventually incorporated into milk fat (Hagemeister et al., Reference Hagemeister, Franzen, Barth and Precht1991). In the current trial, the greater availability of cis-9, cis-12, cis-15 18:3 with FLAX did not affect the total secretion of other members of the n-3 family when compared with CONT. The regulation appears to be at the elongation steps, as most of Δ5- and Δ6-desaturase indexes were not different between CONT and FLAX, but both elongase indexes were significantly lower with FLAX than with CONT. The increased proportions of preformed FA with FLAX were mainly compensated by lower concentrations of 10:0, 12:0, 14:0, and 16:0 which were respectively decreased by 1.1, 1.2, 1.8 and 5.2 percentage units as compared with CONT. These results corroborate the observations from a meta-regression carried out by Martínez Marín et al. (Reference Martínez Marín, Núñez Sánchez, Garzón Sigler, Peña Blanco and de la Fuente2015) showing that dietary unsaturated plant lipids have a more pronounced negative effect on medium chain saturated FA contents in milk fat of dairy goats as their chain length increases.
Fat supplementation (PALM and FLAX) reduced milk fat concentration of odd-chain FA, and dietary FLAX decreased few branched-chain FA relative to CONT. Milk odd- and branched-chain FA are mainly derived from bacteria leaving the rumen (Vlaeminck et al., Reference Vlaeminck, Fievez, Cabrita, Fonseca and Dewhurst2006). Variations in their concentrations were, therefore, suggested to reflect rumen function. In the current experiment, fat supplements appear to have had minor impact on microbial activity, as fermentation parameters were not different between treatments. Limited effects on bacterial populations are, therefore, suspected. Lower concentrations of several milk odd- and branched-chain FA following fat supplementation could then be explained by a reduced de novo synthesis of FA by ruminal bacteria, a phenomenon that has been observed previously in dairy cows (Weisbjerg et al., Reference Weisbjerg, Børsting and Hvelplund1992). Alternatively, lower levels of odd-chain FA with both fat supplements could be explained by a dilution effect due to a greater uptake of preform FA of dietary origin by mammary gland for incorporation into milk fat.
In conclusion, we have compared the effects of two lipid supplements greatly differing in their FA profile when added to the diet of early lactating dairy goats. Both PALM and FLAX increased milk fat concentration, but only PALM had a minor (non-significant) stimulatory effect on milk fat yield (+22 g/d) as compared with CONT. Actual and fat-corrected milk yields were similar between treatments. It could be hypothesised that the basal control with a forage-to-concentrate ratio of 45:55 offered enough energy to support optimal lactation performances, so that the extra energy provided by lipid supplements could not be used to further improve milk production. Feeding FLAX increased milk fat concentration of cis-9, cis-12, cis-15 18:3 and total n-3 FA at the expense of shorter chain saturated FA (e.g. 10:0, 12:0, 14:0, and 16:0). Such changes have been reported to have positive impacts on blood lipid profile in human subjects. On the other hand, dietary PALM increased milk fat concentration of 16:0 also at the expense of shorter chain FA (i.e., 10:0, 12:0, and 14:0). However, the impact of such modifications to the FA profile on the nutritive value of milk remains to be determined.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0022029922000784
Acknowledgements
We acknowledge the financial support from the Programme Innov'Action Agroalimentaire from the agreement Cultivons L'Avenir 2 concluded between the Ministère de l'Agriculture, des Pêcheries et de l'Alimentation du Québec and Agriculture and Agri-Food Canada. We also thank the manager and staff of Centre de Recherche en Sciences Animales de Deschambault for their supports.